Abstract
Background
Iron deficiency anemia (IDA)-induced reactive thrombocytosis can occur in children and adults. The underlying mechanism for this phenomenon is indeterminate. Traditional cytokines such as thrombopoietin (TPO), interleukin-6 (IL-6), and IL-11 involved in megakaryopoiesis have not been shown to be the cause. Recent studies suggest that growth factors and signaling molecules involved with angiogenesis influence the proliferation and differentiation of megakaryocytes.
Methods
We investigated the possible association between angiogenic cytokines with reactive thrombocytosis due to IDA in an iron-deficient (ID) rat model. Complete blood count, iron panels, and TPO levels were measured at baseline and 5 weeks later in both control (C) and ID rats. Angiogenic cytokines were evaluated in the bone marrow in all rats.
Results
We successfully induced IDA in our rats by phlebotomy and reduced iron diet. We did not find an increase of TPO in ID rats. A review of the bone marrow showed an increase in the number of megakaryocytes, vascular structures, as well as increased intensity of stain for vascular endothelial growth factor (VEGF), and CXC chemokine receptor 4 (CXCR4) in rats with IDA compared to controls.
Conclusions
Our results of histological bone marrow data suggest an important role for angiogenesis in the development of IDA-induced thrombocytosis.
Impact
-
Thrombocytosis is common with IDA in both children and adults, but the mechanism is unclear.
-
We confirmed that TPO is not the major driver of iron deficiency-associated thrombocytosis.
-
We confirmed the increase in the number of megakaryocytes in the bone marrow despite stable TPO levels.
-
We provided evidence supporting an important role of angiogenesis in megakaryocytopoiesis/thrombopoiesis with increased vascular structures and angiogenic cytokines in the bone marrow of iron-deficient rats.
-
The demonstration that angiogenesis may play an important role in secondary thrombocytosis could lead to a new approach in treating symptomatic reactive thrombocytosis by targeting angiogenesis.
Similar content being viewed by others
Introduction
Thrombocytosis is a frequent complication of iron deficiency anemia (IDA) in children and adults.1,2 Although in most children with IDA thrombocytosis is an asymptomatic manifestation, the mechanism responsible for the thrombocytosis in IDA is not clear. Platelets are the final cytoplasmic organelle product of megakaryocytes. Therefore, platelet production, or thrombopoiesis, is dependent on megakaryocyte proliferation and differentiation from hematopoietic stem cells or megakaryocytopoiesis. Hematopoietic stem cells in turn are the product of differentiation of haemangioblast, which gives rise to both vascular and blood precursors.3 Platelets have multiple physiologic functions, including hemostasis, angiogenesis, wound healing, and cancer metastasis. Megakaryocytopoiesis supplies about 1011 platelets per day with a turnover every 8–9 days.4 Thrombopoietin (TPO), the ligand for megakaryocytic growth factor receptor (c-mpl), is the main contributor of megakaryopoiesis and thrombopoiesis. TPO is the principal regulator of these processes but is not essential. Mice deficient in TPO or its receptor c-mpl have ~10–20% of normal platelet count and do not show any abnormalities in platelet or megakaryocyte structure.5 In parallel, congenital amegakaryocytic thrombocytopenia, a rare congenital thrombocytopenia that is due to mutations in c-mpl (TPO surface receptor), results in severe thrombocytopenia, but not a platelet count of zero.6 In contrast, NF-E2- (a transcription factor that controls terminal megakaryocyte maturation) deficient mice have profound thrombocytopenia, but normal TPO signaling.7 These findings suggest that other receptors and ligands may be able to support megakaryopoiesis.
Megakaryocytes may increase >10-fold in certain circumstances causing elevated platelet counts. There are three major categories of thrombocytosis. These include familial thrombocytosis, clonal thrombocytosis, and reactive or secondary thrombocytosis such as that associated with infection, postsurgical state, malignancy, or IDA. Serum TPO levels are elevated in some forms of secondary thrombocytosis.8
The processes involved in the development of thrombocytosis associated with IDA are not well understood. Previous studies assessed the role of thrombopoietic cytokines, such as erythropoietin, TPO, interleukin-11, interleukin-6, and leukemia inhibitory factor in patients with IDA and reactive thrombocytosis. The results reported by Akan et al.9 did not show any relationship of these cytokines to thrombocytosis as to be responsible for the reactive thrombocytosis associated with IDA.
Recent studies suggest that growth factors and signaling molecules involved with angiogenesis influence the proliferation and or differentiation of megakaryocytes. A recent animal study demonstrated that a vascular endothelial growth factor (VEGF) receptor 1-mediated pathway stimulates up-regulation of CXC chemokine receptor 4 (CXCR4) on megakaryocytes, which results in enhanced platelet production via redistribution of megakaryocytes in the bone marrow.10 Another study demonstrated that platelet-derived growth factor activated mesenchymal stromal cells to produce VEGF through the phosphatidylinositol 3-kinase-dependent pathway.11 A third study also reported that the FMS-like tyrosine kinase-3, in in vitro cultures, stimulated the secretion of VEGF in ex vivo cultured acute lymphoblastic leukemia xenograft cells.12 These findings suggest an alternative process that may be involved in the generation of secondary thrombocytosis.
In this study, we investigated the possible association between angiogenic cytokines with reactive thrombocytosis due to IDA in an iron-deficient (ID) rat model.
Methods
Animals
This research was performed in accordance with the guidelines and approval of the Institutional Animal Care and Use Committee of the University of Illinois College of Medicine Peoria, Illinois (Protocol number 594480-6). Six-week-old male Sprague–Dawley rats with jugular vein cannulas were purchased from Harlan Laboratories (Indianapolis, IN). C rats (N = 10) were fed a diet containing 50 p.p.m. of iron (standard Purina chow) and ID rats (N = 20) were fed a diet containing 7–8 p.p.m iron in Purina chow. In addition, 1.5 mL of blood was drawn from ID rats at baseline to further induce anemia.
Blood collection and processing
All blood samples were drawn at baseline and 5 weeks later. For complete blood count (CBC), 0.75 mL of whole blood was collected in an EDTA tube and shipped to the University of Illinois at Urbana-Champaign College of Veterinary Medicine. CBC was estimated using the Abbott Cell-Dyn 3700 hematology analyzer. In the iron panel, 0.5 mL of whole blood was placed in lithium heparin tubes, immediately spun at 3500 r.p.m. for 10 min to collect plasma, and shipped to Cornell University to run commercially available iron panel (serum iron, saturation, and transferrin available to bind iron (TIBC)) using the Roche/Hitachi Mod P chemistry analyzer. For the TPO assay, 0.5 mL of whole blood was collected in heparin (10% of final volume), immediately centrifuged at 3000 r.p.m. for 20 min at 2–8 °C to obtain plasma, and stored in −80 °C or used immediately for testing.
Measurement of TPO by sandwich enzyme-linked immunosorbent assay (ELISA)
TPO concentrations were measured in duplicate from C and ID rats by sandwich ELISA using a commercial kit (MyBiosource, San Diego, CA) according to the manufacturer’s protocols. Manufacture protocol included incubating samples in pre-coated microtiter plates with anti-TPO antibodies. TPO was measured using biotin-conjugated anti-TPO antibody, followed by horseradish peroxidase-conjugated avidin and appropriate substrate. Optical density absorbance was read at 450 nm in a microplate reader.
Hematoxylin and eosin (H&E) staining and immunohistochemical staining
A total of 29 rats were evaluated (10 C and 19 ID rats). Femurs were collected, fixed in formalin, following decalcification using RapidCal. Tissues were then dehydrated in an ethanol gradient, followed by xylene gradient, and then set in paraffin blocks to be sectioned longitudinally. Sections at an average thickness of 4 µM were collected on subbed slides as per standard protocols. The sections were then deparaffinized using a xylene–ethanol gradient, followed by an ethanol water gradient. Prior to blocking, antigen retrieval was done by immersing the rehydrated section in 85 °C phosphate-buffered saline (PBS) for 120 s, followed by cooling in 4 °C PBS. Native peroxidases were inactivated by dipping the sections in 0.3% H2O2 in PBS for 60 s. The heat-shocked and peroxidase-inactivated sections were then blocked with blocking solution [1× PBS) + 1% bovine serum albumin] for 30 min at room temperature. Blocked sections were washed and incubated with one of the following primary antibody (1:100 dilution in blocking solution) [peroxidase linked anti-factor VIII (Santa Cruz Biotechnology, Inc., Dallas, TX), VEGF (Santa Cruz, Dallas, TX), and CXCR4 (Abcam, Cambridge, MA)] for 1 h at room temperature, followed by washing in PBS (3× 10 min each). The slides were then incubated with secondary antibody (1:500 dilution in blocking solution) for 1 h at room temperature in PBS. Following secondary antibody incubation, the slides were washed in PBS (3× 10 min each). Immunoperoxidase staining was done according to standard protocols (DAB (3,3'-diaminobenzidine) staining, Vector Labs, Burlingame, CA). The stained slides were counter-stained with hematoxylin, followed by microscopy. Two femur sections per rat were used for all IHC staining procedures and each section was analyzed in duplicate for all markers. The microscopic images were then analyzed using ImageJ Fiji software using the IHC toolbox plugin.13,14
For H&E staining to quantitate the number of megakaryocytes, deparaffinized and rehydrated slides were stained with hematoxylin and eosin as per standard protocols. The slides were evaluated under ×40 microscopy. The number of megakaryocytes in the selected area was visually quantitated and used for analysis.
Statistics
Our statistical analysis was obtained using the SAS software and GraphPad Prism v8. We compared each outcome between C and ID groups using two-tailed t test if normality was held. Otherwise, we used Wilcoxon rank-sum tests. When values were not all numerical, we created binary variables 0 and 1 and used the McNemar test. A P value < 0.05 was considered statistically significant.
Results
Low iron diet with phlebotomy induces iron deficiency, anemia, and thrombocytosis in rats
We induced iron deficiency in the ID rats by feeding them a low iron diet and day-zero phlebotomy of 1.5 mL of blood. Iron studies from the ID rats (N = 18) documented iron deficiency with a low mean serum iron of 67.2 ± 13.4 μg/dL on day 35 of the study, and a decrease from a mean of 188.0 ± 126.0 μg/dL at day 0 (P < 0.0001). TIBC increased to a mean level of 709.5 ± 54.5 μg/dL on day 35, from a baseline mean level of 519.2 ± 24.3 μg/dL on day 0 (P = 0.0006) (Table 1).
ID rats (N = 18) developed anemia with a mean hemoglobin of 11.6 ± 1.1 g/dL on day 35 compared to the C rats (N = 9) that had a mean hemoglobin of 13.9 ± 0.6 g/dL (P < 0.0001). The mean corpuscular volume (MCV) also decreased in the ID rats with a mean of 43.6 ± 2.6 fl compared to the C rats with a mean of 55.6 ± 1.2 fl (P < 0.0001) (Table 1).
Thrombocytosis developed in the ID rats with a mean platelet count value in the ID rats of 1,263,300 ± 190,000 per dL on day 35 compared to the mean value in C rats of 444,100 ± 81,000 per dL (P < 0.0001) (Table 1).
Plasma TPO was not found to be related to thrombocytosis associated with IDA
There was no significant difference in baseline plasma TPO between groups (P = 0.08), with a mean of 36.98 ± 12.89 pg/mL in ID rats (N = 19) and 47.49 ± 10.29 pg/mL in C rats (N = 6). There was no significant difference in plasma TPO at 35 days between groups (P = 0.48), with a mean of 44.23 ± 21.83 pg/mL in ID rats (N = 19) and 39.10 ± 12.58 pg/mL in C rats (N = 6). The difference from baseline and final plasma TPO was not significantly different among ID and C rats (P = 0.55).
IDA increased megakaryocytes and vessels in the bone marrow
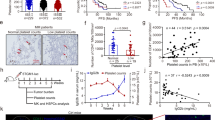
H&E-stained bone marrow slides from ID and C rats were studied under ×40 microscopy. There was a significant difference in the number of megakaryocytes among groups (P < 0.0001), with an average of 4.3 ± 0.79 megakaryocytes in C rats and 11.0 ± 0.8342 in ID rats (Fig. 1). There was a significant difference in factor VIII-stained slides (P < 0.0001) between groups, with quantitative analysis of peroxidase-stained megakaryocytes in C rats containing 334,928 ± 49,583 pixels, while staining in the ID rats was 1,404,573 ± 96,642 pixels (Fig. 2a, b). When the analysis was carried out looking at vessel staining, there was a significant difference between C and ID rats (P < 0.0001), with 5.4 ± 1.16 vessels in C rats and 33 ± 2.40 vessels in ID rats (Fig. 2c) suggesting increased angiogenesis under iron deficiency.
Femurs from rats were collected, decalcified, and embedded in paraffin. Slides from thin sliced sections of bone were stained with hematoxylin and eosin (H&E). a Slides were evaluated under ×40 microscopy and megakaryocytes (marked with yellow arrows) were visually quantitated in control rats (n = 10) and iron-deficient rats (n = 19). b The graph shows the number of megakaryocytes and is represented as mean ± SEM. *P < 0.0001.
a Slides from the bone marrow of control rats (n = 10) and iron-deficient rats (n = 17) were stained with peroxidase-linked anti-factor VIII and pictures were taken in a bright field microscope. b Factor VIII staining was quantitated using ImageJ Fiji software. The graph shows the area (in pixels) stained for factor VIII and data are expressed as mean ± SEM. *P < 0.0001. c Vessel formation as shown marked with yellow arrows were visually counted in both control and low iron slides and data are expressed as mean ± SEM. *P < 0.0001.
IDA was associated with increased VEGF and CXCR4 in the bone marrow
In the VEGF-stained slides, there was a significant difference of quantitation of peroxidase stain between groups (P = 0.0127), with a 2.9-fold increased intensity of staining per cell in the ID rats (area 233,011 ± 47,723) compared to the C rats (area 80,475 ± 16,671) (Fig. 3a, b). In the CXCR4-stained slides, there was a significant difference in the quantitation of CXCR4 staining among groups (P = 0.0004), with a 2.13-fold increase in ID rats (area 70,235 ± 6978) compared to C rats (area 32,941 ± 2027) (Fig. 4a, b).
a Slides from the bone marrow of control rats (n = 9) and iron-deficient rats (n = 11) were stained with peroxidase-linked VEGF antibody and pictures were taken using bright-field microscopy. b The intensity of VEGF staining was quantitated using ImageJ Fiji software. The graph shows the area (in pixels) stained for VEGF and data are expressed as mean ± SEM. **P = 0.0127.
Discussion
In this study, we successfully induced ID anemia in an animal model with co-existing thrombocytosis using an iron-depleted diet and day-zero phlebotomy. We did not find any association of serum levels of TPO with IDA-induced thrombocytosis. Evaluation of bone marrow histology on H&E-stained slides documented an increase in megakaryocyte numbers. We also documented a marked increase in vessel numbers, suggesting active angiogenesis in the ID rats. We demonstrated the increased intensity of VEGF stain on the surface of megakaryocytes in the ID rats as compared to C rats. We found increased CXCR4 on megakaryocytes of ID rats as compared to C rats. Moreover, we also demonstrated an increase in the number of small CXCR4-positive cells in the bone marrow of ID rats, which we speculate represent an increase of early megakaryocyte-precursor cells.
Previous studies have used the rat model to investigate the development of IDA by restricting iron from their diets. Younger rats are typically more amendable to induction of IDA, given their rapid growth rate, and a greater percentage of iron is derived from diet alone. Dallman et al.15 were able to successfully induce IDA in 36-day-old rats by ID diets alone. We aimed to follow the same time frame at inducing IDA with an ID diet. However, since our rat model included placement of jugular vein cannulas, the earliest the experimental animal was available to induce an ID diet was at 42 days of life; therefore, we decided to additionally phlebotomize our rat model to ensure IDA. Our method was able to induce IDA and thrombocytosis, which was evident with lower hemoglobin, lower MCV, higher TIBC, and higher platelet count in ID rats when compared to C rats, as well as lower iron levels in the ID rats when compared at day 0 to day 35 of study.
Traditional hematopoietic growth factors have not been shown to be associated with IDA-induced thrombocytosis. A recent study by Evstatiev et al.16 assessed the mRNA expression of several hematopoietic growth factors, including TPO, IL-6, and IL-11 in the liver, kidney, and spleen of ID rats, and did not find statistical differences between the ID and C rats.
In addition to not finding an increase in traditional hematopoietic growth factors, Evstatiev et al.16 also found a decreased megakaryocyte proliferation, and changes in megakaryopoiesis that included enhanced progenitor expansion, increased megakaryocyte ploidy, and overall augmented megakaryocyte differentiation. They went on to further investigate potential genes involved in the process of megakaryocyte differentiation and found increased hypoxia-inducible factor 1 alpha subunit protein expression in megakaryocytes from ID rats, and a higher concentration of VEGF-A in ID culture supernatants.17 A study by Xavier-Ferrucio et al.18 that involved mice with acquired IDA did not show a difference in frequency of CD41 megakaryocytes when compared to controls, but found that low iron biases the commitment of megakaryocytic erythroid progenitors toward the megakaryocyte lineage .
In contrast to the above studies, we were able to find an increased number of megakaryocytes in the bone marrow of the rats with IDA when compared to the C group. We similarly found increases in VEGF; however, this was on the surface of the megakaryocytes of our ID rat model rather than cell supernatants from cord blood in an ID state reported by previous studies.17 In addition, we were able to demonstrate an increase in other cytokines associated with angiogenesis on the surface of megakaryocytes. Moreover, we also demonstrated a clear increase in vessels within the bone marrow of the rats with IDA and thrombocytosis, which we believe is associated with active angiogenesis. Platelets are involved in multiple physiological processes. Many of these processes are associated with active angiogenesis like wound healing, metastatic cancer development, and inflammation. How IDA relates to these processes remains unclear, but we have demonstrated increased megakaryocytes in the bone marrow and an association of angiogenic factors with the presence of IDA and thrombocytosis in our rat model. Angiogenesis may be induced by a hypoxic state based on increased hypoxia-inducible factor 1 alpha subunit identified by other researchers.17 However, we believe that there are other pathways associated with angiogenesis given that other angiogenic cytokines aside from VEGF were also increased that are not directly regulated by hypoxia-inducible factors. How iron deficiency is associated with angiogenesis remains to be elucidated and needs further study.
References
Chiarello, P., Magnolia, M., Rubino, M., Liguori, S. A. & Miniero, R. Thrombocytosis in children. Miner. Pediatr. 63, 507–513 (2011).
Hsieh, R. W. et al. Etiologies of extreme thrombocytosis: a contemporary series. Mayo Clin. Proc. 94, 1542–1550 (2019).
Fernandez, K. S. & de Alarcon, P. A. Development of the hematopoietic system and disorders of hematopoiesis that present during infancy and early childhood. Pediatr. Clin. N. Am. 60, 1273–1289 (2013).
Kaushansky, K. Determinants of platelet number and regulation of thrombopoiesis. Hematol. Am. Soc. Hematol. Educ. Program. 1, 147–152 (2009).
Italiano, J. E. & Shivdasani, R. A. Megakaryocytes and beyond: the birth of platelets. J. Thromb. Haemost. 1, 1174–1182 (2003).
Geddis, A. E. Congenital amegakaryocytic thrombocytopenia. Pediatr. Blood Cancer 57, 199–203 (2011).
Deutsch, V. R. & Tomer, A. Megakaryocyte development and platelet production. Br. J. Haematol. 134, 453–466 (2006).
Ceresa, I. F., Noris, P., Ambaglio, C., Pecci, A. & Balduini, C. L. Thrombopoietin is not uniquely responsible for thrombocytosis in inflammatory disorders. Platelets 18, 579–582 (2007).
Akan, H. et al. Thrombopoietic cytokines in patients with iron deficiency anemia with or without thrombocytosis. Acta Haematol. 103, 152–156 (2000).
Pitchford, S. C., Lodie, T. & Rankin, S. M. VEGFR1 stimulates a CXCR4-dependent translocation of megakaryocytes to the vascular niche, enhancing platelet production in mice. Blood 120, 2787–2795 (2012).
Ding, W. et al. Platelet-derived growth factor (PDGF)-PDGF receptor interaction activates bone marrow-derived mesenchymal stromal cells derived from chronic lymphocytic leukemia: implications for an angiogenic switch. Blood 116, 2984–2993 (2010).
Markovic, A., MacKenzie, K. L. & Lock, R. B. Induction of vascular endothelial growth factor secretion by childhood acute lymphoblastic leukemia cells via the FLT-3 signaling pathway. Mol. Cancer Ther. 11, 183–193 (2012).
Crowe, A. R. & Wei, Yue Semi-quantitative determination of protein expression using immunohistochemistry staining and analysis: an integrated protocol. Bio Protoc. 9, e3465 (2019).
Schindelin et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 576–582 (2012).
Dallman, P. R., Refino, C. & Yland, M. J. Sequence of development of iron deficiency in the rat. Am. J. Clin. Nutr. 35, 671–677 (1982).
Evstatiev, R. et al. Iron deficiency alters megakaryopoiesis and platelet phenotype independent of thrombopoietin. Am. J. Hematol. 89, 524–529 (2014).
Jimenez, K. et al. Increased expression of HIF2α during iron deficiency-associated megakaryocytic differentiation. J. Thromb. Haemost. 13, 1113–1127 (2015).
Xavier-Ferrucio, J. et al. Low iron promotes megakaryocyte commitmentof megakaryocytic-erythroid progenitorsin humands and mice. Blood 134, 1547–1557 (2019).
Acknowledgements
We thank the Laboratory Animal Care Facility staff at the University of Illinois College of Medicine at Peoria for their excellent care of the animals, Christopher Gondi, Ph.D., and Maria Thomas, Ph.D., for their technical assistance. This study was supported by the endowed William H. Albers Professorship fund.
Author information
Authors and Affiliations
Contributions
All authors meet the Pediatric Research authorship requirements. Substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data were made by J.G., P.M., M.G., and P.A.d.A. Drafting the article or revising it critically for important intellectual content was made by J.G., M.G., and P.A.d.A. Final approval of the version to be published was given by P.A.d.A.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Garcia, J., Mankin, P., Gnanamony, M. et al. Evaluation of angiogenic signaling molecules associated with reactive thrombocytosis in an iron-deficient rat model. Pediatr Res 90, 341–346 (2021). https://doi.org/10.1038/s41390-020-01318-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-020-01318-0