Abstract
Background
Two meta-analyses concluded that jaundice was associated with an increased risk of autism. We hypothesize that these findings were due to methodological limitations of the studies included. Neonatal jaundice affects many infants and risks of later morbidity may prompt physicians towards more aggressive treatment.
Methods
To conduct a systematic literature review and a meta-analysis of the association between neonatal jaundice and autism with particular attention given to low risk of bias studies. Pubmed, Scopus, Embase, Cochrane, and Google Scholar were searched for publications until February 2019. Data was extracted by use of pre-piloted structured sheets. Low risk of bias studies were identified through predefined criteria.
Results
A total of 32 studies met the inclusion criteria. The meta-analysis of six low risk of bias studies showed no association between neonatal jaundice and autism; cohort studies risk ratio 1.09, 95% CI, 0.99–1.20, case-control studies odds ratio 1.29 95% CI 0.95, 1.76. Funnel plot of all studies suggested a high risk of publication bias.
Conclusions
We found a high risk of publication bias, selection bias, and potential confounding in all studies. Based on the low risk of bias studies there was no convincing evidence to support an association between neonatal jaundice and autism.
Impact
-
Meta-analysis of data from six low risk of bias studies indicated no association between neonatal jaundice and autism spectrum disorder.
-
Previous studies show inconsistent results, which may be explained by unadjusted confounding and selection bias.
-
Funnel plot suggested high risk of publication bias when including all studies.
-
There is no evidence to suggest jaundice should be treated more aggressively to prevent autism.
Similar content being viewed by others
Introduction
Autism spectrum disorder (ASD) is a disease defined by symptoms in the following three domains; social interaction, communicative disorders, and stereotyped, repetitive or restricted behavior.1 This review focuses on ASD, including all subtypes. The prevalence of ASD is 1−2%, and has increased since the 1940s.2,3,4 ASD is more than four times as prevalent in boys than in girls.2 The etiology of ASD is unknown, but studies indicate involvement of both genetic5,6,7 and non-inheritable factors.8,9 ASD is a disease with long-term consequences for both the child and the family.10 Accordingly, there is a need to identify preventable causes of ASD. Neonatal jaundice occurs in some 80% of neonates.11 Unconjungated bilirubin crosses the blood−brain barrier in the newborn and high levels may cause acute bilirubin-induced encephalopathy and permanent brain damage.12 The most common neuropathological findings in children with ASD are a decreased number of purkinje cells in the cerebellum, decreased neuronal cell size, and increased cell packing density in the cerebral cortex.13,14 These areas may also be damaged by bilirubin deposition in brain tissue.12,15,16 Accordingly, an association between hyperbilirubinemia and ASD seems plausible.17 Reviews by Amin et al.16 and Jenabi et al.18 concluded that neonatal jaundice was associated with an increased risk of ASD. In the review by Amin et al. no structured quality assessment was performed and the conclusion was based on a meta-analysis of all studies regardless of their quality. Jenabi et al. rated 19 out of 21 studies as high quality despite methodological limitations of some studies including no adjustment for confounders. The purpose of this systematic review was to compile and critically review the existing evidence of the association between jaundice and ASD and to base the conclusion only on studies with low risk of bias.
Methods
Search strategy
This study is conducted in accordance with the PRISMA guideline (see PRISMA checklist). A systematic literature search was carried out according to the review protocol published in PROSPERO, protocol number: CRD42016025927. Pubmed, Scopus, Embase, Cochrane, and Google Scholar were searched for publications until February 2019. The search terms included autism, autistic disorder, pervasive developmental disorder (PDD), ASD, Asperger, hyperbilirubinemia, jaundice, icterus, bilirubin, newborn/perinatal/neonatal risk factor (s), phototherapy. MESH terms were used whenever available. The full search strategy can be found in Supplementary Text S1 (online). References of included studies and other relevant reviews were screened to identify additional studies.
Inclusion/exclusion criteria
All case−control and cohort studies examining the association between jaundice, hyperbilirubinemia, or phototherapy and ASD, that provided absolute numbers were eligible.
Exposure measures had to be either neonatal hyperbilirubinemia or jaundice based on clinical assessment, parental report, laboratory confirmation by estimating serum bilirubin during the neonatal period (within 28 days after birth), or phototherapy treatment.
The outcome measure was ASD, which include childhood/infantile autism, autistic disorder, pervasive developmental disorder—not otherwise specified, and Asperger’s. In the literature the terms autism, ASD, and PDD are often used interchangeably; thus, all were included.
To be able to tease out the details of each study, only studies in English peer-reviewed journals were included. Conference abstracts and studies without a reference group such as case series or case reports were excluded.
Studies that adjusted for confounding factors, but did not include the adjusted results, were excluded from the meta-analysis. Studies that investigated preterm infants only were included in a sub-analysis of preterm infants.
Study selection and data extraction
Titles and abstracts of all identified records were screened for eligibility according to the inclusion and exclusion criteria. If immediate exclusion based on title and abstract was not possible, the full text was assessed for eligibility. Structured sheets piloted prior to the search were used for data extraction from each study (see Table 1).
Low risk of bias studies
Studies passed the threshold for strong methodological quality, if they met the following criteria: ASD diagnosis based on International Classification of Diseases/Diagnostic and Statistical Manual of Mental Disorder (ICD/DSM), jaundice was based on TSB measurement or jaundice diagnosis from medical records, and adjustment for at least sex2 and either gestational age (e.g. term vs. preterm or gestational week at birth) or birth weight.19 These quality criteria were defined after the development of the PROSPERO protocol, but prior to data extraction. Studies that met the quality criteria were defined as low risk of bias studies. Only low risk of bias studies were subjected to further quality assessment.
Quality assessment
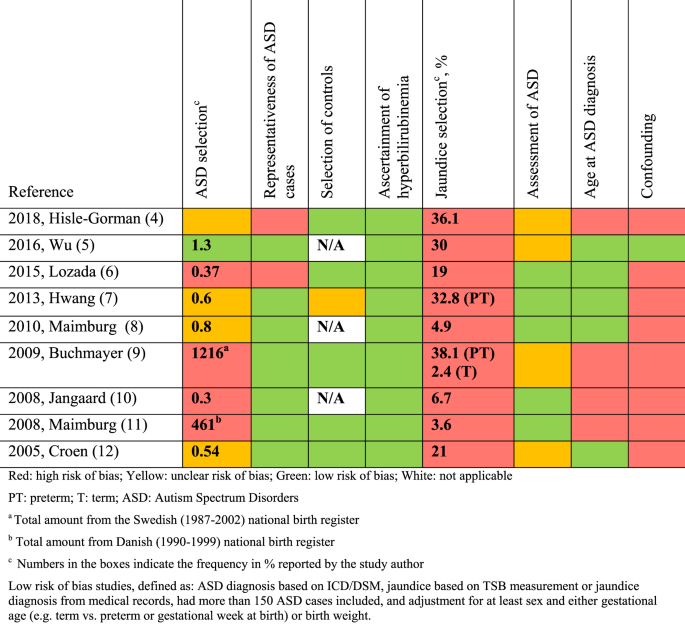
The quality-assessment was guided by the Cochrane Handbook for systematic reviews of interventions,20 the STROBE checklist21 (STrengthening the Reporting of OBservational studies in Epidemiology), and the Newcastle-Ottawa Scale.22 We defined essential confounders as: sex,2 gestational age19 or birth weight,19 birth year,4 and Apgar score.19 According to current evidence, these may likely influence the association and should be adjusted for.23 Other potential confounding factors such as pregnancy complications, parental age, education, and socioeconomic status were also considered, but not deemed essential due to the paucity of studies between these variables and ASD. To further evaluate the quality of the low risk of bias studies, the risk of bias in predefined areas (ASD selection, representativeness of ASD cases, selection of controls, ascertainment of hyperbilirubinemia, jaundice selection, assessment of ASD, age at ASD assessment, confounding) were rated as low, high or unclear risk of bias (Fig. 1). This assessment aimed to show the quality of the studies without suggesting how that might influence the effect estimates. The quality-assessment was based on a risk of bias table (Supplementary Table S2 (online)) and assessment of confounders (Supplementary Table S3 (online)) made a priori by the authors.
Qualitative assessment of low risk of bias studies based on predefined criteria (Supplementary Table S2).
Literature search, inclusion, data extraction, selection of low risk of bias studies, and quality assessment of low risk of bias studies were conducted independently by two authors (M.L.K. and M.V.P.). In case of discrepancy between the two authors, a third author (T.B.H.) was conferred.
Data analysis
Data were analyzed using the Cochrane Collaboration Review Manager Software (RevMan version 5.3).24 Adjusted effect measures were used when available. The unadjusted risk ratio (RR) or odds ratio (OR) was calculated from absolute numbers with 95% confidence intervals (CIs) if adjusted estimates were unavailable. Effect measures were entered into RevMan using the “generic inverse variance” outcome. OR and RR were analyzed separately in the meta-analysis because case−control and cohort studies are heterogenic and may have different challenges related to methodology. A random-effects model was used to analyze the included studies as a random sample of a hypothetical population of studies. Between-study heterogeneity was assessed using I2, which describes the percentage of variation across studies that is due to heterogeneity rather than chance.25,26 A forest plot and meta-analyses using a logarithmic scale were made for all studies, the low risk of bias studies, and for preterm infants. A funnel plot was used to assess selective reporting.
Results
Literature search
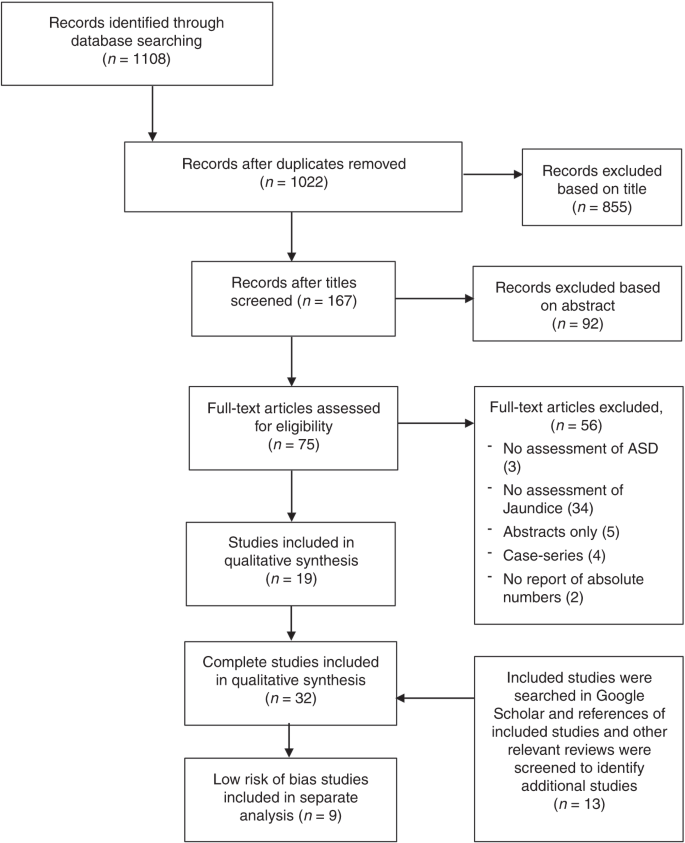
Literature search was conducted in February 2019 (PRISMA flow chart in Fig. 2) identifying a total of 32 studies to be included in this review. Two studies by Maimburg et al.27,28 were both included, despite overlapping by 5 years. However, they also represent 10 years without overlap.
Study characteristics
Table 1 shows the main characteristics and effect estimates from all 32 included studies. The earliest study dates back to 1979. The total number of children with ASD across all studies was 29,299. Differences in the definition of jaundice (parental assessment by self-administered questionnaires, clinical diagnosis, diagnosis by TSB levels, the need for treatment by phototherapy) and the definition of ASD (diagnosis by ICD-8, 9 or 10 or DSM-III, IV or V) compromised overall comparability.
Nine studies met the low risk of bias criteria. The low risk of bias studies included 24,440 children with ASD. The studies that were not included in the low risk of bias studies failed to adjust for any potential confounding factors or they based the information on jaundice on parental recall. Fig. 1 shows the quality assessment of each of these nine studies and Supplementary Table S3 (online) shows the potential confounders adjusted for. As seen in Fig. 1 even the studies we considered low risk of bias studies had several limitations. Of the nine studies two reported an increased risk of ASD with jaundice,28,29 the seven remaining studies showed no association between jaundice and ASD.27,30,31,32,33,34,35 These nine studies were thoroughly reviewed and their main characteristics are summarized in the following narrative syntheses ordered according to their weight in the meta-analyses, with cohort studies first.
Narrative description of low risk of bias studies
Wu et al.31 based their cohort study on 457,855 children born 1995–2011 at 15 Kaiser Permanente Northern California hospitals (KPNC) covering 40% of the insured population. They found no association between jaundice and ASD (RR 1.07, 95% CI, 0.98–1.17). Neonatal jaundice was found in 30% and ASD in 1.3% of the included population. Jaundice was defined as TSB > 10 mg/dL, and 51% of all newborns in the study had TSB measured. ASD was defined according to ICD-9 and retrieved from the KPNC registry. Children were either diagnosed at autism evaluation centres, by a clinical specialist outside the ASD center, or by a general pediatrician. The study adjusted for all our predefined essential confounders. They estimated the effect of phototherapy, and found that use of phototherapy did not change the association between jaundice and ASD.
Maimburg et al.27 (revised ASD selection36) based their cohort study on all Danish children born 1994–2004. They found no association between jaundice and ASD (RR 1.07, 95% CI, 0.94–1.21). They included 733,826 children, 5% were jaundiced and 0.8% had ASD. Jaundice was defined according to ICD-10 retrieved from the National Patient Registry. Several neurodevelopmental disorders (F80−F84.9 and F88−F88.9), including autism/pervasive developmental disorders, were studied. ASD was defined by ICD-10 from the Danish Psychiatric Central Register (in- and outpatients). Results were adjusted for all the predefined essential confounders except birth year. In children born preterm no association was found (RR 1.05, 95% CI, 0.83–1.33).
Jangaard et al.32 based their cohort study on the Canadian Nova Scotia Atlee Perinatal Database including 94% of all newborns 1994–2000 in the province. They found an association between jaundice and ASD (RR 1.60, 95% CI, 1.00–2.56). A total of 56,019 children were included, 7% were jaundiced and 0.33% had ASD. The study assessed the association between serum bilirubin levels and four outcomes including autism. Jaundice was defined as TSB level above 13.5 mg/dL. The Medical Service Insurance (physician billings) and the hospital Discharge Abstract Database provided ASD diagnosis by ICD-9. The study adjusted for all predefined essential confounders except birth year and Apgar score.
Lozada et al.29 found that neonatal jaundice was associated with an increased risk of ASD (OR 1.18, 95% CI, 1.06–1.31). This case−control study was based on data from the United States (US) Military Health System database. It included 2917 cases born 2000–2009 and 8751 controls matched by sex and age. Jaundice and ASD was defined according to ICD-9-CM; only inpatient diagnoses were used for jaundice. Eighteen percent of infants in the control group were jaundiced. ASD was ascertained from a minimum of one outpatient visit to a pediatric specialist, with no description of how children were referred. ASD was found in 0.37% of 783,047 births recorded. Our defined essential confounders apart from Apgar score and birth year were assessed. When studying preterm children only, the association disappeared (OR 1.06, 95% CI, 0.77–1.46).
Buchmayer et al.33 based their case−control study on the Swedish Medical Birth Register and included 1216 ASD cases born 1987–2002 and 6080 controls matched by sex, birth year and birth hospital. They found no association between jaundice and ASD (OR 1.18, 95% CI, 0.83–1.68). Jaundice was one of many perinatal factors studied. Jaundice and ASD was defined by ICD-9 and ICD-10 from inpatient medical records, 5% of infants in the control group were jaundiced. ASD was verified by a child psychiatrist. The study adjusted for all the predefined essential confounders and 16 other risk factors. When preterm infants were assessed no association was seen (OR 0.70, 95% CI, 0.50–0.98).
Croen et al.30 based their case−control study on children born at one of the KPNC hospitals in Northern California covering 30% of the insured population. They found no association between jaundice and ASD (OR 0.67, 95% CI, 0.43−1.04). It included 338 ASD cases born 1995–1998 and 1718 controls matched by sex, birth year, and hospital of birth. Jaundice was defined as TSB > 15 mg/dL, 28% of cases and controls had TSB measured and 12% of infants in the control group were jaundiced. ASD was defined by ICD-9-CM and obtained from the outpatient databases. The study adjusted for all our predefined essential confounders except Apgar score.
Maimburg et al.28 found that TSB > 17.5 mg/dL (300 µmol/L) was associated with increased odds of ASD (OR 3.70, 95% CI, 1.30–10.53). Maimburg et al. based their case−control study on all children born in Denmark 1990–1999. The study included 461 cases and 461 controls from the national civil registration system matched by sex, birth year and county of birth. The study assessed the association between seven neonatal risk factors and ASD. TSB values were retrieved from medical records; 18% of cases and 13% of controls had a TSB measured, jaundice frequency was 3.6%. ASD was defined by psychiatrists’ ICD-8 and ICD-10 codes. ASD cases were ascertained from the Danish Psychiatric Central Register including all inpatients in Denmark 1990–1995 and in- and outpatients 1995–1999. Apgar score was the only essential confounder not adjusted for. When preterm infants were considered the association disappeared (OR 1.00, 95% CI, 0.06–16.67).
Hilse-Gorman et al.35 based their case−control study on the US Military Health System. They included 8760 ASD cases born 2000–2013. They claimed to find no association in the adjusted analyses. However, the adjusted results were not presented. Each case was matched by three controls by age, sex, and enrollment time frame. Jaundice was one of 28 different risk factors studied. Information on jaundice and ASD was based on ICD-9 from inpatient and outpatient data. Thirty-six percent of infants in the control group were jaundiced (highest rate in any study in this review). All essential confounders were adjusted for. Adjusted results were not shown, and therefore not included in our meta-analysis.
Hwang et al.34 based their case−control study on Taiwan National Health Insurance Research Database covering 99% of Taiwanese population. They found no association between jaundice in preterm neonates and ASD (OR 0.99, 95% CI, 0.81–1.21). The aim was to identify neonatal risk factors for autism in preterm children. The study included 411 ASD cases and 29,614 controls born 1998–2001. Jaundice was defined by ICD-9-CM from in- and outpatient databases, ASD was only from outpatient databases, 33% of infants in the control group were jaundiced. All predefined essential confounders except for Apgar score were adjusted for.
Meta-analysis and funnel plot
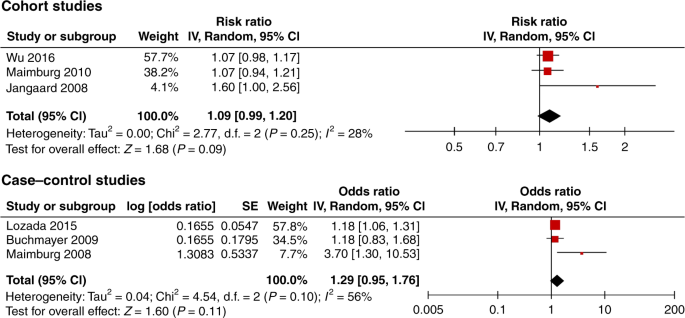
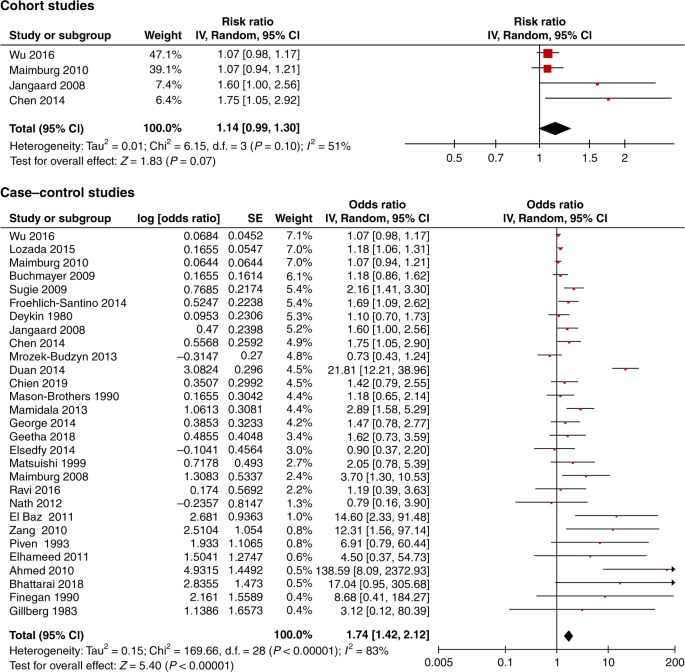
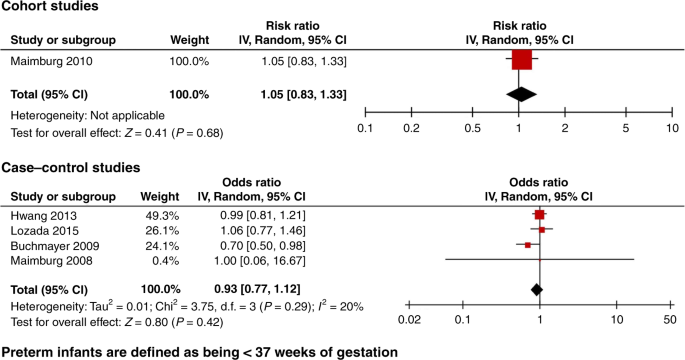
When restricting the analysis to the low risk of bias studies, there was no significant association between neonatal jaundice and ASD. Three case−control studies were excluded from the meta-analysis, one only studied preterm infants34 and one did not show the adjusted OR.35 The third study had an overlapping population with that of Wu et al.31 Croen et al.30 included one KNPC hospital while Wu et al.31 included 15 KNPC hospitals of Northern California. The meta-analysis of the three low risk of bias cohort studies revealed an RR of 1.09 (95% CI, 0.99–1.20), and of the three low risk of bias case−control studies an OR of 1.29 (95% CI, 0.95–1.76) (Fig. 3). If the study by Croen et al.30 was included in the meta-analysis of the case−control studies, the OR was 1.14 (95% CI, 0.80–1.61). In addition, we found no statistically significant association from the meta-analysis of all four cohort studies (RR 1.14, 95% CI, 0.99–1.30), while the meta-analysis of all 29 case−control studies showed an association OR 1.74 (95% CI, 1.42–2.12) (Fig. 4). The meta-analysis based on preterm infants only showed no significant association (OR 0.93, 95% CI, 0.77–1.12) (Fig. 5). The meta-analysis of all studies found a high degree of heterogeneity (I2 of 51% (cohort studies) and 83% (case−control studies)). Furthermore, funnel plots (Fig. 6) indicated selective reporting of studies that found an association.
Discussion
We identified 32 studies that qualified for this review of the association between neonatal jaundice and ASD. In the meta-analysis of all studies we found an association between neonatal jaundice and ASD. A funnel plot demonstrated a high risk of publication bias. Due to the large variation in the quality of the studies, a meta-analysis of all studies should be interpreted with caution. The low risk of bias studies were based on ICD/DSM and not on parental recall, and most of them had a predefined primary aim to study jaundice and ASD, making publication bias and type 1 errors less likely. Although not significant, our meta-analysis restricted to the three low risk of bias cohort studies showed an increased risk of ASD of 9% (RR 1.09, 95% CI, 0.99–1.20). If not due to random variation, this could be explained by methodological limitations such as residual confounding and selection bias even in the low risk of bias studies (Fig. 1).
A challenge in all studies was a reliable jaundice diagnosis. No studies defined the criteria for diagnosing jaundice or measuring TSB level, e.g., referral criteria. In most settings bilirubin testing is not used as a screening procedure for all newborns, and since jaundice often develops some days after birth, discharged newborns may be less likely to be diagnosed. Accordingly, the neonate who has been discharged may rarely have a diagnosis of hyperbilirubinemia from the hospital system37,38,39; at nine KPNC hospitals the number of infants with TSB 15–19.9 mg/dL increased by 56% after implementation of universal bilirubin screening.40 This suggests that the jaundice diagnosis is an indicator of being hospitalized rather than having a bilirubin level different from non-hospitalized newborns, in particular when jaundice is defined by the lower cut-off levels of bilirubin. In our low risk of bias studies we included jaundice based on medical records and even among studies using serum values28,30,31,32,41,42 highly variable definitions of jaundice were seen resulting in frequencies differing between 1 and 36%. In conclusion, availability and criteria of TSB testing and TSB cut-off values may influence the frequency of jaundice, the risk of selection bias, and the interpretation of the exposure in the studies. All studies qualified as low quality on jaundice selection, because they did not explain which infants had TSB measured or controlled for hospitalization or in other ways reflected on the frequency of TSB measurement/hyperbilirubinemia.
If hospitalized children are more likely to be categorized as exposed, interpretation of results may be difficult. Compared to the background population, hospitalized newborns may differ in several ways: they are more likely to be the first child, to have had a complicated delivery, to be of low birth weight, or to be preterm. These are all factors associated with ASD. Comparing children hospitalized in the newborn period who may much more often be diagnosed with jaundice to non-hospitalized children with a much lower risk of being diagnosed with jaundice might lead to bias towards an association between jaundice and ASD. We have illustrated this by a directed acyclic graph (DAG)43; if hospitalization is a cause of jaundice diagnosis it opens numerous potential biasing pathways (Supplementary Fig. 5). According to the DAG, studies of a causal relationship should either adjust for all covariates causing both neonatal hospitalization and ASD, should be based on exposures obtained from universal bilirubin screening, or should adjust for hospitalization for reasons other than suspected hyperbilirubinemia. Using a conservative cut-off level may decrease but not eliminate this bias.
Accordingly, studies in preterm newborns that are all hospitalized after birth may illustrate the points made on jaundice and hospitalization; in six of the included studies, preterm neonates were analyzed independently. Five of these studies were low risk of bias and all showed no association between jaundice and ASD in preterm newborns (Fig. 5).
Confounding factors may influence the relationship between bilirubin levels and ASD. Potential confounders could be newborn infections, asphyxia, parental age, and complicated delivery; however, other factors such as genetic and socioeconomic factors may also be involved. Whether it is possible to fully adjust for all potential confounders is questionable.
Several studies used parental recall of neonatal jaundice as the exposure, which may result in recall bias. None of these studies were considered low risk of bias studies in this review.
The Autism and Development Disabilities Monitoring Network suggested an increase in estimated prevalence of ASD by roughly 123% since 2002, which is supported by several other sources.4,44,45,46 This is thought to be explained by other factors than a true increase, i.e., diagnostic criteria, service availability, increased funding, and population awareness.3,46,47,48,49 Furthermore, new guidelines on the diagnosis of hyperbilirubinemia (one particularly from 199437) have emerged, and contributed to an increase in admissions for neonatal jaundice.32,50,51 The majority of studies collected data over time periods of some 15 years. Therefore, if time is not adjusted for, changes in diagnostic practices, could bias results related to the association between jaundice and ASD.
The majority of included studies offered no description on how infants with ASD were referred for diagnostic evaluation. Reported frequencies of ASD were as low as 0.3%29,32 and as high as 1.3%31 (the latter being close to the expected prevalence2.) The low number of ASD cases seen in some studies could be due to the use of hospital-based databases.28,30,33,34,35,52 In somatic hospital databases only children with somatic diseases will be admitted to the hospital and an additional ASD diagnosis may depend on availability of patient history from other contacts e.g., general practice or history taken from parents. While studies with small numbers of children with autism argue that they have more severe cases, the cases might also differ in other aspects. Thus, studies with a low frequency that did not provide valid arguments for the occurrence were rated as low quality on ASD selection.
Maimburg et al. published two studies based on information from Danish health registries with overlapping study periods. They differed substantially in the number of identified cases; a case−control study including 461 cases born 1990−199928 and a cohort study including 6171 cases born between 1994 and 2004.27 The case−control study showed a threefold increased risk of ASD with jaundice, while the cohort study found no association. Thus, selection bias might contribute significantly to the associations seen.
The study by Wu et al.31 investigated the effect of phototherapy and found no indication of a protective effect. So, even if there would be an association between jaundice and ASD, it does not seem to be affected by the use of phototherapy.
Strengths and limitations
Our inclusion criteria were broad to allow for a high number of studies. Consequently, we made no restrictions to studies with particular methodological strengths. Many studies examined a variety of newborn complications with no a priori hypotheses related to jaundice.41,42,53,54,55,56,57,58,59,60,61,62,63,64,65,66 A number of studies had other methodological weaknesses such as the use of parents’ information to diagnose neonatal jaundice55,56,57,58,59,61,62,63,67,68,69,70,71 and no adjustment for confounding factors.41,42,53,54,55,56,57,58,60,61,63,66,67,69,70,72,73 However, we were able to restrict our main analysis to include only low risk of bias studies. The low risk of bias studies were identified based on a priori defined quality criteria. Thus, providing a reliable final conclusion based on low risk of bias studies. Our criteria could have been stricter, since the low risk of bias studies also had limitations.
Conclusion
We identified a high risk of publication bias in all studies on jaundice and ASD. We also pointed out selection and information bias and lack of adjustment for potential confounding factors in a number of studies, which may explain previous findings. When restricting the meta-analysis to low risk of bias studies, we found no convincing evidence of an association between neonatal jaundice and ASD. Furthermore, one study investigated the effect of phototherapy and found no indication of a protective effect. However, further high-quality studies are warranted to provide more firm conclusions. A more aggressive use of phototherapy to lower any potential risk of ASD in jaundiced infants should not be encouraged based on current evidence.
References
WHO. International Statistical Classification of Diseases and Related Health Problems (WHO, 2018).
Baio, J. et al. Prevalence of autism spectrum disorder among children aged 8 years—autism and developmental disabilities monitoring network, 11 sites, United States, 2014. MMWR Surveill. Summ. 67, 1–23 (2018).
Hansen, S. N., Schendel, D. E. & Parner, E. T. Explaining the increase in the prevalence of autism spectrum disorders: the proportion attributable to changes in reporting practices. JAMA Pediatr. 169, 56–62 (2015).
Nevison, C., Blaxill, M. & Zahorodny, W. California autism prevalence trends from 1931 to 2014 and comparison to National ASD Data from IDEA and ADDM. J. Autism Dev. Disord. 48, 4103–4117 (2018).
Bill, B. R. & Geschwind, D. H. Genetic advances in autism: heterogeneity and convergence on shared pathways. Curr. Opin. Genet. Dev. 19, 271–278 (2009).
Chaste, P. & Leboyer, M. Autism risk factors: genes, environment, and gene−environment interactions. Dialogues Clin. Neurosci. 14, 281–292 (2012).
Bailey, A. et al. Autism as a strongly genetic disorder: evidence from a British twin study. Psychol. Med. 25, 63–77 (1995).
Guinchat, V. et al. Pre-, peri- and neonatal risk factors for autism. Acta Obs. Gynecol. Scand. 91, 287–300 (2012).
Gardener, H., Spiegelman, D. & Buka, S. L. Perinatal and neonatal risk factors for autism: a comprehensive meta-analysis. Pediatrics 128, 344–355 (2011).
Karst, J. S. & Van Hecke, A. V. Parent and family impact of autism spectrum disorders: a review and proposed model for intervention evaluation. Clin. Child Fam. Psychol. Rev. 15, 247–277 (2012).
Bhutani, V. K. et al. Predischarge screening for severe neonatal hyperbilirubinemia identifies infants who need phototherapy. J. Pediatr. 162, 477–482.e1 (2013).
Shapiro, S. M. Bilirubin toxicity in the developing nervous system. Pediatr. Neurol. 29, 410–421 (2003).
Bauman, M. L. & Kemper, T. L. Neuroanatomic observations of the brain in autism: a review and future directions. Int. J. Dev. Neurosci. 23, 183–187 (2005).
Palmen, S. J., van Engeland, H., Hof, P. R. & Schmitz, C. Neuropathological findings in autism. Brain 127, 2572–2583 (2004).
Hansen, T. W. & Bratlid, D. Bilirubin and brain toxicity. Acta Paediatr. Scand. 75, 513–522 (1986).
Amin, S. B., Smith, T. & Wang, H. Is neonatal jaundice associated with autism spectrum disorders: a systematic review. J. Autism Dev. Disord. 41, 1455–1463 (2011).
Verhoeven, J. S., De Cock, P., Lagae, L. & Sunaert, S. Neuroimaging of autism. Neuroradiology 52, 3–14 (2010).
Jenabi, E., Bashirian, S. & Khazaei, S. Association between neonatal jaundice and autism spectrum disorders among children: a meta-analysis. Clin. Exp. Pediatr. 63, 8–13 (2020).
Schieve, L. A., Clayton, H. B., Durkin, M. S., Wingate, M. S. & Drews-Botsch, C. Comparison of perinatal risk factors associated with autism spectrum disorder (ASD), intellectual disability (ID), and co-occurring ASD and ID. J. Autism Dev. Disord. https://doi.org/10.1007/s10803-015-2402-0 (2015).
Higgins, J. P. T. & Green, S. Cochrane Handbook for Systematic Reviews of Interventions. 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from https://training.cochrane.org/handbook/archive/v5.1/ (2011).
von Elm, E. et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J. Clin. Epidemiol. 61, 344–349 (2008).
Wells, G. A. et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa Hospital Research Institute. http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm (2009).
Gardener, H., Spiegelman, D. & Buka, S. L. Prenatal risk factors for autism: comprehensive meta-analysis. Br. J. Psychiatry 195, 7–14 (2009).
Review Manager 5 (RevMan 5) [computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration (2014).
Higgins, J. P. & Thompson, S. G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 21, 1539–1558 (2002).
Higgins, J. P., Thompson, S. G., Deeks, J. J. & Altman, D. G. Measuring inconsistency in meta-analyses. Bmj 327, 557–560 (2003).
Maimburg, R. D., Bech, B. H., Væth, M., Møller-Madsen, B. & Olsen, J. Neonatal jaundice, autism, and other disorders of psychological development. Pediatrics 126, 872–878 (2010).
Maimburg, R. D. et al. Neonatal jaundice: a risk factor for infantile autism? Paediatr. Perinat. Epidemiol. 22, 562–568 (2008).
Lozada, L. E. et al. Association of autism spectrum disorders with neonatal hyperbilirubinemia. Glob. Pediatr. Heal 2, 2333794x15596518 (2015).
Croen, L. A., Yoshida, C. K., Odouli, R. & Newman, T. B. Neonatal hyperbilirubinemia and risk of autism spectrum disorders. Pediatrics 115, e135–e138 (2005).
Wu, Y. W. et al. Risk of autism associated with hyperbilirubinemia and phototherapy. Pediatrics 138. https://doi.org/10.1542/peds.2016-1813 (2016).
Jangaard, K. A., Fell, D. B., Dodds, L. & Allen, A. C. Outcomes in a population of healthy term and near-term infants with serum bilirubin levels of ≥325 μmol/L (≥19 mg/dL) who were born in Nova Scotia, Canada, between 1994 and 2000. Pediatrics 122, 119–124 (2008).
Buchmayer, S. et al. Can association between preterm birth and autism be explained by maternal or neonatal morbidity? Pediatrics 124, e817–e825 (2009).
Hwang, Y. S., Weng, S. F., Cho, C. Y. & Tsai, W. H. Higher prevalence of autism in Taiwanese children born prematurely: a nationwide population-based study. Res. Dev. Disabil. 34, 2462–2468 (2013).
Hisle-Gorman, E. et al. Prenatal, perinatal, and neonatal risk factors of autism spectrum disorder. Pediatr. Res. https://doi.org/10.1038/pr.2018.23 (2018).
Maimburg, R. D., Bech, B. H., Væth, M., Møller-Madsen, B. & Olsen, J. [personal communication]. Response from authors. Comment to Neonatal jaundice, autism, and other disorders of psychological development. Pediatrics. 126, 872–878 (2010).
American Academy of Pediatrics Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics 114, 297−316 (2004).
Bjerre, J. V. & Petersen, J. R. & Ebbesen, F. Surveillance of extreme hyperbilirubinaemia in Denmark. A method to identify the newborn infants. Acta Pediatr. https://doi.org/10.1111/j.1651-2227.2008.00879.x (2008).
Lain, S. J., Roberts, C. L., Bowen, J. R. & Nassar, N. Early discharge of infants and risk of readmission for jaundice. Pediatrics https://doi.org/10.1542/peds.2014-2388 (2015).
Kuzniewicz, M. W., Escobar, G. J. & Newman, T. B. Impact of universal bilirubin screening on severe hyperbilirubinemia and phototherapy use. Pediatrics 124, 1031–1039 (2009).
Gillberg, C. & Gillberg, I. C. Infantile autism: a total population study of reduced optimality in the pre-, peri-, and neonatal period. J. Autism Dev. Disord. 13, 153–166 (1983).
Finegan, J. A. & Quarrington, B. Pre-, peri-, and neonatal factors and infantile autism. J. Child Psychol. Psychiatry 20, 119–128 (1979).
Williams, T. C., Bach, C. C., Matthiesen, N. B., Henriksen, T. B. & Gagliardi, L. Directed acyclic graphs: a tool for causal studies in paediatrics. Pediatr. Res. 84, 487–493 (2018).
Idring, S. et al. Changes in prevalence of autism spectrum disorders in 2001–2011: findings from the Stockholm Youth Cohort. J. Autism Dev. Disord. 45, 1766–1773 (2014).
Rutter, M. Incidence of autism spectrum disorders: changes over time and their meaning. Acta Paediatr. 94, 2–15 (2005).
Elsabbagh, M. et al. Global prevalence of autism and other pervasive developmental disorders. Autism Res. 5, 160–179 (2012).
Nassar, N. et al. Autism spectrum disorders in young children: effect of changes in diagnostic practices. Int. J. Epidemiol. 38, 1245–1254 (2009).
Williams, J. G., Higgins, J. P. & Brayne, C. E. Systematic review of prevalence studies of autism spectrum disorders. Arch. Dis. Child 91, 8–15 (2006).
Lundström, S., Reichenberg, A., Anckarsäter, H., Lichtenstein, P. & Gillberg, C. Autism phenotype versus registered diagnosis in Swedish children: prevalence trends over 10 years in general population samples. BMJ 350, h1961 (2015).
Burgos, A. E., Schmitt, S. K., Stevenson, D. K. & Phibbs, C. S. Readmission for neonatal jaundice in California, 1991−2000: trends and implications. Pediatrics 121, e864–e869 (2008).
Burke, B. L. et al. Trends in hospitalizations for neonatal jaundice and kernicterus in the United States, 1988−2005. Pediatrics 123, 524–532 (2009).
Rosti, L., Lambertini, L., Stucchi, I. & Condo, V. Neonatal jaundice: should we go crazy? Pediatrics 127, e859–e860 (2011). author reply e860−1.
Mrozek-Budzyn, D., Majewska, R. & Kieltyka, A. Prenatal, perinatal and neonatal risk factors for autism. Cent. Eur. J. Med. 8, 424–430 (2013).
Nath, S., Roy, R. & Mukherjee, S. Perinatal complications associated with autism—a case control study in a neurodevelopment and early intervention clinic. J. Indian Med. Assoc. 110, 526–529 (2012).
Zhang, X. et al. Prenatal and perinatal risk factors for autism in China. J. Autism Dev. Disord. 40, 1311–1321 (2010).
Piven, J. et al. The etiology of autism: pre-, peri- and neonatal factors. J. Am. Acad. Child Adolesc. Psychiatry 32, 1256–1263 (1993).
Ravi, S., Chandrasekaran, V., Kattimani, S. & Subramanian, M. Maternal and birth risk factors for children screening positive for autism spectrum disorders on M-CHAT-R. Asian J. Psychiatry https://doi.org/10.1016/j.ajp.2016.04.001 (2016).
Geetha, B., Sukumar, C., Dhivyadeepa E., Reddy, J. K. & Balachandar, V. Autism in India: a case–control study to understand the association between socio-economic and environmental risk factors. Acta Neurol. Belg. https://doi.org/10.1007/s13760-018-01057-4 (2018).
Deykin, E. Y. & MacMahon, B. Pregnancy, delivery, and neonatal complications among autistic children. Am. J. Dis. Child. 134, 860–864 (1980).
El-Baz, F., Ismael, N. A. & El-Din, S. M. N. Risk factors for autism: an Egyptian study. Egypt J. Med. Hum. Genet. 12, 31–38 (2011).
Elhameed, M. A. A., Elbaky, A. & Kamel, E. A. A. A controlled study of the risk factors and clinical picture of children with autism in an Egyptian sample. Egypt J. Neurol. Neurosurg. Psychiatry 48, 271–276 (2011).
Froehlich-Santino, W. et al. Prenatal and perinatal risk factors in a twin study of autism spectrum disorders. J. Psychiatr. Res. 54, 100–108 (2014).
George, B., Padmam, M. S. R., Nair, M. K. C., Leena, M. L. & Russell, P. S. S. CDC Kerala 13: Antenatal, natal and postnatal factors among children (2null6 y) with autism null: a case control study. Indian J. Pediatr. 81, 133–137 (2014).
Juul-Dam, N., Townsend, J. & Courchesne, E. Prenatal, perinatal, and neonatal factors in autism, pervasive developmental disorder—not otherwise specified, and the general population. Pediatrics 107, E63 (2001).
Lord, C., Mulloy, C., Wendelboe, M. & Schopler, E. Pre- and perinatal factors in high-functioning females and males with autism. J. Autism Dev. Disord. 21, 197–209 (1991).
Matsuishi, T. et al. Brief report: incidence of and risk factors for autistic disorder in neonatal intensive care unit survivors. J. Autism Dev. Disord. 29, 161–166 (1999).
Bhattarai, A. et al. Prenatal and perinatal risk factors for autism at National Children’s Hospital. J. Gandaki Med. Coll. 11, 67–73 (2018).
Mamidala, M. P. et al. Prenatal, perinatal and neonatal risk factors of Autism Spectrum Disorder: a comprehensive epidemiological assessment from India. Res. Dev. Disabil. 34, 3004–3013 (2013).
Mason-Brothers, A. et al. The UCLA-University of Utah epidemiologic survey of autism: prenatal, perinatal, and postnatal factors. Pediatrics 86, 514–519 (1990).
Elsedfy, G. O. & Abdelraheem, T. High autism risk in children. Middle East Curr. Psychiatry 21, 106–112 (2014).
Chien Chien, Y. L., Gau, S. S. F. & Wu, Y. Y. Prenatal and perinatal risk factors in autism spectrum disorders and their association on autistic symptoms severity. Eur. Child Adolesc. Psychiatry 22, S262 (2013).
Ahmed, E. S., Helaly, M. & Gemeay E. M. Effect of neonatal hyperbilirubinemia on the occurrence of Autism in children. AAMJ 8 (2010).
Sugie, Y. & Sugie, H. Perinatal and neonatal risk factors for autism spectrum disorders. Seishin Shinkeigaku Zasshi 111, 1397–1403 (2009).
Duan, G., Yao, M., Ma, Y. & Zhang, W. Perinatal and background risk factors for childhood autism in central China. Psychiatry Res 220, 410–417 (2014).
Chen, M. H. et al. Is neonatal jaundice associated with autism spectrum disorder, attention deficit hyperactivity disorder, and other psychological development? A nationwide prospective study. Res. Autism Spectr. Disord. 8, 625–632 (2014).
Author information
Authors and Affiliations
Contributions
Each author has met the Pediatric Research authorship requirements listed below: Substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; Drafting the article or revising it critically for important intellectual content; and final approval of the version to be published. M.L.K., J.P.P., and T.B.H. contributed substantially to conception and design, M.L.K. and M.V.P. contributed substantially to the acquisition of data. All authors contributed substantially to the analysis and interpretation of data; M.L.K. drafted the article; the remaining authors contributed in revising it critically for important intellectual content. All authors have approved the final version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Kujabi, M.L., Petersen, J.P., Pedersen, M.V. et al. Neonatal jaundice and autism spectrum disorder: a systematic review and meta-analysis. Pediatr Res 90, 934–949 (2021). https://doi.org/10.1038/s41390-020-01272-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-020-01272-x
This article is cited by
-
The association between post-term births and autism spectrum disorders: an updated systematic review and meta-analysis
European Journal of Medical Research (2023)