Abstract
Background
Preterm birth (PTB) is the leading cause of perinatal morbimortality worldwide. Genetic and environmental factors could raise PTB risk. The aim of this study was to analyze the contribution of the statistical interaction between genes and vaginal–urinary tract infections (VI-UTI) to the risk of PTB by clinical subtype.
Methods
Twenty-four SNPs were genotyped in 18 candidate genes from 352 fetal triads and 106 maternal triads. Statistical interactions were evaluated with conditional logistic regression models based on genotypic transmission/disequilibrium test.
Results
In PTB-idiopathic subtype mothers exposed to UTI, fetal SNPs rs11686474 (FSHR), rs4458044 (CRHR1, allele G), rs883319 (KCNN3), and maternal SNP rs1882435 (COL4A3) showed a nominal significant increment in prematurity risk. In preterm premature rupture of membranes (PPROM), fetal SNP rs2277698 (TIMP2) showed a nominal significant risk increment. In mothers exposed to VI, fetal SNP rs5742612 (IGF1) in PTB-PPROM and maternal SNP rs4458044 (CRHR1, allele C) in spontaneous PTB showed nominal significant increment in prematurity risk.
Conclusions
Certain maternal and fetal genes linked to infectious/inflammatory and hormonal regulation processes increase prematurity risk according to clinical subtype when mothers are exposed to UTI or VI. These findings may help in the understanding of PTB etiology and PTB prevention.
Impact
-
Preterm birth is a major cause of perinatal morbimortality worldwide and its etiology remains unknown.
-
This work provides evidence on the statistical interaction of six genes with gestational vaginal or urinary infections leading to the occurrence of preterm births. Statistical interactions vary according to infection type, genotype (maternal and fetal), and clinical subtype of prematurity.
-
Certain maternal and fetal genetic variants of genes linked to infectious/inflammatory and hormonal regulation processes would increase the risk of prematurity according to clinical subtype and infection type.
-
Our findings may help in the study of etiology of preterm birth and its prevention.
Similar content being viewed by others
Introduction
Preterm birth (PTB) is defined as a conceptus born before 37 weeks of gestational age. PTB is responsible for a neonatal estimated incidence of 5–12% mortality and morbidity rates depending on a given geographic region.1 In 2018, an Argentinean 8.83% PTB rate was estimated.2
PTB has a multifactorial etiology and maternal factors (e.g., short inter-pregnancy interval, multiple gestations), genetic mechanisms associated with recurrent previous PTB (e.g., familial aggregation), and environment aspects (e.g., infections, low socio-economic status) as well as interactions between them are involved; however, their isolated or connected role persists uncertain.3
In epidemiology, gene–environment interaction (GxE) studies attempt to assess the way a set of genetic and environmental factors may influence the risk of developing a complex disease. This approach allows identifying factors that by themselves do not represent a health risk but they do so when occurring together.4 In PTB, the available evidence suggests that infection and/or inflammation may be a key mechanism leading to PTB and that it may contribute to 25% of occurring cases.3 To this respect, vaginal infections (VIs) and urinary tract infections (UTIs) have been widely studied.5 Vulvovaginal candidiasis, trichomoniasis, and bacterial vaginosis are the most common VIs; among them, bacterial vaginosis is the most frequently associated with PTB.6 In addition, previous studies have identified interactions between bacterial vaginosis and maternal genetic variants (genes: tumor necrosis factor, PRKCA, FLT1, and interleukin (IL)-6) in PTB cases.7,8 On the other hand, UTI refers to the presence of microbial pathogens in any part of the urinary tract, including the kidneys, ureters, bladder, or urethra.9 UTI have also been identified in association with genes such as IL-8, transforming growth factor, and Toll-like receptor.10
In previous studies,11,12 a population from Argentina was epidemiologically characterized and genetic variants were identified according to PTB clinical subtypes (idiopathic, preterm premature rupture of the membranes, and medically induced).13,14 However, the interaction between genes and VI or UTI in PTB was not analyzed; therefore, we consider it pertinent to deepen the study of these genetic variants in relation to urogenital tract infections.
The aim of this study was to analyze the contribution of the interaction of genes with VI or UTI to the risk of PTB by clinical subtype in the same Argentine population. For the present work, interactions were analyzed through the genotypic transmission/disequilibrium test (gTDT) that allows testing for GxEs.15
Methods
Ethics statement
Study protocols were approved by the Centro de Educación Médica e Investigaciones Clínicas (CEMIC) Ethics Committee (IRB 00001745–IORG 0001315) and the University of Iowa Institutional Review Board (IRB 200411759). Parents provided written informed consent for themselves and for the neonates.
Clinical subtypes
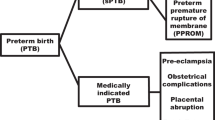
Ananth et al.,16 collecting and registering perinatal record data on the onset of labor on a regular basis, developed research guidelines for conducting PTB epidemiological genetic studies and defined three clinical subtypes:
-
1.
PTB-I: preterm labor leading to PTB (idiopathic), diagnosed as cervical change (dilation and effacement) due to regular uterine activity. A rupture of membranes was only included in this category when the rupture occurred during active labor.
-
2.
PTB-PPROM: preterm premature rupture of the membranes (PPROM) diagnosed by pelvic examination or obvious leakage of fluid from the cervix into the posterior fornix. If it was unclear whether PPROM had occurred, an ultrasound was performed to identify oligohydramnios. Membrane rupture had to occur before the onset of labor regardless of the means of delivery.
-
3.
PTB-M: an existing medical condition that required delivery before 37 gestational weeks (e.g., preeclampsia).
The subtypes PTB-I and PTB-PPROM were together designated as spontaneous PTB (PTB-S). Two independent researchers classified PTB cases into subtypes. If independent researchers disagreed on classification, the principal investigator reviewed cases.
Population
The current study included families of neonates (probands) previously analyzed in Gimenez et al. research work.13 Probands were born between July 2005 and December 2010 at the Instituto de Maternidad y Ginecología Nuestra Señora de Las Mercedes in Tucumán, Argentina. The inclusion criteria for the study was singleton neonates delivered at <37 weeks of gestational age, corresponding to PTB-PPROM and PTB-I clinical subtypes. Exclusion criteria were neonates with major congenital anomalies, multiple gestation, or maternal age <14 years.
Genotyping
DNA samples were obtained from placental blood cord and, for parents, from a peripheral blood vessel or saliva. Twenty-four single-nucleotide polymorphisms (SNPs) in 18 candidate genes were used (F3, IL6R, potassium calcium-activated channel subfamily N member 3 (KCNN3), CR1, follicle-stimulating hormone receptor (FSHR), IL1B, FN1, COL4A3, PON1, TRAF2, F2, SERPINH1, PGR, insulin-like growth factor (IGF1), INF2, IGFIR, corticotropin-releasing hormone receptor 1 (CRHR1), and tissue inhibitor of metalloproteinase 2 (TIMP2)) (Supplementary Table S1 online). These SNPs were genotyped in Gimenez et al. work.13 Candidate genes were selected based on previous research reporting that these genes are involved in some of the main PTB biological mechanisms. Lockwood and Kuczynski have described four mechanisms17: (i) activation of maternal or fetal hypothalamic–pituitary–adrenal axis, (ii) inflammation and infection, (iii) decidual hemorrhage, and (iv) uterine distention. Additionally, selection of SNPs for the present study was also based on previous literature that has examined them in other populations around the world, particularly in Latin American communities.13 Genotyping of SNP markers was performed using Taqman probes and Fluidigm SNP Genotyping platform (192.24 Dynamic Array).
Maternal exposure assessment
Maternal exposures were ascertained following a detailed review of a standard perinatal clinical record developed by the Pan American Health Organization at the Latin American Centre for Perinatology. In all cases, the diagnosis of symptomatic UTI and VI was made before the delivery admission and were diagnosed using the standard clinical criteria. UTI was diagnosed either when an asymptomatic bacteriuria was detected during prenatal control or by clinical diagnosis of cystitis during an emergency consultation. Cystitis diagnosis was based on symptoms, such as dysuria, increased micturition frequency, and/or lower abdominal or suprapubic pain, without fever. In these cases, the presence of pyuria (>10 leukocytes per high‐power microscopic field) is usually sufficient to indicate medication without requesting a urine culture. Pyelonephritis, also considered as UTI, was diagnosed when an acute episode of fever, costovertebral tenderness, pyuria, and a positive urine culture were detected. On the other hand, women reporting at least one of these symptoms: abnormal vaginal discharge; vaginal itching, burning, or irritation; and vaginal odor, during routine or emergency clinic visits, were considered positive for VI. Women with positive cultures for group B streptococcus between 35 and 37 weeks of pregnancy were also included. Subjects were excluded when they were concurrently exposed to both infections (VI and UTI).
Maternal schooling stratification
Maternal education was stratified into three levels: low, which includes from illiteracy to complete primary education (<7 years of school education); medium, which includes incomplete or complete secondary education (7–12 years of school education); and high, which includes complete or incomplete tertiary or universitary education (>12 years of education).
Data analysis
Genotyping quality controls were performed before completing the analysis [e.g., missing genotype rate by individual and by SNP, minor allele frequency (0.05), Hardy–Weinberg equilibrium failures for each SNP, and Mendelian error rate]. We used a gTDT to test genetic association of each SNP to an environmental factor. The gTDT can be formulated as a conditional logistic regression model (CLRM) where the three not transmitted parental genotypes act as pseudo-controls.15 With an additive model of inheritance, the CLRM of the ith trio is defined as: logit[P(case i)] = bG(Gi) + bGxE(GixEi), where G = 0, 1, or 2 is the case number of risk alleles and E = 0 or E = 1 reflects unexposed or exposed to the environmental factor, respectively. To determine the model significance we used a 2 degree of freedom (df) likelihood ratio test (LRT), which examines the inherited effect of the SNP after considering the GxE interaction effects. We also used a 1 df Wald test to assign the statistical significance for each term in the model. We considered 0.05 as P value significance threshold; this P value was adjusted through a false discovery rate. Four models were implemented for the combinations between proband and maternal triads with UTI or VI as environmental factors. For the proband triads, the proband is represented by the PTB case and the mother’s exposure to an infection as the environment factor. To estimate the relative risk (RR) of being a proband carrying one copy of the risk allele (compared to non-carriers), in the absence of maternal infection, we used exp(bG); to estimate the RR of a proband carrying one copy of the risk allele in the presence of maternal infection we used exp(bG + bGxE). On the other hand, in maternal triads, the case represented the proband’s mother and the environment, her exposure to an infection. Analogously, we estimated the RR of being a mother carrying one copy of the risk allele in the absence of infection with exp(bG) and, in the presence of infection, with exp(bG + bGxE). To determine the significance of the difference in RR between mothers exposed to VI or UTI, we used an analogous model where the parameter E = 0 or E = 1 represents mothers unexposed or exposed to VI and UTI, respectively.
All analyses were performed using the R package Trio (v3.20.0).15
Results
From a 499 proband triad cohort, 352 triads passed genotyping quality controls and had certainty of exposure only to VI or UTI; from them, 164 were classified as PTB-I and 188 as PTB-PPROM. Moreover, we studied 106 proband’s mother triads classified by the proband subtype as PTB-I (n = 48), and PTB-PPROM (n = 58). We compared demographic data between triads exposed and not exposed to UTI or VI. Differences were only found across groups in less than five prenatal visits, fetal sex ratio, and primiparous (Table 1). The statistical interactions between 24 SNP of 18 genes and UTI or VI were analyzed.
The SNP showing nominal significant differences in prematurity risk from maternal exposure to UTI in PTB-PPROM subtype was rs2277698 (TIMP2) of fetal genotype, with its RR increased from 0.53 (not exposed) to 2.57 (exposed). In PTB-I subtype, fetal genotype were rs11686474 (FSHR), RR from 0.89 to 2.67; rs4458044 (CRHR1, allele G), RR from 1.00 to 2.54; and rs883319 (KCNN3), RR from 0.76 to 2.88 (Fig. 1; Supplementary Table S2 online). Meanwhile, in the same subtype, maternal genotype was rs1882435 (COL4A3), RR from 0.38 to 2.88.
VI vaginal infections, UTI urinary tract infections, PTB-PPROM premature rupture of the membranes preterm birth, PTB-S spontaneous preterm birth, PTB-I idiopathic preterm birth, RR relative risk, A risk allele, Freq frequency of the allele in the stratum, P. GxE P value of 1 df Wald test of interaction term on the model, P. LRT P value of 2 df likelihood ratio test of the model. Asterisk (*) indicates that false discovery rate was <0.1.
On the other hand, the SNPs showing nominal significant differences in prematurity risk from maternal exposure to VI in PTB-PPROM subtype were rs5742612 (IGF1) of fetal genotype, RR from 0.42 (not exposed) to 2.50 (exposed) and in PTB-S subtype rs4458044 (CRHR1, allele C) of maternal genotype, RR increased from 0.59 to 2.00 (Fig. 1; Supplementary Table S3 online).
When considering maternal exposure to UTI or VI (excluding cases exposed to both infections), the SNPs showing nominal significant differences in prematurity risk were rs1882435 (COL4A3) in PTB-I of maternal genotype, RR from 0.38 (not exposed) to 2.14 (exposed) and in PTB-PPROM of fetal genotype rs2277698 (TIMP2), RR from 0.53 to 2.33 and rs5742612 (IGF1), RR from 0.42 to 1.22 (Fig. 1, Supplementary Table S4 online).
The SNPs rs11686474 (FSHR), rs883319 (KCNN3), and rs4458044 (CRHR1, allele G) showed a nominal significant difference in prematurity risk according to exposure to UTI with respect to VI in fetal genotype and PTB-I subtype (Fig. 2, Supplementary Table S5 online). Although the SNPs rs5742612 (IGF1) (fetal genotype) and rs4458044 (CRHR1, allele C) (maternal genotype) in PTB-PPROM subtype showed a difference in prematurity risk from maternal exposure to UTI with respect to VI, this difference was not significant according to the criteria assumed in the present work.
RR relative risk, VI vaginal infections, UTI urinary tract infections, A risk allele, Freq frequency of the allele in the stratum, P. GxE P value of 1 df Wald test of interaction term on the model, P. LRT P value of 2 df likelihood ratio test of the model. Asterisk (*) indicates that false discovery rate was <0.1.
Discussion
In the present study, we used the gTDT to evaluate the influence of GxEs. We were able to identify specific interactions across PTB clinical subtypes that modified the PTB risk in mothers exposed to UTI or VI.
In UTI exposition cases, a RR variation in FSHR, CRHR1, KCNN3, and COL4A3 with PTB-I and in TIMP2 with PTB-PPROM was found. Meanwhile, in VI exposition cases we found RR variations in IGF1 with PTB-PPROM and CRHR1 with PTB-S.
KCNN3 is involved in uterine relaxation maintenance until full-term gestation. It retains uterine quiescence during early pregnancy and leads to increasing uterine excitability at the time of labor.18 Lower urinary tract role of KCNN3 is related to bladder regulation.19
Corticotropin-releasing hormone (CRH) is involved in stress response as well as in regulation of inflammation and immune reactions. Decidua, fetal membranes, and placenta produce CRH during pregnancy.20 In addition, Ryckman et al. found an association of CRHR1 and CRH-binding protein (CRHBP) to bacterial vaginosis.21 In their work, they determined that variants rs17689966 (CRHR1) and rs10055255 (CRHBP) increased the risk for bacterial vaginosis, odds ratio 4.0 and 5.4, respectively.21 These results may be associated with our data since the rs4458044 (CRHR1, allele C) in the maternal genotype would increase the risk of PTB in the presence of VI. On the other hand, the expression of placental CRHR1 increases as gestation advances, in association with changes in fetal cortisol concentrations, fetal lung maturation, amniotic fluid proteins, phospholipids, and expression of myometrium receptors that merge to provoke labor and delivery.22
FSHR is required for ovarian and follicular development. It is mainly expressed in the ovary and Stilley et al. identified extra gonadal FSHR expression in endothelial cells of the fetal portion of the placenta, the umbilical cord, epithelial cells of the amnion, and myometrium.23,24 Previous studies have identified associations of maternal genetic variants of FSHR to PTB that could be related to placental angiogenesis and initiation of labor as well as to gestational duration by altering the time of ovulation and menstrual cycle length.25,26 Future studies are necessary to elucidate the biological mechanisms linked to fetal genetic variants of FSHR.
TIMP2 is expressed in the amnion and plays an important role in the activity regulation of matrix-degrading enzymes. TIMP2 has been implicated in the metabolism of type IV collagen.27 Type IV collagen precursor (COL4A3) is the major structural component of glomerular basement membranes.28 Previous studies have shown that DNA variants of this genes increase the risk of PTB-I and PTB-PPROM.29,30 These variants would influence the activity of matrix metallopeptidases, promoting extracellular matrix degradation associated with labor.29,30
IGF1 is structurally and functionally related to insulin, but it also has a higher growth-promoting activity. It is the major hormonal mediator of in utero growth.31 Bound to the alpha subunit of IGF1 receptor, it initiates a cascade of downstream signaling events leading to activation of the Ras-mitogen-activated protein kinase pathway, which is linked to inflammatory responses.32 Dementis et al. suggested that intrauterine infections decrease IGF1 activity reducing the fetus capacity to handle distress.33 In addition, the association of IGF1 with PTB-S in maternal genotype and its interaction with gestational diabetes mellitus status has been reported.34
Literature data report the relationship between infection/inflammation–hormonal regulation pathways and PTB clinical subtypes. Subtype PPROM is more frequently associated with infection than subtype PTB-I.35 In our research, frequencies between subtypes were similar for PPROM and PTB-I in mothers exposed to VI or UTI. Capece et al. suggest that autoimmune/hormonal regulation pathways would be associated with PTB-I, while hematologic/coagulation function disorder pathways, collagen metabolism, matrix degradation, and local inflammation could be responsible for PPROM.36 Our data indicate that genes IGF1, COL4A3, and TIMP2 related to a local inflammation and immune response show an RR variation in both subtypes PPROM and PTB-I (IGF1 and TIMP2 with PTB-PPROM, COL4A3 with PTB-I). Besides, FSHR and CRHR1 SNPs in relation to hormonal regulation only presented an RR variation in PTB-I, which is in accordance with previous data mentioning a relationship between hormonal regulation–infection/inflammation pathways and the PTB-I subtype.36 These findings suggest that both subtypes would be associated with genital tract infections through local inflammation pathways and also in PTB-I through hormonal regulation pathways.
On the other hand, the SNPs of KCNN3, FSHR, and CRH1R of the fetal genotype and subtype PTB-I presented a significant difference in RR between triads exposed and not to UTI (Fig. 1). No differences were found between triads exposed and unexposed to VI and even presented a reverse RR when compared to UTI (Fig. 2). This would indicate a specific effect of KCNN3, FSHR, and CRH1R SNPs on UTI, suggesting a relationship with hormonal pathways as it has been previously reported by Robinson et al.37 These authors described that alterations in hormonal regulation constitute one of the main risk factors for UTI.37 Therefore, it would be possible to hypothesize that there are different etiopathogenic mechanisms for each type of infection and that KCNN3, FSHR, and CRH1R SNPs would be markers of genetic susceptibility to PTB associated with UTI.
Through our findings and the ones described in the literature, we can support that infections and inflammatory etiologies are closely related to the activation of immune responses and PTB. This assumption indicates that the risk of prematurity could be modified according to PTB clinical subtype in mothers exposed to conditions, such as UTI and VI. However, we did not analyze the direction of causality between infections and PTB, a subject that is still uncertain.38 Other studies are necessary to consolidate our findings and its relevance in the study of PTB etiology and prevention.
When analyzing the sociodemographic and clinical data, differences were found among groups in the number of prenatal visits, primiparity, and fetal sex ratio (male/female). The number of prenatal visits is an indicator of access to health services, which would be associated with socioeconomic status. Previous studies have described the contribution of the lack of access to health services to the risk of PTB.12 Additionally, a higher percentage of UTI and VI primiparous mothers was observed. In line with this finding, Lee et al. has described that primiparity would be a risk factor for UTI during pregnancy.39 Furthermore, the fetal sex ratio was higher in the VI group. It has been previously suggested that the male fetus develops in a relatively more “pro-inflammatory environment” than the female one.40 Future studies are necessary to delve into these aspects and possible differences between infection types.
Strengths and limitations
The strengths of this work are the number of triads, the design of a family-based study that allows the bias control by the population structure, and the discrimination of PTB subtypes.
However, when interpreting the results, it is necessary to consider the following limitations: individuals were recruited from a single maternity hospital, thus limiting the results generalization; only 24 SNPs were considered, and studies with a higher number of SNPs may yield other results; mothers’ exposure to infections was determined from review of prenatal records; future studies should use more reliable diagnostic methods.
Conclusions
In this work, we studied the interaction between genes associated with PTB and maternal genital tract infections. The results suggest that certain maternal and fetal genes linked to infectious/inflammatory and hormonal regulation processes increase the risk of prematurity according to its clinical subtype when the mother is exposed to UTI or VI. These findings may help in the understanding of PTB etiology and prevention. However, more studies are needed, in different populations, and with more accurate infection diagnostic methods to consolidate the present findings.
References
Goldenberg, R. L., Culhane, J. F., Iams, J. D. & Romero, R. Epidemiology and causes of preterm birth. Lancet 371, 75–84 (2008).
Ministerio de Salud, Argentina. Dirección de Estadísticas e Información de Salud, 2019. Estadísticas vitales Información Básica 2018. http://www.deis.msal.gov.ar/wp-content/uploads/2020/01/Serie5Nro62.pdf (2019). Accessed 17 Apr 2020.
Muglia, L. J. & Katz, M. The enigma of spontaneous preterm birth. N. Engl. J. Med. 362, 529–535 (2010).
Manuck, S. B. & McCaffery, J. M. Gene-environment interaction. Annu. Rev. Psychol. 65, 41–70 (2014).
Romero, R. et al. The role of inflammation and infection in preterm birth. Semin. Reprod. Med. 25, 21–39 (2007).
Meis, P. J. et al. The preterm prediction study: significance of vaginal infections. Am. J. Obstet. Gynecol. 173, 1231–1235 (1995).
Gómez, L. M. et al. Evidence of a gene-environment interaction that predisposes to spontaneous preterm birth: a role for asymptomatic bacterial vaginosis and DNA variants in genes that control the inflammatory response. Am. J. Obstet. Gynecol. 202, 386–e1-6 (2010).
Macones, G. A. et al. A polymorphism in the promoter region of TNF and bacterial vaginosis: preliminary evidence of gene-environment interaction in the etiology of spontaneous preterm birth. Am. J. Obstet. Gynecol. 190, 1504–1508 (2004).
Kalinderi, K., Delkos, D., Kalinderis, M., Athanasiadis, A. & Kalogiannidis, I. Urinary tract infection during pregnancy: current concepts on a common multifaceted problem. Am. J. Obstet. Gynecol. 38, 448–453 (2018).
Zaffanello, M. et al. Genetic risk for recurrent urinary tract infections in humans: a systematic review. J. Biomed. Biotechnol. 2010, 321082 (2010).
Krupitzki, H. B. et al. Environmental risk factors and perinatal outcomes in preterm newborns, according to family recurrence of prematurity. Am. J. Perinatol. 30, 451–462 (2013).
Gimenez, L. G. et al. Maternal and neonatal epidemiological features in clinical subtypes of preterm birth. J. Matern. Fetal Neonatal Med. 29, 3153–3161 (2016).
Gimenez, L. G. et al. Association of candidate gene polymorphisms with clinical subtypes of preterm birth in a Latin American population. Pediatr. Res. 82, 554–559 (2017).
Mann, P. C. et al. Polymorphisms in the fetal progesterone receptor and a calcium-activated potassium channel isoform are associated with preterm birth in an Argentinian population. J. Perinatol. 33, 336–340 (2013).
Schwender, H., Taub, M. A., Beaty, T. H., Marazita, M. L. & Ruczinski, I. Rapid testing of SNPs and gene–environment interactions in case–parent trio data based on exact analytic parameter estimation. Biometrics 68, 766–773 (2012).
Ananth, C. V., Ananth, C. V. & Vintzileos, A. M. Epidemiology of preterm birth and its clinical subtypes. J. Matern. Fetal Neonatal Med. 19, 773–782 (2006).
Lockwood, C. J. & Kuczynski, E. Risk stratification and pathological mechanisms in preterm delivery. Paediatr. Perinat. Epidemiol. 15, 78–89 (2001).
Brainard, A. M., Korovkina, V. P. & England, S. K. Potassium channels and uterine function. Semin. Cell Dev. Biol. 18, 332–339 (2007).
Gopalakrishnan, M. & Shieh, C. C. Potassium channel subtypes as molecular targets for overactive bladder and other urological disorders. Expert Opin. Ther. Targets 8, 437–458 (2004).
Kalantaridou, S. N. et al. Corticotropin-releasing hormone, stress and human reproduction: an update. J. Reprod. Immunol. 85, 33–39 (2010).
Ryckman, K. K., Simhan, H. N., Krohn, M. A. & Williams, S. M. Predicting risk of bacterial vaginosis: the role of race, smoking and corticotropin-releasing hormone-related genes. Mol. Hum. Reprod. 15, 131–137 (2009).
Smith, R. Parturition. N. Engl. J. Med. 356, 271–283 (2007).
George, J. W., Dille, E. A. & Heckert, L. L. Current concepts of follicle-stimulating hormone receptor gene regulation. Biol. Reprod. 84, 7–17 (2011).
Stilley, J. A. et al. FSH receptor (FSHR) expression in human extragonadal reproductive tissues and the developing placenta, and the impact of its deletion on pregnancy in mice. Biol. Reprod. 91, 74–71 (2014).
Chun, S., Plunkett, J., Teramo, K., Muglia, L. J. & Fay, J. C. Fine-mapping an association of FSHR with preterm birth in a Finnish population. PLoS ONE 8, e78032 (2013).
Dominguez-Lopez, P. et al. The follicle-stimulating hormone receptor Asn680Ser polymorphism is associated with preterm birth in Hispanic women. J. Matern. Fetal Neonatal Med. 31, 580–585 (2018).
Frappart, L. et al. Basement membrane of the uterine cervix: immunofluorescence characteristics of the collagen component in normal or atypical epithelium and invasive carcinoma. Gynecol. Oncol. 13, 58–66 (1982).
Mariyama, M., Leinonen, A., Mochizuki, T., Tryggvason, K. & Reeders, S. T. Complete primary structure of the human alpha 3 (IV) collagen chain. Coexpression of the alpha 3 (IV) and alpha 4 (IV) collagen chains in human tissues. J. Biol. Chem. 269, 23013–23017 (1994).
Romero, R. et al. Identification of fetal and maternal single nucleotide polymorphisms in candidate genes that predispose to spontaneous preterm labor with intact membranes. Am. J. Obstet. Gynecol. 202, 431. e1–34 (2010).
Romero, R. et al. A genetic association study of maternal and fetal candidate genes that predispose to preterm prelabor rupture of membranes (PROM). Am. J. Obstet. Gynecol. 203, 361.e1–361.e30 (2010).
Hellström, A. et al. Insulin‐like growth factor 1 has multisystem effects on foetal and preterm infant development. Acta Paediatr. 105, 576–586 (2016).
Hakuno, F. & Takahashi, S. I. 40 years of IGF1: IGF1 receptor signaling pathways. J. Mol. Endocrinol. 61, T69–T86 (2018).
Demendi, C. et al. Gene expression patterns of insulin-like growth factor 1, 2 (IGF-1, IGF-2) and insulin-like growth factor binding protein 3 (IGFBP-3) in human placenta from preterm deliveries: influence of additional factors. Eur. J. Obstet. Gynecol. Reprod. Biol. 160, 40–44 (2012).
He, J. R. et al. Maternal IGF 1 and IGF 1R polymorphisms and the risk of spontaneous preterm birth. J. Clin. Lab. Anal. 31, e22125 (2017).
Moutquin, J. M. Classification and heterogeneity of preterm birth. BJOG 110, 30–33 (2003).
Capece, A., Vasieva, O., Meher, S., Alfirevic, Z. & Alfirevic, A. Pathway analysis of genetic factors associated with spontaneous preterm birth and pre-labor preterm rupture of membranes. PLoS ONE 9, e108578 (2014).
Robinson, D., Toozs-Hobson, P. & Cardozo, L. The effect of hormones on the lower urinary tract. Menopause Int. 19, 155–162 (2013).
McClure, E. M. & Goldenberg, R. L. Use of antibiotics to reduce preterm birth. Lancet Glob. Health 7, e18–e19 (2019).
Lee, A. C. et al. Urinary tract infections in pregnancy in a rural population of Bangladesh: population-based prevalence, risk factors, etiology, and antibiotic resistance. BMC Pregnancy Childbirth 20, 1 (2020).
Challis, J., Newnham, J., Petraglia, F., Yeganegi, M. & Bocking, A. Fetal sex and preterm birth. Placenta 34, 95–99 (2013).
Acknowledgements
We acknowledge Mrs. Mariana Piola for her constant efficient support. This study was funded by a grant of the Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT) PICT 2016–0952.
Author information
Authors and Affiliations
Contributions
Each author has met the Pediatric Research authorship requirements. D.E. and L.G. made substantial contributions to design, acquisition of data, analysis and interpretation of data, drafting the article, and approving the final manuscript as submitted. F.P., H.C., J.G., J.R., M.P., M.R., M.R.S., R.U., V.C. and C.S. made substantial contributions to design, acquisition of data, analysis and interpretation of data, and approving the final manuscript as submitted. S.L.H. made draft review and manuscript edition. E.G., H.K., and J.S.L.C. made substantial contributions to design, acquisition of data, critical manuscript revision for important intellectual content, and approving of the final version as submitted.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics statement
Parents provided written informed consent for themselves and for the neonates.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Elias, D., Gimenez, L., Poletta, F. et al. Preterm birth and genitourinary tract infections: assessing gene–environment interaction. Pediatr Res 90, 678–683 (2021). https://doi.org/10.1038/s41390-020-01200-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-020-01200-z
This article is cited by
-
Genetic susceptibility for retinopathy of prematurity and its associated comorbidities
Pediatric Research (2024)
-
Preterm birth etiological pathways: a Bayesian networks and mediation analysis approach
Pediatric Research (2022)
-
Genes, exposures, and interactions on preterm birth risk: an exploratory study in an Argentine population
Journal of Community Genetics (2022)