Abstract
Background
Noonan Syndrome with Multiple Lentigines (NSML) and Noonan Syndrome (NS) can be difficult to differentiate clinically in early childhood. This study aims to describe characteristics of the ventricular septum that may differentiate NSML from NS. We hypothesize that the shape of the ventricular septum determined by echocardiography correlates with genotype and may distinguish patients with NSML from those with NS.
Methods
We analyzed data from 17 NSML and 67 NS patients. Forty normal and 30 sarcomeric hypertrophic cardiomyopathy (HCM) patients were included as controls. Septal morphology was qualitatively evaluated, and septal angle was measured quantitatively at end diastole. We recorded the presence of a ventricular septal bulge (VSB) and reviewed genetic testing results for each patient.
Results
The most important findings were a sigmoid septum (71%) and VSB (71%) in NSML. NSML septal angle was decreased compared to the normal and sarcomeric HCM control groups, respectively (149 ± 13 vs. 177 ± 3, p < 0.001; 149 ± 13 vs. 172 ± 7, p < 0.001). NS septal angle was similar to the controls (176 ± 6 vs. 177 ± 3, p > 0.5; 176 ± 6 vs. 172 ± 7, p > 0.5). NSML-linked pathogenic variants were associated with sigmoid septum and VSB.
Conclusions
These findings provide novel phenotypic evidence to clinicians that may offer incremental diagnostic value in counseling families in ambiguous NSML/NS cases.
Impact
-
Characteristics of the ventricular septum are linked to specific gene variants that cause NSML and NS.
-
Sigmoid septum and VSB are associated with NSML.
-
This novel echocardiographic association may help clinicians distinguish NSML from NS in ambiguous cases.
-
Early distinction between the two may be important, as syndrome-specific therapies may become available in the near future.
-
This study may encourage further research into genotype–phenotype associations in other forms of HCM.
Similar content being viewed by others
Introduction
Noonan Syndrome with Multiple Lentigines (NSML) is a rare condition characterized by facial dysmorphisms, skin findings, short stature, and cardiac defects. The term “lentigines” refers to spots of concentrated melanin expression that gradually develop as irregular macules across the entire body.1 When the condition was described in a 1969 paper by Gorlin et al., the term LEOPARD syndrome was coined as a convenient acronym describing the clinical presentation of the condition: multiple Lentigines, ECG abnormalities, Ocular hypertelorism, Pulmonic Stenosis, Abnormal genitalia, Retardation of growth, and sensorineural Deafness.1,2,3,4 However, in more recent years the disease has been referred to as NSML.
From a cardiovascular standpoint, hypertrophic cardiomyopathy (HCM) is the most commonly observed cardiac anomaly associated with NSML, occurring in 60–70% of patients,2 and is known to occur in conjunction with a variety of external morphologic features.3,4 The HCM in NSML may have the same clinical implications as those noted in the more common form of HCM, resulting from sarcomeric protein mutations. Both have a propensity for developing left ventricular (LV) outflow tract obstruction, mitral regurgitation, and life-threatening arrhythmias.
Like the more common Noonan Syndrome (NS), NSML is a RASopathy, a term used to describe a group of phenotypically related disorders arising from variants in genes within the Ras/mitogen-activated protein kinase (Ras/MAPK) pathway.5 The exact incidence of NSML is unknown, but it is considered to be a rarer RASopathy phenotype.
A number of genes can cause both NS and NSML phenotypes, including PTPN11, BRAF, and RAF1. However, the two disorders differ in their underlying pathogenesis. Specifically, NS arises from gain-of-function mutations in RAS pathway proteins, including PTPN11-encoded SHP2, which upregulates MAPK signaling.6,7 Conversely, NSML mutations are often hypomorphic alleles that lead to decreased SHP2 activity, which results in increased phosphoinositide-3 kinase-AKT-mammalian target of rapamycin (MTOR) pathway signaling.8 These molecular differences have important implications for potential treatment strategies, as NSML patients may benefit from drugs targeting the AKT-MTOR pathway,9,10 while NS patients may benefit from drugs targeting the MAPK signaling pathway.11
NS has many similarities to NSML, albeit NS is more prevalent, estimated to occur in 1/1000–2500.12 Like its counterpart, NS is characterized by the presence of short stature, craniofacial anomalies, musculoskeletal defects, and heart disease, although it is not associated with lentigines. In spite of its outward similarity to NSML, NS has a somewhat different cardiac phenotype. There is a significantly higher incidence of pulmonary stenosis (PS) (~60%) and a lesser incidence of HCM (~20%) in NS than in NSML (35 and 71%, respectively).3,4,13 Due to the external phenotypic similarities, and the fact that lentigines may develop later in life, NSML has often been misdiagnosed as NS in early childhood.13 In the modern era, the vast majority of pediatric Noonan-spectrum patients are diagnosed via genetic testing. However, the results of this testing may reveal novel or ambiguous variants. In these situations, genotype–phenotype association by echocardiography may be useful in establishing a clinical diagnosis. Furthermore, establishing this diagnosis early in life (prior to receiving results of genetic testing) may have treatment implications on emerging pharmacological therapies that may target HCM in NS or NSML.
In adults with sarcomeric HCM, the morphology of the ventricular septum, evaluated by echocardiography, has provided clues regarding genotype.14,15 In adults, the presence of a biconvex ventricular septum is more commonly associated with the presence of a pathogenic sarcomeric gene variant, i.e., genotype-positive type of HCM. In contrast, the sigmoidal shape is commonly linked to genotype-negative HCM in adults.14,15 Similar echocardiographic associations may provide supplemental evidence to clinicians in RASopathies that may be used in conjunction with the results of genetic testing.
We conducted this descriptive study to determine the genotype–phenotype association between the morphology of the ventricular septum and specific mutations in NSML. We aim to describe characteristic phenotypes of the ventricular septum that are commonly affiliated with NSML and NS in children and young adults. We hypothesize that the shape of the ventricular septum determined by echocardiography may be associated with genotype and may distinguish patients with NSML from those with NS.
Materials and methods
Study population
This was a retrospective study of 17 NSML and 67 NS patients between 2 months and 34 years of age that was conducted at a single center, the Children’s Hospital of Philadelphia. All NSML patients with available data were included, and a total of 80 NS patients were selected by random number generator from a master list. However, 13 of these NS patients were excluded because they had no history of genetic testing. NSML and NS diagnoses were confirmed by the center’s electronic medical record software and from their genetic evaluation notes. Virtually all patients in our NSML cohort showed previously reported pathogenic mutations associated with NSML. One young adult with NSML did not undergo genetic testing but was still included because of a clear-cut clinical diagnosis. Moreover, her offspring had positive genetic testing for NSML. This finding in the offspring increased our confidence regarding the inclusion of the mother in our NSML cohort. For this study, we used two sets of controls. First, we studied 40 control patients who were evaluated for innocent heart murmurs, vasodepressor syncope, non-cardiac chest pain, and family history of congenital or acquired heart disease but were found to have no cardiac lesions. In order to avoid the bias introduced by the presence of cardiomyopathy itself and to control for a similar phenotype associated with other HCM genes, we also included an additional 30 control patients with sarcomeric HCM, all of whom had undergone genetic testing and were found to have previously reported variants of the genes causing sarcomeric protein mutation type of HCM (MYH7, MYBPC3, TNNI3, etc.). They were all negative for the RASopathy genes.
Echocardiographic measurements
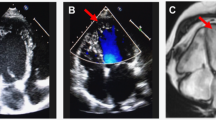
Conventional echocardiographic data were recorded from official echocardiographic reports. In addition, the shape of the ventricular septum was confirmed visually in the apical view by three investigators (H.K., M.D.Q., and A.B.), who were blinded to the genetic diagnosis. The three septal shapes were described as “sigmoid,” “biconvex,” and “neutral.” The biconvex shape has been referred to as “reverse curvature” HCM in adult literature14 The reverse curvature septum shows a mid-septal convexity that bulges toward the LV cavity in contrast to the normal septum, which bulges away from the LV cavity toward the right ventricular cavity, hence the label “reverse”. We chose to use the term biconvex, as a more intuitive descriptor. The angle of the ventricular septum was measured quantitatively, by measuring the angle between the crux of the heart to the middle of the septum and then from the middle of the septum to the apex of the ventricular septum at end diastole (Fig. 1). For the neutral septum, the highest angle was arbitrarily chosen to be 180°.
In addition, the presence of a distinct subaortic muscular ridge of variable size was recorded. In concordance with Binder et al.,14 we refer to this ridge as a ventricular septal bulge (VSB). In a very recent publication, Neubauer et al. refers to this finding in adults as “localized basal septal hypertrophy.”15 VSB can be observed via echocardiography in both the apical and parasternal long axis views but is best visualized in the parasternal long axis view (Fig. 2). A septal angle of 167° was used as a cutoff value for the curved septa. This number was derived from a value that was <−2 standard deviations (SD) from the mean for the neutral group. In our study, HCM was defined as maximal septal or LV posterior wall thickness exceeding Boston pediatric z scores of 2 at end diastole. For patients who underwent myomectomy, echocardiographic analysis was limited to studies that took place before the myomectomy procedure.
Genetics review
A review of genetics records was performed for each patient, including results of RASopathy molecular diagnostic panels. Genetic testing was completed using a multigene Noonan panel at one of a variety of clinical diagnostic laboratories, including GeneDx and The Children’s Hospital of Philadelphia. Exact composition of the panel varied by laboratory and the year when the testing was sent, but all panels included PTPN11. Pathogenic variants, including gene and allele data, were abstracted for use in analysis of genotype–phenotype relationships.
Statistics
Results of septal angulation are expressed as mean ± SD, and categorical data are expressed as rates (positive individuals/total population). The septal angle was compared between groups using Mann–Whitney test, while Fisher’s exact test, which is useful for small sample sizes, was utilized to demonstrate associations between categorical variables. Both samples were compared to a control group of normal patients. A two-tailed p value of <0.05 was considered statistically significant. Intraclass correlation coefficients (ICCs) were used to assess intraobserver and interobserver variability. For increasing the robustness of the ICC, we included three observers, instead of the traditional two.
Results
The study cohort comprised 154 subjects (17 NSML, 67 NS, 40 Normal, 30 sarcomeric HCM) and the demographic data are depicted in Table 1. Three distinct shapes of the interventricular septa were observed: sigmoid, biconvex, and neutral (Fig. 1). The echocardiographic features of each syndromic group (NS vs. NSML) are shown in Table 2. The most important findings were that a sigmoid septum (71%) and a VSB (71%) were noted predominantly in NSML patients (p < 0.001). In contrast, a biconvex septum was more common in NS patients with HCM (94%), and the neutral or normal appearing septum was observed in NS patients without HCM (92%) (Fig. 1). The septal angles for all NSML, NS, and normal patients are displayed in Fig. 3. The average septal angle in all NSML patients was significantly decreased compared to the normal and sarcomeric HCM control groups, respectively (Table 2). In addition, we found that the average septal angle in NS patients was similar to the control groups (Table 2). In our series, 6 NSML patients had undergone LV myomectomy procedure, due to severe left ventricular outflow tract (LVOT) obstruction (Table 3).
Septal angle is represented in degrees on the x axis, and the y axis is composed of each individual analyzed in the study, where y = 1–40 are normal patients, y = 41–70 are sarcomeric HCM patients, y = 71–137 are NS patients, and y = 138–154 are NSML patients. The vertical line is at the cutoff value of 167° (=−2SD), used to differentiate a curved septum from a neutral septum. The septal angle in two NSML patients measured less than the cutoff line; however, their septa were visually evaluated as a variant of neutral and not sigmoid. Mann–Whitney test p values are listed in the box. NSML Noonan Syndrome with Multiple Lentigines, NS Noonan Syndrome, HCM hypertrophic cardiomyopathy.
Longitudinal follow-up echocardiographic data were available for 13/17 patients with NSML and the follow-up duration was 5.1 ± 4.2 years (range: 3 months–12 years). Notably, the shape of the septum continued to resemble the pattern noted in the original study for all NSML patients up until their most recent examination or prior to their LV myomectomies. The shape of the septum could be determined as early as 1 month of age.
Each of the cardiac phenotypes depicted in Fig. 1 showed association with specific genotypes. PTPN11 variants are the most common cause of Noonan-spectrum cases16,17,18 and the most frequent molecular etiology in our population. The relationship between specific PTPN11 variants and echocardiographic findings are shown in Table 4. Of note, variants located in the same exon often were associated with similar septal morphologies. RAF1 and SOS1 mutations were also observed, along with variants in a number of more recently described NS genes including LZTR1 and A2ML1, which occurred at very low frequencies. The genetic and cardiac features of these cases are included in Supplemental Table 1. In addition, the full molecular results from all cases are shown in Supplemental Table 2. Notably, six of the patients included in our study showed variants that have not been previously reported in ClinVar, but each was predicted to be pathogenic or likely pathogenic by the reporting clinical laboratory.
Both sigmoid septum and VSB had a frequency of 71% in NSML. However, they did not exclusively occur together. The full cardiac phenotypes of all NSML patients are shown in Table 3. Sigmoid septum and VSB were shown to occur concurrently in 10/17 NSML patients. Two more NSML individuals showed VSB but lacked sigmoid septum. An additional two patients showed sigmoid septum, but lacked VSB (Table 3). Moreover, the presence of VSB was not always linked to HCM. In our study, 9/12 NSML patients with a VSB showed HCM (Table 3).
The clearest genotype–cardiac phenotype association occurred in patients harboring variants within specific exons of PTPN11. Specifically, pathogenic variants in exons 7 and 13 of PTPN11 were associated with a NSML clinical phenotype and a sigmoid-shaped septum, while variants in exons 3, 4, and 8 of PTPN11 were associated with a NS clinical phenotype and a neutral shaped septum. Interestingly, out of 5 patients with straight septa, 2 had mutations in PTPN11 exon 12.
A comparison of septal angles is depicted in Fig. 3. These data show that NSML cases were distinct from normal (p < 0.0001), sarcomeric HCM (p < 0.0001), and NS (p < 0.0001) with respect to septal angle. In contrast, the normal and NS groups were similar (p = 0.0654). To describe the association between NS and sigmoid septum, we grouped PTPN11 exons 3 (1/12), 4 (0/4), 8 (1/12), 13 (0/3), and SOS1 (1/8) for a total of 3/39 cases of sigmoid septum. For NSML, we grouped PTPN11 exons 7 (3/4), 12 (2/4), 13 (6/8) and RAF1 (1/1) for a total of 12/17 cases of sigmoid septum. Fisher’s exact test showed a highly significant association between the NSML mutations (7, 12, and 13 grouped together) and the sigmoid septum (p < 0.0001).
Intraobserver and interobserver variability
Interobserver and intraobserver variability for septal angle measurements were assessed in all 17 NSML patients. Based on analysis of variance data analysis and three-group ICC, a high degree of both interobserver and intraobserver reliability was found between septal angle measurements. For interobserver variability, the average measure ICC was 0.97 with a 95% confidence interval from 0.74 to 0.98. For intraobserver variability, the average measure ICC was 0.98, with a 95% confidence interval from 0.91 to 0.99.
Discussion
This study sought to identify genotype–phenotype associations that may assist in differentiating two main Noonan-spectrum disorders. The resulting data demonstrate that mutations in a specific set of exons within PTPN11 (7, 12, 13) are associated with NSML. Furthermore, we observed that specific mutations within another set of PTPN11 exons (3, 4, 8, 13) are affiliated with NS. However, the major phenotypic conclusion of this study is that the presence of a sigmoid-shaped ventricular septum and a VSB are typically associated with the diagnosis of NSML. In contrast, patients with NS displayed biconvex septa when HCM was present.
Echocardiographic characteristics
Investigations in adults have suggested that curved or sigmoid septa are far more common in older patients (>50 years), possibly resulting from a gradual adaptation to stimuli such as hypertension, rather than genetics.14,15 In fact, they suggest that the sigmoid shape is predominantly associated with negative genetic testing for sarcomeric variants.14 In our study, of all the molecularly confirmed NSML patients examined, 71% had a sigmoid septal morphology. In contrast, only 4% of NS patients showed sigmoid septa. Typically, NS patients with HCM displayed a biconvex septal morphology (85%). Based on our study, another echocardiographic finding that supports the diagnosis of NSML is the presence of a VSB. Bulges of variable size and LVOT encroachment were noted from the mid to basal septum, a trait that was heavily associated with NSML (71%) and limited to only a single individual in NS populations. Importantly, the VSB occurred without a significant septal angle in some. This observation shows that, while both septal findings may be linked to NSML, these two characteristics do not always occur concomitantly. It is noteworthy that 35% of the NSML patients underwent surgical myomectomy procedures in response to severe LVOT obstruction (Table 3).15 All of these patients had rather large basally positioned septal bulges, as well as LV–aorta pressure gradients that increased over time. In contrast to NSML, only a small portion (3/67) of patients underwent LV myomectomy in our NS cohort. From this finding, we speculate that the septal morphology associated with NSML carries a greater risk of significant LVOT obstruction, which may require future myomectomy. The average septal angle was reduced by −26.0° (−29° to −23°) in patients with NSML compared to the normal + HCM + NS group. This significant decrease in angle is also an objective measure of sigmoidness of the septum and could be used as a clinical marker for patient follow-up or to establish disease severity.
Genetic characteristics
While molecular diagnostic panels for RASopathies continue to expand, PTPN11 variants remain the most common cause of Noonan-spectrum disorders. Our data show that NSML and NS variants occur in distinct exons within PTPN11. Prior studies have also noted an association of NSML with PTPN11 exons 7, 12, and 13, as well as NS with 3, 4, 8, and 13. Similar to prior studies, we noted that variants in exon 13 were present in both NSML/NS phenotypes.4,7,12,17,18 However, it is notable that the mutations within exon 13 were unique to each syndrome at the amino acid level, which specifies an amino acid base change in the protein product of PTPN11. Mutations in Gln510 and Arg498 were specific to NSML, while the remainder of exon 13 mutations were specific to NS. From our observations, we speculate that genetic testing for Noonan-spectrum disorders can provide information regarding their cardiac phenotype. For example, our study noted a very high association of HCM to PTPN11 exon 7 and PS/atrial septal defect to PTPN11 exon 3. Therefore, individuals who are genotype positive for mutations in these exons should be monitored for these conditions.
Clinical implications
In clinical practice, the characteristic findings in the ventricular septum are important as they may serve as tools for early differentiation between NSML and NS patients. These findings may be detected in a screening echocardiogram even before genetic testing is performed and before the development of lentigines. Genotype–cardiac phenotype associations are particularly valuable in NSML vs. NS, because these syndromes are difficult to distinguish based on clinical examination early in life.
Most Noonan-spectrum patients receive genetic testing. However, the results of this testing may reveal novel variants or variants of uncertain significance. For example, our study found six previously unreported variants in NS-associated genes (Supplemental Table 2). In cases such as these, echocardiography may provide incremental information to clinicians and families during the decision-making process. Moreover, the novel concept of “echocardiography-guided genetic testing” may help with genetic counseling due to the higher pre-test probability of a positive genetic test.
Furthermore, syndrome-specific treatments may become readily available in the near future.7,17,18,19,20,21,22 Therapies such as mitogen-activated extracellular signal-regulated kinase (MEK) inhibitors and rapamycin analogs, which target NS and NSML, respectively, have been shown to block upregulated signaling cascades and subsequently reduce cardiomyopathy in mice.10,22,23 These treatments have also been implemented in several small human studies, and results from these studies have suggested that early administration is of paramount importance in order for them to function as efficiently as possible.9,24,25 Therefore, a characteristic cardiac phenotype determined by early echocardiography may have a potential role in early diagnosis, leading to early treatment. Currently, rapamycin analog therapies for HCM in NSML are limited to animal modeling studies. However, an early phase human clinical trial has been initiated by Novartis (Clinical Trial Identifier: NCT01556568) for the use of MEK inhibitors to treat NS-related cardiomyopathy.21,26 Should these therapies become approved for widespread clinical use, echocardiographic phenotype analysis may help point to one or the other diagnosis and may provide valuable insight as to which approach would be the most effective.
This small pilot study has shown that the echocardiographic evaluation of septal morphology in NS and NSML can provide clues regarding genotype. Notably, the vast majority of NS and NSML patients have their genotypes confirmed by genetic testing, which is also the case in most other syndromic cardiomyopathies. However, in non-syndromic cases of HCM, genetic testing may not be performed for various reasons. In the clinical arena, we have observed that, despite the recommendation from physicians to perform genetic testing, many parents refuse so that future insurability or employment opportunities of the child are not jeopardized. A recent large study by Neubaur et al., in >2700 adults with sarcomeric HCM, has shown that basal septal hypertrophy (VSB-type septum) causes more LVOT obstruction, but these patients have infrequent genetic mutations.15 This finding in adults stands in contrast to our findings in children with VSB, where there was a strong correlation with specific mutations. Another adult study conducted in 2006 by the Ackerman group analyzed genotype–phenotype associations in Z-disk HCM. Interestingly, this study found a strong association between sigmoid septum and variants that cause Z-disk HCM.27 A follow-up review by Ackerman et al. in 2009, raised the concept of “pharmacogenomics.” This concept suggests that, based on experiments on transgenic models of sarcomeric HCM, therapies may someday emerge that can potentially halt the progression of HCM in humans.28 Establishing genotype–phenotype associations may help guide such therapies.
In contrast to adults, in children with sarcomeric HCM, large-scale genotype–phenotype correlations like the Neubaur and Ackerman studies are lacking. Our small pilot study may encourage future larger investigations in sarcomeric HCM in children. We speculate that, if correlation between cardiac phenotype and genotype were to be established, it would be of incremental value in clinical counseling of sarcomeric HCM patients who do not undergo genetic testing for reasons mentioned previously.
Limitations
The small sample size of NSML patients is an important limitation of this study. NSML is a very rare disorder, and even for a single tertiary-care institution, this is perhaps the maximal achievable number. Due to low numbers in all subgroups of exon mutations, further correlations between individual exon mutations and phenotypes were not attempted as it would not yield meaningful statistics. Instead important and relevant exon mutations were grouped together to determine associations between mutations and septal phenotype.
Conclusions
This study has shown that patients with NSML have a clear association with sigmoid septum and VSB morphology, while patients with NS have an approximately straight septal orientation, with the occurrence of biconvex septum in the setting of HCM. This observation is important as it provides novel, additional phenotypic evidence to clinicians, which may offer incremental diagnostic value and help with counseling families in ambiguous cases, even prior to genetic testing. Furthermore, it may encourage future investigation into the role of echocardiography as a predictor of genotype in non-syndromic, sarcomeric HCM in children, many of whom may never undergo genetic testing.
References
Gorlin, R. J., Anderson, R. C. & Moller, J. H. The LEOPARD (multiple lentigines) syndrome revisited. Birth Defects Orig. Artic. Ser. 7, 110–115 (1971).
Grant, A. R. et al. Assessing the gene‐disease association of 19 genes with the RASopathies using the ClinGen gene curation framework. Hum. Mutat. 39, 1485–1493 (2018).
Gelb, B. D. & Tartaglia, M. Noonan Syndrome with Multiple Lentigines. GeneReviews® [Internet] (eds Adam, M. P. et al.) (University of Washington, Seattle, 2007).
Sarkozy, A., Digilio, M. & Dallapiccola, B. LEOPARD syndrome. Orphanet J. Rare Dis. 3, 13 (2008).
Feng, G. S. & Pawson, T. Phosphotyrosine phosphatases with SH2 domains: regulators of signal transduction. Trends Genet. 10, 54–58 (1994).
Nakamura, T., Gulick, J., Pratt, R. & Robbins, J. Noonan Syndrome is associated with enhanced pERK activity, the repression of which can prevent craniofacial malformations. Proc. Natl Acad. Sci. USA 106, 15436–15441 (2009).
Calcagni, G., Digilio, M. C., Marino, B. & Tartaglia, M. Pediatric patients with RASopathy-associated hypertrophic cardiomyopathy: the multifaceted consequences of PTPN11 mutations. Orphanet J. Rare Dis. 14, 163 (2019).
Hanna, N. et al. Reduced phosphatase activity of SHP-2 in LEOPARD Syndrome: Consequences for PI3K binding on Gab1. FEBS Lett. 580, 2477–2482 (2006).
Hahn, A. et al. Rapidly progressive hypertrophic cardiomyopathy in an infant with Noonan syndrome with multiple lentigines: palliative treatment with a rapamycin analog. Am. J. Med. Genet. A. 167a, 744–751 (2015).
Wang, J. et al. In vivo efficacy of the AKT inhibitor ARQ 092 in Noonan syndrome with multiple lentigines-associated hypertrophic cardiomyopathy. PLoS ONE 12, e0178905 (2017).
Gross A. et al. Advancing RAS/RASopathy therapies: an NCI-sponsored intramural and extramural collaboration for the study of RASopathies. Am. J. Med. Genet. A 182, 866–876 (2020).
Tartaglia, M., Gelb, B. D. & Zenker, M. Noonan syndrome and clinically related disorders. Best Pract. Res. Clin. Endocrinol. Metab. 25, 161–179 (2011).
Tartaglia, M. et al. PTPN11 mutations in Noonan Syndrome: molecular spectrum, genotype-phenotype correlation, and phenotypic heterogeneity. Am. J. Hum. Genet. 70, 1555–1563 (2002).
Binder, J. et al. Echocardiography-guided genetic testing in hypertrophic cardiomyopathy: septal morphological features predict the presence of myofilament mutations. Mayo Clin. Proc. 81, 459–467 (2006).
Neubaur, S. et al. Distinct subgroups in hypertrophic cardiomyopathy in the NHLBI HCM Registry. J. Am. Coll. Cardiol. 74, 2333–2345 (2019).
Tartaglia, M. et al. Mutations in PTPN11, encoding the protein tyrosine phosphatase SHP-2, cause Noonan syndrome. Nat. Genet. 29, 465–468 (2001).
Sarkozy, A. et al. Correlation between PTPN11 gene mutations and congenital heart defects in Noonan and LEOPARD syndromes. J. Med. Genet. 40, 704–708 (2003).
Lee, C. L. et al. Cardiac manifestations and gene mutations of patients with RASopathies in Taiwan. Am. J. Med. Genet. 182, 357–364 (2020).
Krenz, M., Yutzey, K. E. & Robbins, J. Noonan syndrome mutation Q79R in Shp2 increases proliferation of valve primordia mesenchymal cells via extracellular signal-regulated kinase 1/2 signaling. Circ. Res. 97, 813–820 (2005).
Schramm, C., Fine, D. M., Edwards, M. A., Reeb, A. N. & Krenz, M. The PTPN11 loss-of-function mutation Q510E-Shp2 causes hypertrophic cardiomyopathy by dysregulating mTOR signaling. Am. J. Physiol. Heart Circ. Physiol. 302, H231–H243 (2012).
Gelb, B. D., Roberts, A. E. & Tartaglia, M. Cardiomyopathies in Noonan syndrome and the other RASopathies. Prog. Pediatr. Cardiol. 39, 13–19 (2015).
Marin, T. M. et al. Rapamycin reverses hypertrophic cardiomyopathy in a mouse model of LEOPARD syndrome-associated PTPN11 mutation. J. Clin. Investig. 121, 1026–1043 (2011).
WU, X. et al. MEK-ERK pathway modulation ameliorates disease phenotypes in a mouse model of Noonan syndrome associated with the Raf1(L613V) mutation. J. Clin. Investig. 121, 1009–1025 (2011).
Andelfinger, G. et al. Hypertrophic cardiomyopathy in noonan syndrome treated by MEK-inhibition. J. Am. Coll. Cardiol. 73, 2237 (2019).
Calcagni, G., Digilio, M. C., Marino, B. & Tartaglia, M. Pediatric patients with RASopathy-associated hypertrophic cardiomyopathy: the multifaceted consequences of PTPN11 mutations. Orphanet J. Rare Dis. 14, 163 (2019).
Calcagni, G. et al. Clinical presentation and natural history of hypertrophic cardiomyopathy in RASopathies. Heart Fail. Clin. 14, 225–235 (2018).
Theis, J. L. et al. Echocardiographic-determined septal morphology in Z-disc hypertrophic cardiomyopathy. Biochem. Biophys. Res. Commun. 351, 896–902 (2006).
Bos, J. M., Towbin, J. A. & Ackerman, M. J. Diagnostic, prognostic, and therapeutic implications of genetic testing for hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 54, 3 (2009).
Author information
Authors and Affiliations
Contributions
Each author has met the Pediatric Research authorship requirements.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent
This is a retrospective study for which consent is not required by the institutional review board of the Children’s Hospital of Philadelphia.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Kauffman, H., Ahrens-Nicklas, R.C., Calderon-Anyosa, R.J.C. et al. Genotype–phenotype association by echocardiography offers incremental value in patients with Noonan Syndrome with Multiple Lentigines. Pediatr Res 90, 444–451 (2021). https://doi.org/10.1038/s41390-020-01292-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-020-01292-7