Abstract
Background
Eighty milliliter per kilogram of polyethylene glycol (PEG) for bowel preparation (BP) has been recommended, but the amount of liquid orally without nasogastric intubation is difficult to achieve. This study is to compare the efficacy and tolerability of two different low-volume PEG electrolyte solutions for BP in children.
Methods
The randomized, double‐blind, controlled trial enrolled 150 children aged 6–18 years undergoing colonoscopy in our center. Patients were randomly assigned to receive 60 ml/kg (PEG-ELS 60) or 40 ml/kg (PEG-ELS 40) of PEG electrolytes (PEG-ELS) 4000. The Boston Bowel Preparation Scale was used for bowel cleansing evaluation. Primary end point was overall colon cleansing. Tolerability was also evaluated.
Results
PEG-ELS 40 and PEG-ELS 60 had similar efficacy in bowel cleansing for both whole colon and various colonic segments. The proportions of patients experiencing any adverse symptoms, or those who were willing to have BP repeated if necessary were similar in both groups. More patients considered the BP solution easy to take and be satisfied with the preparation in PEG-ELS 40 than PEG-ELS 60.
Conclusions
Low volume of PEG-ELS for BP has good efficacy in bowel cleansing. PEG-ELS with 40 ml/kg volume was not inferior to that of 60 ml/kg.
Impact
-
PEG-ELS 40 and PEG-ELS 60 had similar efficacy in bowel cleansing for whole and various colonic segments.
-
The proportions of patients experiencing any adverse symptoms, or those who were willing to have BP repeated if necessary were similar in both groups.
-
More patients considered BP solution easy to take and be satisfied with the preparation in PEG-ELS 40 than PEG-ELS 60.
-
This study showed that low-volume PEG-ELS monotherapy was effective in bowel cleansing and explored a possibly feasible BP method for pediatrics in China that PEG-ELS 40 was comparable to PEG-ELS 60 regimen.
Similar content being viewed by others
Introduction
Colonoscopy is an important procedure for the diagnosis and management of gastrointestinal disorders.1 Adequate bowel preparation is the premise to ensure the proper conduction of the procedure. In different studies, up to 20–30% of incomplete colonoscopies are due to inadequate bowel cleanout, often attributed to the significant discomfort associated with a variety of preparation regimens.2,3 Ideal preparation should be of low volume, tasty, effective, convenient, and safe. Despite several studies reported, standardized protocol of bowel preparation for pediatric patients is still lacking and the practice varies in different medical centers. Currently, many commonly used agents, such as sodium phosphate, are not approved for children in China for safety issues. Only one agent, Polyethylene Glycol Electrolyte Powder (IV) is officially approved for bowel preparation in pediatrics in China. Its efficacy has been verified by a large volume of data. American Society of Gastrointestinal Endoscopy (ASGE) Standards of Practice Committee and North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) endoscopy and procedures committee recommended 80 ml/kg polyethylene glycol for bowel preparation.1,4 However, a significant proportion of pediatric patients cannot tolerate the large volume orally without the insertion of a nasogastric tube.5,6 Low volume of PEG with a stimulant (bisacodyl, senna) regimens have also been studied,7,8,9,10 but the data for low volume of PEG monotherapy for bowel preparation is scarce.
In our study, we compared the efficacy and tolerability of different low-volume regimens to explore a possibly feasible bowel preparation method in China.
Methods
Study design
This is a single-center, randomized, double-blind study conducted in China. The study was approved by the local Ethical Committee. Written informed consent from the patients’ legal guardian was obtained for all patients.
Patients
Eligible patients for this study were children aged 6–18 years scheduled for elective colonoscopies in our center. Exclusion criteria included (1) bowel obstruction, (2) gastric retention, (3) gastrointestinal perforation, (4) toxic colitis, (5) known allergy or hypersensitivity to the active or other ingredients, (6) severe cardiac, liver, and renal diseases. All patients involved were hospitalized.
The study was conducted in the Pediatric Gastroenterology Unit of the First Hospital of Jilin University, Changchun, China, from December 2018 to November 2019.
Interventions
All enrolled participants were given low fiber diet for one day before colonoscopy. Patients in Group A received a total dose of 60 ml/kg of polyethylene glycol electrolytes (PEG-ELS) solution (maximum 2250 ml), while Group B received 40 ml/kg PEG-ELS (maximum 2250 ml). The preparation was given in two doses. The first dose (half of total dose) was given at 7 p.m. the day before colonoscopy, and the second dose was given at 2 a.m. if colonoscopy was performed in the morning, or at 6 a.m. if performed in the afternoon. The two doses were instructed to complete in 1 h. Dimethicone was also given according to age: 6–10 years olds were given 10 ml, while 11–18 years olds was given 20 ml.
The preparations were distributed by a pharmacist who carefully explained how the preparation should be taken, emphasizing the importance of completely ingesting the solution to ensure the effectiveness of colonoscopy. Besides, each patient was provided with low fiber diet instructions for the day before colonoscopy.
Preparation failure was defined as not having watery stool 2 h before colonoscopy, as identified by a gastroenterologist not involved in the trial. In this situation, enema with 0.9% sodium chloride was given. Preparation failures were also included in primary outcomes intention-to-treat (ITT) analysis.
Assessment of bowel preparation
Efficacy
Boston Bowel Preparation Scale (BBPS) was used for the assessment of bowel preparation. BBPS score is a 10-point score ranging from minimum 0 to maximum 9. In the score system, colon was divided into three regions: right colon, transverse colon, and left colon. Each segment was evaluated by the blinded endoscopist: a score 0–3 given to each segment according to the criteria as listed in Table 1.11 Summation of all three segments yields the total score: 8–9 points was accepted as excellent cleansing, 6–7 points as good cleansing, 4–5 as poor cleansing, and 0–3 as inadequate cleansing. Every segment should score at least 2. Excellent and good cleansing was considered as successful, while poor or inadequate as a failure. Before the study, two endoscopists performed an evaluation exercise on the assessment of bowel cleansing using BBPS score system to ensure evaluation consistency.
Patients’ questionnaire
Each patient was asked to complete a standardized questionnaire about his/her experience on colonoscopy. The questionnaire was carried out on the day after colonoscopy. Patients aged ≥10 years completed the questionnaire independently, and those aged 6–10 years was completed by parents and children. The questionnaire, in which the endoscopist was not allowed to participate, included patient tolerability of the procedure. Tolerability evaluation included the occurrence of gastrointestinal symptoms, such as abdominal pain, bloating, nausea, and vomiting.
The palatability of the solution was scored as follows: 3 = very difficult; 2 = a bit difficult; 1 = not difficult.
Willingness to repeat the same method for bowel preparation was also recorded: 0 = undecided, 1 = yes, 2 = no.
Satisfaction was also graded according to score scale: 1 = excellent, 2 = good, 3 = general, 4 = poor.
Study end points
Primary end point of efficacy was defined by “excellent” or “good” bowel cleansing, which total BBPS score ≥6. Secondary end points included (1) the proportion of occurrence of symptoms associated with side effects of laxative solutions; (2) the proportion of patients having no difficulty or a bit difficulty taking the solution; (3) the proportion of patients who are willing to repeat the same protocol for bowel preparation in future if necessary; (4) satisfaction evaluation: proportion of children who responded as “excellent” or “good.”
Randomization and blinding
A randomization table was generated by a statistician unrelated to the study. Eligible patients with written consent obtained were assigned 1:1 to receive one of the two bowel preparations. Randomization was performed in block sizes of 6 and was not revealed to the research team prior to allocation. Opaque, sealed, and signed envelopes were prepared and numbered according to the randomization table. The cleanout regimen was dispensed directly to the family by using the numbered envelope, and the envelope was opened only after getting written consent. Endoscopists were blinded and were not allowed to participate in all activities associated with preparation protocol before and after colonoscopy.
Statistical analysis
The sample size for this study was chosen on the assumption that the two low-volume regimens are of equivalent efficacy. Published studies showed that a successful cleansing rate of 80% should be achieved in the patients. A difference of 20% in efficacy between the two low-volume regimens was assumed to be clinically relevant. It was estimated that an initial sample size of 61 patients would be sufficient to reveal a difference in the treatment effect of 20% setting an α, the probability of a Type I error at 0.05, and a power of 80% (=1 − β). Seventy-two children accounted for ~15% withdrawals or losses.
The statistical analysis was performed by using IBM SPSS Statistics software package (version 20.0; IBM Co., Armonk, NY, USA). Student’s t test, χ2 test, and Mann–Whitney test were used as indicated. Statistical significance was considered at p < 0.05.
Results
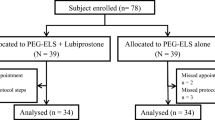
Figure 1 showed the study flow. One hundred and fifty of 196 patients were randomized to PEG-ELS 40 or PEG-ELS 60 protocol. Twenty-five patients were excluded from per-protocol analysis (colonoscopy not performed 4, insufficient compliance 9, and enema requirement 12).
The baseline data and clinical characteristics were shown in Table 2, which were similar in two groups.
Efficacy
No difference in each parameter was found in two groups. At per-protocol analysis, successful bowel cleansing rate (i.e., total BBPS score ≥6 points) in the PEG-ELS 40 group was 82.3% compared to 82.5% in the PEG-ELS 60 group (p = 0.602). The PEG-ELS 40 method was also comparable to PEG-ELS 60 in different colonic segments. The results were similar at ITT analysis with successful bowel cleansing rate of 68% (PEG-ELS 40) vs. 69.3% (PEG-ELS 60) (p = 0.860). Cecal intubation rate was 100% in both groups. Details of variables were shown in Table 3.
Tolerability
Table 4 showed the results of tolerability. Nausea, bloating, vomiting, and abdominal pain were the main symptoms reported. No significant difference in incidence was found in PEG-ELS 40 and PEG-ELS 60 groups (79.0% vs. 88.9%, p = 0.133).
There is a higher percentage of “ease of administration” in PEG-ELS 40 (79.0%) compared with PEG-ELS 60 (61.9%). The difference has not attained statistical significance (p = 0.055).
The proportions of children who claimed they would be willing to repeat the same protocol were similar in PEG-ELS 40 and PEG-ELS 60 groups (53.2% vs. 47.6%, p = 0.749).
Children (59.7%) in the PEG-ELS 40 group declared “satisfaction” as “excellent or good” compared with that of 38.1% in the PEG-ELS 60 group, which was statistically significantly different (p = 0.007).
Discussion
Colonoscopy is a standard procedure for diagnosis and therapy of pediatric gastrointestinal disorders. Finding a suitable bowel preparation for children who is safe and tolerable might be difficult. Since oral sodium phosphate was not approved for pediatrics and sodium picosulfate was not on the market in China, PEG-ELS is the most commonly used agent. Previous clinical trials showed a good cleansing effectiveness with high volumes of PEG or low volumes PEG with an active laxative.4,5,10 However, nasogastric tube insertion, which was unacceptable to most parents in China, would be needed in high-volume regimens for a proportion of pediatric patients, especially in younger ones. Efficacy of low-volume PEG-ELS as monotherapy has not been well tested.
Our study showed that PEG-ELS 40 monotherapy was comparable to PEG-ELS 60 in bowel cleansing efficacy. There were two strengths to our study. First, the bowel preparation was performed in an in-patient setting to reduce the bias of outpatient compliance, which could influence effectiveness. Second, each patient was informed elaborately by a pharmacist via verbal and written instructions. However, in contrast with high efficacy rate (i.e., ≥90%) in previous studies,7,10 our results showed 82.5% in the PEG-ELS 60 group at per-protocol analysis and 69.3% at ITT analysis. This could be attributed to several factors. First, we did not insert nasogastric tubes for children with vomiting that resulted in inadequate amount of liquid being ingested. Second, in an involved 8819 patients study, opiate use within 3 days of colonoscopy, colonoscopy performed after 12:00 p.m., and solid diet the day before colonoscopy were identified to have an association with inadequate bowel preparation.12 In another recent study by Fuccio and colleagues,13 several factors, involving bed-ridden status, constipation, diabetes mellitus, use of anti-psychotic drugs, and 7 or more days of hospitalization before colonoscopy, have been considered to increase the risk of inadequate colon cleansing in adult population. In our study, a proportion of children involved had a history of constipation who might respond to the regimens poorly, although Adamiak et al.14 suggested that history of constipation did not significantly alter the success rate of colonoscopy in his retrospective study, which still needs to be validated. We believe the risk factors of bowel cleansing in children may differ from adults. In the future, we would like to collect more data to find the difference.
In our study, the most common uncomfortable symptoms are nausea, followed by bloating, abdominal pain, and vomiting. Most children claimed the salty taste made them feel sick, while others described the taste as rusty or detergent-like. Compared with the PEG-ELS 60 protocol, PEG-ELS 40 did not reduce the incidence of occurrence of gastrointestinal symptoms. In satisfaction evaluation, a much higher proportion of children claimed “excellent” or “good” in the low-volume group (p = 0.007). As for ease of administration, despite without significant difference, there were more patients who found the solution to be not difficult or just a bit difficult to take in the low-volume group (p = 0.055). Only ~50% children admitted willingness to have the procedure repeated in both groups. Results indicated that (1) the incidence of side effects was not dose-dependent; (2) lower volume could improve tolerance in some degree, but the unpleasant taste was still a significant factor for limiting PEG-ELS use.4,8 PEG 3550 without ELS seemed to have a relative high acceptance rate at 67%.15 In addition, PEG without electrolytes mixed with a sports drink or fruit juice for bowel preparation has been tested. A retrospective study reported by Adamiak et al.14 showed that 93% (252/272) of patients had adequate bowel preparation, but there was no tolerance evaluation. Abbas et al.16 carried a prospective small sample size study with an efficacy rate of 77% (33/43) and regimen acceptable rate as 64% (29/45). Therefore, efficacy and tolerance of PEG-ELS mixed with sports drink still needs to be validated in well-designed, larger, and prospective randomized controlled studies, which would provide a direction for our future research.
There are several limitations. First, since this is a single tertiary center study, the result may not be applicable to other centers. Multicentered, well-designed, prospective studies with a larger number of patients are needed. Second, all the patients were hospitalized, the external validity of outpatients is limited. Third, biochemical adverse effects related to bowel preparation were not evaluated. However, previous studies showed no statistically significant difference in laboratory values, including kidney and liver function tests or electrolytes disturbances, pre- and post treatment.7,17,18 Fourth, the procedure was performed both in the morning and afternoon, but unfortunately the exact interval between bowel preparation and colonoscopy was not calculated, which resulted in variability. European Society of Gastrointestinal Endoscopy guideline emphasized the delay between the last dose of bowel preparation and the beginning of colonoscopy should be within 5 h.19 An interval of 3–5 h could be considered most optimal for good colonoscopic examination.20 In our study, the interval was all within 6 h. Last, patients who underwent colonoscopy in the morning had to wake up at 2 a.m. to drink the second dose, which might strongly limit the acceptability and use of the protocol. More patients in the PEG-ELS 60 group received colonoscopy in the morning than in the PEG-ELS 40 group, which inevitably created selection bias.
In spite of some shortcomings above, this is a rigorous design. Allocation was done strictly and properly. In addition, we strictly maintained blinding to persons who evaluated the outcomes, and processed data management and data analysis throughout the study.
In conclusion, the search for the best regimen for bowel preparation in pediatrics is a challenging problem, but PEG-ELS, as the only approved for pediatrics in China, is still the most commonly used agent for bowel preparation. Our study showed that low-volume PEG-ELS monotherapy was effective in bowel cleansing. PEG-ELS 40 was comparable to PEG-ELS 60 regimen, which might be an attractive alternative in pediatric bowel preparation.
References
Lightdale, J. R. et al. Modifications in endoscopic practice for pediatric patients. Gastrointest. Endosc. 79, 699–710 (2014).
Belsey, J., Epstein, O. & Heresbach, D. Systematic review: oral bowel preparation for colonoscopy. Aliment. Pharm. Ther. 25, 373–384 (2007).
Barkun, A. et al. Commonly used preparations for colonoscopy: efficacy, tolerability, and safety—a Canadian Association of Gastroenterology position paper. Can. J. Gastroenterol. 20, 699–710 (2006).
Pall, H. et al. Bowel preparation for pediatric colonoscopy: report of the NASPGHAN endoscopy and procedures committee. J. Pediatr. Gastroenterol. Nutr. 59, 409–416 (2014).
Gordon, M., Karlsen, F., Isaji, S. & Teck, G. O. Bowel preparation for elective procedures in children: a systematic review and meta-analysis. BMJ Paediatr. Open 1, e000118 (2017).
Yoshioka, S. et al. Study to determine guidelines for pediatric colonoscopy. World J. Gastroenterol. 23, 5773–5779 (2017).
Di Nardo, G. et al. Bowel preparations for colonoscopy: an RCT. Pediatrics 134, 249–256 (2014).
Berger, T. et al. Bowel preparation in pediatric colonoscopy: results of an open observational study. Endosc. Int. Open 4, E820–E827 (2016).
Turner, D. et al. Evidence-based recommendations for bowel cleansing before colonoscopy in children: a report from a national working group. Endoscopy 42, 1063–1070 (2010).
Kierkus, J. et al. High- versus low-volume polyethylene glycol plus laxative versus sennosides for colonoscopy preparation in children. J. Pediatr. Gastroenterol. Nutr. 57, 230–235 (2013).
Lai, E. J., Calderwood, A. H., Doros, G., Fix, O. K. & Jacobson, B. C. The Boston bowel preparation scale: a valid and reliable instrument for colonoscopy-oriented research. Gastrointest. Endosc. 69, 620–625 (2009).
Garber, A. et al. Modifiable factors associated with quality of bowel preparation among hospitalized patients undergoing colonoscopy. J. Hosp. Med. 14, 278–283 (2019).
Lorenzo, F. et al. Factors that affect adequacy of colon cleansing for colonoscopy in hospitalized patients. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association. Clin. Gastroenteral. Hepatol. S1542-3565(20)30342-30346 (2020).
Adamiak, T. et al. One-day bowel preparation with polyethylene glycol 3350: an effective regimen for colonoscopy in children. Gastrointest. Endosc. 71, 573–577 (2010).
Jibaly, R., LaChance, J., Lecea, N. A., Ali, N. & Weber, J. E. The utility of PEG3350 without electrolytes for 2-day colonoscopy preparation in children. Eur. J. Pediatr. Surg. 21, 318–321 (2011).
Abbas, M. I., Nylund, C. M., Bruch, C. J., Nazareno, L. G. & Rogers, P. L. Prospective evaluation of 1-day polyethylene glycol-3350 bowel preparation regimen in children. J. Pediatr. Gastroenterol. Nutr. 56, 220–224 (2013).
Turner, D. et al. Pico-Salax versus polyethylene glycol for bowel cleanout before colonoscopy in children: a randomized controlled trial. Endoscopy 41, 1038–1045 (2009).
Hunter, A. & Mamula, P. Bowel preparation for pediatric colonoscopy procedures. J. Pediatr. Gastroenterol. Nutr. 51, 254–261 (2010).
Hassan, C. et al. Bowel preparation for colonoscopy: European Society of Gastrointestinal Endoscopy (ESGE) Guideline—update 2019. Endoscopy 51, 775–794 (2019).
Seo, E. H. et al. Optimal preparation-to-colonoscopy interval in split-dose PEG bowel preparation determines satisfactory bowel preparation quality: an observational prospective study. Gastrointest. Endosc. 75, 583–590 (2012).
Acknowledgements
We thank Professor Ying kit Leung for guidance about research direction.
Author information
Authors and Affiliations
Contributions
S.F. designed the study, collected and interpreted data, drafted the initial manuscript, and reviewed and revised the manuscript. Y.S. enrolled and followed up patients. Y.L. performed the endoscopy. L.W. is the head of the Pediatric Gastroenterology Unit, performed endoscopy, and reviewed and revised the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Fang, S., Song, Y., Liu, Y. et al. Randomized clinical trial: efficacy and tolerability of two different split dose of low-volume polyethylene glycol electrolytes for bowel preparation before colonoscopy in hospitalized children. Pediatr Res 90, 171–175 (2021). https://doi.org/10.1038/s41390-020-01216-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-020-01216-5