Abstract
Background
Chorioamnionitis is associated with increased rates of bronchopulmonary dysplasia (BPD) in ventilated preterm infants. Budesonide when added to surfactant decreased lung and systemic inflammation from mechanical ventilation in preterm lambs and decreased the rates and severity of BPD in preterm infants. We hypothesized that the addition of budesonide to surfactant will decrease the injury from mechanical ventilation in preterm lambs exposed to intra-amniotic (IA) lipopolysaccharide (LPS).
Methods
Lambs at 126 ± 1 day GA received LPS 10 mg IA 48 h prior to injurious mechanical ventilation. After 15 min, lambs received either surfactant mixed with: (1) saline or (2) Budesonide 0.25 mg/kg, then ventilated with normal tidal volumes for 4 h. Injury markers in the lung, liver, and brain were compared.
Results
Compared with surfactant alone, the addition of budesonide improved blood pressures, dynamic compliance, and ventilation, while decreasing mRNA for pro-inflammatory cytokines in the lung, liver, and multiple areas of the brain. LPS caused neuronal activation and structural changes in the brain that were not altered by budesonide. Budesonide was not retained within the lung beyond 4 h.
Conclusions
In preterm lambs exposed to IA LPS, the addition of budesonide to surfactant improved physiology and markers of lung and systemic inflammation.
Impact
-
The addition of budesonide to surfactant decreases the lung and systemic responses to injurious mechanical ventilation preterm lambs exposed to fetal LPS.
-
Budesonide was present in the plasma by 15 min and the majority of the budesonide is no longer in the lung at 4 h of ventilation.
-
IA LPS and mechanical ventilation caused structural changes in the brain that were not altered by short-term exposure to budesonide.
-
The budesonide dose of 0.25 mg/kg being used clinically seems likely to decrease lung inflammation in preterm infants with chorioamnionitis.
Similar content being viewed by others
Introduction
Over half of all extremely preterm infants have been exposed to chorioamnionitis, inflammation of the placenta associated with both preterm deliveries and worse neonatal and long-term morbidities.1,2 Chorioamnionitis increases the risk of development of bronchopulmonary dysplasia (BPD), and mechanical ventilation may further increase BPD risk.3,4 Chorioamnionitis can be experimentally modeled through intra-amniotic (IA) injection of Escherichia coli lipopolysaccharide (LPS) in preterm sheep.5,6 Forty-eight hours after IA LPS, lung inflammation, interstitial edema, and a modest decrease in lung compliance occurs in preterm lambs.7,8 In contrast, by 5–7 days after IA LPS a lung maturation effect with increased lung compliance and surfactant is registered.9 Similar to chorioamnionitis exposure in preterm infants, IA LPS causes persistent changes in the airway response to stimulation.10,11 Systemically, IA LPS causes brain inflammation, which can be worsened by mechanical ventilation.12,13 The ventilated, IA LPS-exposed, preterm lamb model is a relevant model for understanding the pathogenesis of BPD in preterm infants with chorioamnionitis and to test interventions that may decrease BPD.
The combination of budesonide with surfactant therapy at birth has been shown to decrease the rate or severity of BPD compared to surfactant alone, without affecting surfactant’s surface tension properties.14,15,16,17 Budesonide is a very potent glucocorticoid used in asthma and other airway diseases to decrease lung inflammation.18 In preterm lambs, the combination of budesonide and surfactant decreases lung inflammation from injurious and non-injurious ventilation and decreases systemic inflammatory responses in the liver and brain.19,20 Although the preterm lung can form budesonide esters for prolonged release, the majority of the budesonide is released into the systemic circulation by 6 h of ventilation.21 The systemic budesonide, also found in the human studies, may be responsible for alterations in adrenal function found in pilot studies.15,22
In an observational study of infants receiving budesonide and surfactant compared with a historical cohort, infants with documented histological chorioamnionitis appeared to have less improvement in respiratory markers or BPD than infants without chorioamnionitis.17 Using a ventilated preterm lamb model of chorioamnionitis, we tested the hypothesis that budesonide at 0.25 mg/kg with surfactant will decrease the acute lung and brain inflammation from IA LPS and mechanical ventilation compared to surfactant alone.
Methods
Animal management
All animal experiments were performed with the approval of the Animal Ethics Committee of the University of Western Australia.
Date-mated Merino Ewes at 124 + 1 days gestational age (GA; term is about 150 days GA) received IA injection of 10 mg LPS E. coli O55:B5 (Sigma-Aldrich, St. Louis, MO, USA). The IA injections were performed with ultrasound guidance with sampling of the amniotic fluid for electrolyte analysis to verify IA injection.23 Two days after IA LPS at 126 + 1 days GA, ewes were anesthetized with intravenous (IV) ketamine and midazolam (5–10 and 0.25–0.5 mg/kg, respectively) and given spinal anesthesia (lignocaine 60 mg) prior to laparotomy, hysterotomy, and euthanasia.19,20 Immediately before delivery, the lamb was given ketamine 10 mg/kg intramuscular (IM), then a 4.5-mm endotracheal tube was secured in the trachea before gentle aspiration of fetal lung fluid. The lambs were then delivered, weighed, and placed under a radiant warmer. The lambs were ventilated as described below for 15 min, and then assigned to treatment with either: (a) 200 mg/kg surfactant (Poractant alfa, Curosurf® 2.5 mL/kg, Chiesi Farmaceutici, Italy) + 0.5 mL/kg saline (Saline) or (b) 200 mg/kg surfactant + 0.25 mg/kg budesonide (Bud) (0.5 mg/mL, Pulmicort®, AstraZeneca, USA). For surfactant and budesonide dosing, 3 kg was used as the estimated fetal weight. Surfactant was gently mixed with budesonide and then administered through the endotracheal tube with body positioning to assist with distribution to the lungs.20,21 The numbers of lambs per group (n = 5–6) were determined from previous experiments based on multiple markers of injury or inflammation in the lung and liver.24 Unventilated lambs, euthanized at delivery, were used as unventilated controls (UVC) (n = 5).
Mechanical ventilation of lambs
Prior to surfactant treatment and with the intention of initiating lung injury, ventilation was initiated with a Fabian ventilator (Acutronic, Switzerland) with 100% oxygen, peak inspiratory pressure (PIP) of 40 cm H2O, using no positive end expiratory pressure (PEEP), a rate of 50 breaths/min, and an inspiratory time of 0.5 s.19 Tidal volume/kg was monitored and the PIP was adjusted up to a maximum of 50 cm H2O to achieve a target tidal volume (VT)/kg of 12 mL/kg by 15 min.21 After 15 min of ventilation, the animals were treated with the assigned surfactant combination and ventilated using a gentler ventilation strategy for 3 h and 45 min. After surfactant PIP was lowered to maximum 40 cm H2O, PEEP was increased to 5 cm H2O with 40% heated and humidified oxygen to 37 °C (MR850 Humidifier, Fisher & Paykel Healthcare, Auckland, NZ).20 The PIP was adjusted to maintain a VT of ≤8 mL/kg unless the PaCO2 was >50 mm Hg and PIP was less than the maximum of 40 cm H2O. The normal tidal volume of a lamb is approximately 8 vs 4–5 mL/kg in a preterm infant. The lambs were fully sedated with ketamine, so these did not breathe. Oxyhemoglobin saturations were to be >90% monitored by continuous pulse oximetry and oxygen adjusted. Blood gas measurements were performed at 15 and 30 min post-delivery and at 1-h intervals for 4 h and as needed. Immediately following birth, the lamb received a 10 mL/kg transfusion with placental blood to support blood pressure and to allow for the blood sampling. The lamb was continuously monitored for temperature with a rectal thermometer, and the heart rate and blood pressure from an umbilical artery catheter. If mean arterial blood pressure fell <20 mm Hg, lambs received additional red cells (first bolus) or normal saline bolus infusions and volumes were recorded. IV fluid was given at 80 mL/kg/24 h with D51/2NS with 10 units/mL heparin. The lamb was euthanized with 100 mg/kg IV pentobarbital at 4 h.
Tissue sampling
Postmortem inflation and deflation pressure–volume curves were measured with stepwise changes in pressure to a maximum of 40 cm H2O.25 The left lung was used for an alveolar wash and for measurements of residual tissue budesonide.26 Tissues from the right lower peripheral lung and liver were snap frozen for RNA isolation.25 The right upper lobe was inflation fixed at 30 cm H2O with 10% formalin and then paraffin embedded.25 The skull was opened and tissue from the right periventricular white matter (PVWM), right hippocampal region, and right cortical region were snap frozen for RNA isolation. The left hemisphere of the brain was fixed within the skull with 10% formalin for multiple days before being sliced into sagittal sections, with the central segment used for histology.12
Quantitative reverse transcription polymerase chain reaction (RT-PCR)
Total RNA was extracted from the right lower lobe, liver, PVWM, hippocampus, and cortical regions with Trizol.24 Custom TaqMan gene primers (Life Technologies) for ovine sequences for interleukin-1β (IL-1β), IL-6, IL-8, monocyte chemoattractant protein-1 (MCP-1), serum amyloid A3 (SAA3), surfactant protein B (SFTPB), and toll-like receptor 4 (TLR4) were used. Quantitative RT-PCR was performed in triplicate with iTaq Universal mix (Bio-Rad) on a CFX Connect (Bio-Rad). 18S primers (Life Technologies) were used as the internal loading control. Fold increased were determined by ∆∆Cq method (CFX manager, BioRad), and average ∆∆Cq for controls were set as 1, and groups reported as fold increase over UVC.
Immunohistochemistry
Paraffin sections (4 µm) of formalin-fixed right upper lobe tissue were used for hematoxylin and eosin (H&E) staining and immunohistochemistry with 1:250 mouse anti-human inducible nitric oxide synthase (iNOS; BD Biosciences, USA) or no antibody (negative control).27 Blinded slides for iNOS staining had 10 random regions/animal (×40 on Zeiss Axioskop 40) manually counted and scored as 0 = no positive cells, 1 = occasional positive cells, 2 = large number of positive cells. iNOS-positive cells were primarily in airspaces. H&E-stained slides of the lungs were blinded and evaluated for airway epithelial injury, edema, hemorrhage, and inflammation (0–2 points each).28 The fixed brain segments from the central region of the brain were analyzed on H&E for cells with tissue regressing from nucleus (0 = minimal cells, 1 = occasional cells, 2 = multiple cells, 3 = >10 cells per high-power field (HPF; ×20 magnification). Brain tissue was stained with glial fibrillary acidic protein (GFAP) antibody (Sigma G3893 1:400), and blinded slides were counted for positive cells in the white matter (internal capsule) and the cortical tissue. Terminal deoxynucleotidyl transferase-mediated dUTP-fluorescein nick end labeling (TUNEL) staining (ApopTag Peroxidase Apoptosis Kit, Millipore) for apoptosis was performed, and positive cells in the cortical region and vessels were counted on blinded slides. Ki67 (Thermofisher PA5-19462 1:400) staining for proliferation and positive cells/×20 HPF in the peripheral tissue or in the lining of the ventricular system were counted on blinded slides.
Budesonide measurements
Budesonide was measured in the plasma and lung tissue using a previously published protocol.20,24 Lung tissue was hydrolyzed with bovine pancreas cholesterol esterase.20 Budesonide analysis was performed with an Agilent Technologies 1290 Infinity HPLC system and a 6460 Series Triple Quadrupole LC/MS/MS. The limit of quantitation was 1 ng/mL.
Data analysis and statistics
Variables are presented as mean ± standard deviation (SD) and mRNA results as fold increase over control values set to 1. Statistics were analyzed with Prism 6 (GraphPad) using Student’s t test, Mann–Whitney non-parametric, or analysis of variance tests as appropriate. Significance was accepted as p < 0.05.
Results
Physiology during ventilation
There were no statistically significant differences in birth weight, GA, gender, VT per kg achieved at 15 min, or the PIP used at 15 min between the LPS/Saline and LPS/Budesonide lambs (Table 1). The UVC were slightly smaller. The antenatal LPS was associated with lower blood pressures and worsening metabolic acidosis, requiring the additional boluses of fluid early to maintain ventilation. There were no differences in the volume of normal saline/maternal blood needed between LPS/Saline and LPS/Budesonide lambs. After 4 h of ventilation, the mean arterial pressure and systolic blood pressure were higher in LPS/Budesonide lambs. The lambs receiving budesonide had lower pCO2 levels at 4 h and a trend (p = 0.07) for improved ventilation efficiency index (VEI). The LPS/Budesonide lambs had improved dynamic lung compliance at 4 h but no decrease in mean airway pressures (p = 0.16).
Necropsy analysis
The static compliance on pressure volume curves at 40 cm H2O was similar between the LPS/Saline and LPS/Budesonide animals (V40/kg) (p = 0.17) but higher than UVC (Table 1). The lungs in the LPS/Saline group were heavier than the LPS/Budesonide and UVC, but there were no differences between the LPS groups in the weight of the posterior mediastinal lymph node (PMLN) or the number of inflammatory cells in the bronchoalveolar lavage fluid (BALF). Both LPS-exposed groups had more lung inflammation and heavier PMLN then UVC. The number of neutrophils and monocytes in the BALF were similar between the LPS groups (data not shown). On lung histology, both the ventilated LPS groups demonstrated moderate lung injury and inflammation without differences between the two groups on lung injury scores or the number of iNOS-positive inflammatory cells (Table 1). UVC have minimal inflammation, injury scores, or iNOS staining.
Budesonide levels
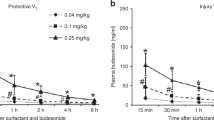
Budesonide was detected in the plasma at 15 min after dose at 76 ± 31 ng/mL and decreased over time to 38 ± 16 ng/mL at 30 min, 28 ± 12 ng/mL at 1 h, 14 ± 7 ng/mL at 2 h, and 8 ± 3 ng/mL at 4 h. The amount of budesonide remaining in the lung at 4 h was low at 1.5 ± 0.9% of initial dose as free budesonide and 1.8 ± 1.4% after hydrolysis to release budesonide esters.
Lung and liver mRNA responses
Mechanical ventilation increased the mRNA values for the pro-inflammatory cytokines in all the ventilated groups compared with controls (UVC set to 1; Fig. 1a–d). The combination of budesonide and surfactant mixed at the bedside decreased mRNA for IL-6 and MCP-1 and had a strong trend for a decrease in IL-β (p = 0.051). IL-8 mRNA increased in the two ventilated groups. LPS and ventilation increased (p < 0.05) SFTPB mRNA, a sign of lung maturation, in both LPS/Saline animals (15 ± 12-fold increase over controls) and LPS/Budesonide animals (16 ± 6-fold increase). TLR mRNA in the lungs was increased (p < 0.05) in LPS/Saline animals 17 ± 6-fold and in LPS/Budesonide animals 11 ± 2-fold compared with controls (p = 0.07 vs LPS/Saline animals). The combination of budesonide and surfactant decreased the mRNA for MCP-1 in the liver (Fig. 1e). IL-6 in the liver (Fig. 1f) was not lower (p = 0.08) with LPS/Budesonide. The acute phase reactant SAA3 mRNA increased in the liver with both LPS/Saline (129 ± 59-fold increase) and LPS/Budesonide (233 ± 111-fold increase) compared to controls.
Brain cytokine mRNA in multiple regions of the brain
In the PVWM, pro-inflammatory cytokine IL-1β mRNA decreased with LPS and mechanical ventilation (Table 2) and further decreases with LPS/Budesonide compared to control animals (values set to 1). PVWM MCP-1 mRNA was decreased in the LPS/Budesonide group compared with controls, whereas the LPS/Saline group was more variable. In the cortical tissue, the LPS/Budesonide lambs had decreased IL-1β and MCP-1 mRNA compared with controls and LPS/Saline animals. In the hippocampal tissue, LPS/Budesonide animals had decreased IL-1β and MCP-1 compared with control and IL-1β vs LPS/Saline.
Brain histology
Exposure to budesonide did not change the histologic effects of LPS, which is likely due to the short-term exposure of the budesonide (4 h) on the structure (Table 3 and Fig. 2). LPS exposure increased the number of cells on H&E staining with perinuclear clearing (Fig. 2a–c and Table 3). TUNEL staining, a marker of apoptosis, was increased in the cortical tissue with LPS/Saline animals compared to controls (Table 3 and Fig. 2d–f), whereas there was no increase seen in the LPS/Budesonide animals. TUNEL staining in the blood vessels were increased with LPS in both groups. Glial cell activation (GFAP-positive cells) was increased in intensity and number in the white matter and the cortical tissue with LPS exposure (Table 3 and Fig. 2g–i), but there was no effect of budesonide on activation. Ki67-positive cells, a marker of proliferation, were similar to controls in the cortical regions (Table 3 and Fig. 2j–l) and the ventricular lining (Table 3) with LPS exposure or exposure to budesonide.
a–c Hematoxylin and eosin (H&E ×20) staining of the cortical regions demonstrating increased regions of separation surrounding nucleus in LPS-exposed animals (inset ×60). d–f TUNEL staining demonstrates increased apoptosis in the e LPS/Saline animals compared with controls (d) (inset ×60). g–i Glial fibrillary acidic protein (GFAP) staining in the cortical region demonstrates increased number and intensity of the neuronal activation with LPS exposure (h, i) compared to controls (g) (insets ×100). j–l Ki67-positive cells demonstrate similar cellular proliferation between groups (inset ×60). Scale bar = 10 nm.
Discussion
In these intentionally injured preterm lambs with lung inflammation from both the IA LPS and the large tidal volume mechanical ventilation, the combination of surfactant and budesonide improved the ventilation physiology and markers of injury in the lungs and systemic organs.28,29 The significant improvements with the addition of budesonide were found in relatively small groups of animals, demonstrating the potent anti-inflammatory effects of budesonide. Although not tested with the current model, this combination would likely have benefits with less aggressive clinical resuscitation in the setting of chorioamnionitis based on previous experiments.20,21
IA LPS exposure caused more severe initial hypotension than observed in previous experiments, and the lambs required additional IV boluses to correct metabolic acidosis. The animals in both treatment groups received similar fluid resuscitations in the first hour of ventilation, but the budesonide-treated lambs had stabilization of their blood pressures by 4 h. Due to the increased combination of the initial lung injury and the deterioration from the LPS exposure, we shortened the experiments to 4 h compared to previous 6-h experiments.19 Previous animals ventilated 4 days after IA LPS did not have early blood pressure problems, whereas receiving a dose of IA LPS 5 days and 2 days prior to delivery had improved blood pressure control.30,31 These findings are consistent with the lung inflammation that occurs after 2 days of IA LPS and the lung and systemic maturation that occurs with IA LPS by 5 days.32,33
The mRNA for SFTPB and TLR4, the innate receptor for LPS endotoxin, were increased in LPS animals compared with the UVC demonstrating both the maturation effects of IA LPS and the upregulation of TLR4 previously demonstrated with IA LPS and mechanical ventilation.6,26 At 133 days GA, intra-tracheal LPS worsened ventilation physiology and lung mRNA values in both mechanical ventilated lambs and lambs maintained on continuous positive airway pressure.34 Mechanical ventilation also releases endogenous TLR4 ligand heat shock protein 70 from the airway epithelial cells and could contribute to the inflammation through further TLR4.28 These findings are consistent with clinical observations of increased lung inflammation after chorioamnionitis and the increased risk of BPD when the infants are mechanically ventilated.3
The plasma levels for budesonide were slightly lower than previously reported, but this may have been due to the initial volume resuscitation needs or LPS-induced leaky lungs.21 The amount of budesonide remaining in the lungs, either as free budesonide or converted to a budesonide ester, was low (<2% on initial results) in the animals consistent with previous reports.21 This release of budesonide into systemic circulation has also been found in preterm infants and could have implications in the systemic responses in preterm infants.15,17,22 We selected the clinically used budesonide dose of 0.25 mg/kg dose for these experiments because our previous experiments with lower dosing (0.1 mg/kg) had less consistent responses. Budesonide is a very potent glucocorticoid and in pilot studies in preterm infant decreased markers of adrenal function in the serum, thus further study is necessary on systemic effects of the combination.22 The effects on the brain could be due to either from a decreased injury mediator signal from the lung itself or from the systemic release of the steroid, but further studies of systemic administration of budesonide or other corticosteroids would be needed. Our previous studies of systemic steroids (dexamethasone or hydrocortisone) given at birth have not shown the lung benefits found with intra-tracheal budesonide.35
We have previously demonstrated changes in the mRNA patterns in the PVWM of the brain of preterm lambs receiving mechanical ventilation, and these changes were partially normalized by exposure to the combination of budesonide and surfactant.19 The combination of surfactant and budesonide also had independent effects of hundreds of genes in the brains and could lead to long-term changes in brain function.19 Similar to the PVWM, the current report demonstrates decreased pro-inflammatory cytokine mRNA in the hippocampal and cortical tissues. Improvements in neuroinflammation with inhaled budesonide were also found in a mouse model of severe asthma compared to saline inhalation.36 Histological evaluation demonstrated increased areas of tissue separation on H&E, increased TUNEL staining (apoptosis), increased neuronal activation (GFAP-positive cells) in the gray and white matter, but no increase in proliferation (Ki67-positive cells). Mechanical ventilation of preterm lambs for 48 h increased microglial activation (amoeboid shape) and cell proliferation (Ki67+), but decreased GFAP staining in the white matter and fewer neurons were found in the cortex.37 Our ventilation period was short at 4 h, thus the effects of mechanical ventilation or budesonide on brain structure may have been limited.
The majority of the structural changes in the brain were likely due to the IA LPS. LPS injection caused increased pro-inflammatory cytokines and inflammation in the brains of rhesus monkeys by 16 h after injection and microglial proliferation by 48 h after LPS.38 The timing of IA LPS administration effects neuronal changes, with glial activation after 2 days, whereas caspase 3 activation was not seen until 8 days and decrease in the number of oligodendrocytes was not observed until closer to 15 days.32 Of interesting note, the combination of IA LPS with betamethasone IM to model pregnant women with chorioamnionitis who have received antenatal steroids can alter the brain inflammation depending on the order of administration.12 With longer ventilation periods, mechanical ventilation of preterm lambs after LPS exposure 2 days prior to birth was associated with white matter astrogliosis (GFAP-positive cells) and cell death (TUNEL-positive cells) with both injurious and protective ventilation strategies.39 Whereas in lambs exposed to LPS 7 days prior to mechanical ventilation, there were no effects of mechanical ventilation on gray matter injury found with IA LPS exposure.13 These findings suggest that the timing of chorioamnionitis to delivery and mechanical ventilation may affect the severity of the brain injury and the location of the injury within the white and gray matter.
The experiment was limited by the small number of animals in each group and the short ventilation period. Small changes in some physiologic and molecular measurements may be hidden within high variance for measurements, but overall significant changes were observed with budesonide in many of the markers of injury with only a few animals. The period of ventilation and exposure to budesonide was only 4 h, so structural changes in the brain may not have been evident at that time point. Since no LPS only animals were examined, the changes in the brain could be due to either ventilation or LPS exposure or the combination. Another limitation of the study for clinicians is the use of intentionally injurious ventilation strategies that may not be used in the delivery room of preterm infants. The ability of budesonide to improve the lung physiology in the 4-h ventilation period, even in these severely injured animals, likely demonstrates the anti-inflammatory effects of this potent steroid. In lambs with less injurious ventilation, budesonide improved the pro-inflammatory cytokines and markers of injury in lambs ventilated for 2 h.20 Since these animals were exposed to LPS, some lung inflammation was present at birth and we may not have been able to differentiate differences in BALF cell counts with budesonide. Measurement of budesonide in the liver or brain was not done, so it is also difficult to determine whether the systemic changes were due to budesonide itself within these tissues.
Conclusions
In preterm lambs exposed to IA LPS injections 2 days prior to delivery, the combination of budesonide and surfactant improved ventilation physiology by 4 h and decreased markers of injury in the lung, liver, and multiple areas of the brain. The histologic changes noted in the brain reinforce the effects of chorioamnionitis on neuroinflammation, but the timing of the budesonide to sacrifice may have been too short to see the relevant effects. Similar to previous experiments, the budesonide was measured in the plasma by 15 min and <2% was still in the lung at 4 h. These findings would suggest that addition of budesonide to surfactant could potentially be beneficial in the preterm exposed to chorioamnionitis.
References
Maisonneuve, E. et al. Association of chorioamnionitis with cerebral palsy at two years after spontaneous very preterm birth: the EPIPAGE-2 cohort study. J. Pediatr. 222, 71.e6–78.e6 (2020).
Venkatesh, K. K. et al. Histologic chorioamnionitis and risk of neurodevelopmental impairment at age 10 years among extremely preterm infants born less than 28 weeks of gestation. Am. J. Obstet. Gynecol. 223, 745.e1–745.e10 (2020).
Villamor-Martinez, E. et al. Association of chorioamnionitis with bronchopulmonary dysplasia among preterm infants: a systematic review, meta-analysis, and metaregression. JAMA Netw. Open 2, e1914611 (2019).
Watterberg, K. L., Demers, L. M., Scott, S. M. & Murphy, S. Chorioamnionitis and early lung inflammation in infants in whom bronchopulmonary dysplasia develops. Pediatrics 97, 210–215 (1996).
Kallapur, S. G. et al. Pulmonary and systemic endotoxin tolerance in preterm fetal sheep exposed to chorioamnionitis. J. Immunol. 179, 8491–8499 (2007).
Hillman, N. H. et al. Toll-like receptors and agonist responses in the developing fetal sheep lung. Pediatr. Res. 63, 388–393 (2008).
Bachurski, C. J., Ross, G. F., Ikegami, M., Kramer, B. W. & Jobe, A. H. Intra-amniotic endotoxin increases pulmonary surfactant components and induces SP-B processing in fetal sheep. Am. J. Physiol. Lung Cell. Mol. Physiol. 280, L279–L285 (2001).
Kramer, B. W., Kramer, S., Ikegami, M. & Jobe, A. Injury, inflammation and remodeling in fetal sheep lung after intra-amniotic endotoxin. Am. J. Physiol. Lung Cell. Mol. Physiol. 283, L452–L459 (2002).
Jobe, A. H. et al. Endotoxin induced lung maturation in preterm lambs is not mediated by cortisol. Am. J. Respir. Crit. Care Med. 162, 1656–1661 (2000).
Lee, A. J. X. et al. Fetal responses to lipopolysaccharide-induced chorioamnionitis alter immune and airway responses in 7-week-old sheep. Am. J. Obstet. Gynecol. 204, 364.e17–364.e24 (2011).
Getahun, D. et al. Effect of chorioamnionitis on early childhood asthma. Arch. Pediatr. Adolesc. Med. 164, 187–192 (2010).
Kuypers, E. et al. Effects of intra-amniotic lipopolysaccharide and maternal betamethasone on brain inflammation in fetal sheep. PLoS ONE 8, e81644 (2013).
Stojanovska, V. et al. Effects of intrauterine inflammation on cortical gray matter of near-term lambs. Front. Pediatr. 6, 145 (2018).
Yeh, T. F. et al. Early intratracheal instillation of budesonide using surfactant as a vehicle to prevent chronic lung disease in preterm infants: a pilot study. Pediatrics 121, e1310–e1318 (2008).
Yeh, T. F. et al. Intratracheal administration of budesonide/surfactant to prevent bronchopulmonary dysplasia. Am. J. Respir. Crit. Care Med. 193, 86–95 (2016).
Ricci, F., Murgia, X., Razzetti, R., Pelizzi, N. & Salomone, F. In vitro and in vivo comparison between poractant alfa and the new generation synthetic surfactant CHF5633. Pediatr. Res. 81, 369–375 (2017).
Kothe, T. B. et al. Surfactant and budesonide for respiratory distress syndrome: an observational study. Pediatr. Res. 87, 940–945 (2020).
Esmailpour, N., Hogger, P. & Rohdewald, P. Binding kinetics of budesonide to the human glucocorticoid receptor. Eur. J. Pharmacol. Sci. 6, 219–223 (1998).
Hillman, N. H. et al. Surfactant plus budesonide decreases lung and systemic responses to injurious ventilation in preterm sheep. Am. J. Physiol. Lung Cell. Mol. Physiol. 318, L41–L48 (2020).
Kothe, T. B. et al. Surfactant plus budesonide decreases lung and systemic inflammation in mechanically ventilated preterm sheep. Am. J. Physiol. Lung Cell. Mol. Physiol. 316, L888–L893 (2019).
Hillman, N. H. et al. Dose of budesonide with surfactant affects lung and systemic inflammation after normal and injurious ventilation in preterm lambs. Pediatr. Res. 88, 726–732 (2020).
McEvoy, C. T. et al. Dose-escalation trial of budesonide in surfactant for prevention of bronchopulmonary dysplasia in extremely low gestational age high-risk newborns (SASSIE). Pediatr. Res. 88, 629–636 (2020).
Kuypers, E. et al. Intra-amniotic LPS and antenatal betamethasone: inflammation and maturation in preterm lamb lungs. Am. J. Physiol. Lung Cell. Mol. Physiol. 302, L380–L389 (2012).
Kothe, T. B. et al. Effects of budesonide and surfactant in preterm fetal sheep. Am. J. Physiol. Lung Cell. Mol. Physiol. 315, L193–L201 (2018).
Jobe, A. H. et al. Endotoxin-induced lung maturation in preterm lambs is not mediated by cortisol. Am. J. Respir. Crit. Care Med. 162, 1656–1661 (2000).
Hillman, N. H. et al. Brief, large tidal volume ventilation initiates lung injury and a systemic response in fetal sheep. Am. J. Respir. Crit. Care Med. 176, 575–581 (2007).
Hillman, N. H. et al. Inflammation and lung maturation from stretch injury in preterm fetal sheep. Am. J. Physiol. Lung Cell. Mol. Physiol. 300, L232–L241 (2011).
Hillman, N. H. et al. Airway injury from initiating ventilation in preterm sheep. Pediatr. Res. 67, 60–65 (2010).
Kallapur, S. G. et al. Pulmonary and systemic endotoxin tolerance in preterm fetal sheep exposed to chorioamnionitis. J. Immunol. 179, 8491–8499 (2007).
Gisslen, T. et al. Repeated exposure to intra-amniotic LPS partially protects against adverse effects of intravenous LPS in preterm lambs. Innate Immun. 20, 214–224 (2014).
Ikegami, M., Kallapur, S. G. & Jobe, A. H. Initial responses to ventilation of premature lambs exposed to intra-amniotic endotoxin 4 days before delivery. Am. J. Physiol. Lung Cell. Mol. Physiol. 286, L573–L579 (2004).
Gussenhoven, R. et al. Chorioamnionitis, neuroinflammation, and injury: timing is key in the preterm ovine fetus. J. Neuroinflammation 15, 113 (2018).
Kramer, B. W. et al. Dose and time response after intraamniotic endotoxin in preterm lambs. Am. J. Respir. Crit. Care Med. 164, 982–988 (2001).
Polglase, G. R. et al. Lung and systemic inflammation in preterm lambs on continuous positive airway pressure or conventional ventilation. Pediatr. Res. 65, 67–71 (2009).
Hillman, N. H. et al. Antenatal and postnatal corticosteroid and resuscitation induced lung injury in preterm sheep. Respir. Res. 10, 124 (2009).
Xia, M. X., Ding, X., Qi, J., Gu, J., Hu, G. & Sun, X. L. Inhaled budesonide protects against chronic asthma-induced neuroinflammation in mouse brain. J. Neuroimmunol. 273, 53–57 (2014).
Nott, F. et al. Brain inflammation and injury at 48 h is not altered by human amnion epithelial cells in ventilated preterm lambs. Pediatr. Res. 88, 27–37 (2020).
Schmidt, A. F. et al. Intra-amniotic LPS causes acute neuroinflammation in preterm rhesus macaques. J. Neuroinflammation 13, 238 (2016).
Barton, S. K. et al. Protective ventilation of preterm lambs exposed to acute chorioamnionitis does not reduce ventilation-induced lung or brain injury. PLoS ONE 9, e112402 (2014).
Acknowledgements
This work was supported by a grant from Chiesi Farmaceutici S.p.A (to A.H.J., N.H.H.).
Author information
Authors and Affiliations
Contributions
Conception and design: N.H.H., A.H.J., M.W.K., G.C.M., F.S.; animal procedures: N.H.H., M.W.K., E.F., J.R.-B., G.C.M., A.H.J.; experimental analysis: N.H.H., A.H.J., M.W.K., E.R., L.A.; manuscript preparation: N.H.H., A.H.J., M.W.K., F.S. All authors approved the final manuscript as submitted.
Corresponding author
Ethics declarations
Animal experiments
Ethical approval was obtained and no consent was necessary.
Competing interests
The authors have no conflicts relevant to this article to disclose. F.S. is an employee of Chiesi Farmaceutici S.p.A, but the data collection and analysis were done independent of Chiesi.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hillman, N.H., Kemp, M.W., Fee, E. et al. Budesonide with surfactant decreases systemic responses in mechanically ventilated preterm lambs exposed to fetal intra-amniotic lipopolysaccharide. Pediatr Res 90, 328–334 (2021). https://doi.org/10.1038/s41390-020-01267-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-020-01267-8
This article is cited by
-
Postnatal budesonide improved lung function in preterm lambs exposed to antenatal steroids and chorioamnionitis
Pediatric Research (2024)
-
Preterm lung and brain responses to mechanical ventilation and corticosteroids
Journal of Perinatology (2023)