Abstract
Background
This study longitudinally characterized the developmental status, growth, and body composition of children who were fed human milk (breastfed, BF), cow’s milk-based (MF), or soy protein-based (SF) infant formula from 3 to 12 months.
Methods
Standardized anthropometrics and dual-energy X-ray absorptiometry were used to characterize growth and body composition at 3, 6, 9, 12, 24, 36, 48, 60, and 72 months (NCT00616395). Preschool Language Scale-3, Children’s Memory Scale Index (CMS), and Wechsler Preschool and Primary Scale of Intelligence were administered at age 72 months. Mixed-effects models adjusting for gestational age, birth weight, child race and sex, parental education, and maternal IQ were performed.
Results
Body Mass index (BMI) was significantly lower between 24 and 72 months in BF children compared to SF children. At 3 and 6 months, BF infants had significantly higher fat mass (FM) than SF infants, whereas BF children had significantly lower FM at 36 and 48 months than SF children. Delayed Recognition Index of the CMS was higher for SF than for MF participants (p = 0.009). There was no other significant difference in developmental outcomes between groups.
Conclusions
In conclusion, BF, MF, and SF support adequate growth and development up to age 6 years.
Impact
-
Although soy protein-based infant formula is reported to support normal infant growth and development compared to cow’s milk-based formula and human milk, there are limited data on the effect of these feeding methods in school-aged children.
-
This study suggests a significant difference in body composition, specifically BMI, after 24 months between infant feeding methods during the first year of life and in early childhood; however, all diets provide adequate nutrients to maintain normal development up to 72 months.
Similar content being viewed by others
Introduction
By 2 months of age, most infants in the U.S.A. have received some form of supplemental formula or have switched completely from human milk (breast milk (BM)) to cow’s milk-based infant formula (MF) or soy protein-based formula (SF).1,2 The American Academy of Pediatrics (AAP) recommends human milk as the ideal source of nutrition for infant feeding during the first 6 months, which should be continued to 12 months of age with the introduction of complementary solid foods.3,4 Understanding the long-term beneficial effects of infant diets is important in terms of both optimizing health and in disease prevention.
All modern SF meet the current nutritional requirements and safety standards of the Infant Formula Act of 1980.5 In 2008, approximately 25% of FF (formula-fed) infants consumed SF for various reasons, most commonly parental preference and perceived gastrointestinal issues.1,6 More recent studies show that, of infants consuming formula, 69% consume MF and 12% consume SF, with the remainder consuming a lactose-reduced or specialty hydrolyzed formula.6 Based on several previous studies, it appears that there are no obvious growth or developmental differences between infants fed SF and MF during the first year of life; although there is very limited research available that compares outcomes beyond the first year of life.7,8
The goal of this study was to evaluate the effect of early infant feeding on growth, body composition, and dietary intake during the first 6 years of life as well as developmental outcomes at age 72 months. We hypothesized that infants fed MF and SF would have greater growth velocity, higher body mass (body Mass Index (BMI)), and higher fat mass (FM) during the first 6 years of childhood compared to breastfed (BF) children. We also hypothesized that SF infants would exhibit similar growth and developmental patterns to MF infants throughout the first 6 years of life. Finally, we hypothesized that children fed human milk as infants would have a slight advantage in neurodevelopment at age 72 months compared to their counterparts fed formula in infancy.
Methods
Participants
Participants were 600 children enrolled in the Beginnings Study between 2002 and 2011 (www.clinicaltrials.gov, ID # NCT00616395). Participants were recruited between ages 1 and 2 months through community outreach conducted by research staff. Participants were contacted via Institutional Review Board (IRB)-approved advertisements placed in various locations such as: physicians’ offices, hospitals, day care centers, churches, grocery stores, newspapers and magazines, health fairs, radio and television commercials, and employee electronic/hard copy newsletters at Arkansas Children’s Hospital and at University of Arkansas for Medical Sciences. Pregnancies were uncomplicated with no medical diagnoses or medications known to affect fetal or infant growth and development. All mothers were non-smokers, denied alcohol use during pregnancy, and reported no use of soy products or other potential estrogenic compounds during pregnancy and/or lactation. Infants were term (>37 weeks), between 2.7 kg (6 lb) and 4.1 kg (9 lb) at birth, had no known medical diagnoses, and had not been administered medications known to affect growth or development. Other exclusion criteria included change of formula after age 2 months and before age 12 months, complementary foods before age 4 months (to comply with the AAP guidelines at the time of the study enrollment), and body weight at age 3 months <5 kg (11 lb). Study visits included in this report were conducted at 3, 6, 9, 12, 24, 36, 48, 60, and 72 months of age. Consent was obtained prior to study procedures. The study was approved by the IRB of the University of Arkansas for Medical Sciences.
Diets
Parents made decisions about which diet to feed their infants prior to enrollment. Those choosing to feed SF based their decision on perceived intolerance, previous sibling receiving SF, or personal choice. Those electing to formula feed chose between standardized Food and Drug Administration-approved milk-based formulas (Similac® Advance® or Enfamil® Lipil®) or soy-based formulas (Similac® Soy Isomil® or Enfamil® Prosobee®). Similac formulas were manufactured by Abbott Nutrition, Columbus, OH and Enfamil formulas were manufactured by Mead Johnson, Evansville, IN. All formulas contained supplemental docosahexaenoic acid and arachidonic acid and were provided free to the parents after enrollment in the study to ensure the standardization of dietary intake during infancy. Parents of infants who were BF received free diapers for the first year of life. All FF infants remained on their selected formula until 12 months of age. For BF infants, breastfeeding was encouraged until age 12 months. If not possible, BF infants were weaned to formula between 6 and 12 months. Among the BF infants, 52% were BF until age 12 months (N = 93), 23% switched to formula between 9 and 12 months (N = 42), and 24% switched to formula before 9 months of age (N = 43) (Table 1). Complementary foods (e.g., juices, cereals, solid foods) could be introduced after age 4 months for all the diet groups as per the AAP recommendations at the time of the study.
Demographics
Family demographics self-reported at enrollment included parental education, child’s race, birth weight, birth length, gestational age, and developmental or mental health disorders (See Table 2: cohort description).
Child anthropometry
Anthropometric measurements were obtained using standardized techniques at each study visit. Infants’ recumbent weight was measured wearing a diaper only to the nearest 0.01 kg and children’s standing weight (≥36 months) wearing only a gown to the nearest 0.1 kg using tared scales (SECA Corp, ON, Canada). Recumbent length was measured to the nearest 0.1 cm on length board (Easy Glide Bearing Infantometer; Perspective Enterprises, Portage, MI), and height (≥24 months) was measured using a standard Stadiometer (Perspective Enterprises) to the nearest 0.1 cm. Weight-for-length z-scores were computed from the World Health Organization’s growth charts using Anthro version 3.2.2 (World Health Organization, Geneva, Switzerland). BMI (weight [kg]/height [m]2) was calculated for children (≥24 months). Head circumference was measured from the occiput to the supraorbital ridges to the nearest 0.1 cm. Triceps skinfolds were measured midway between the acromial process of the scapula and the olecranon process of the ulna to the nearest 0.5 mm.
Child body composition
Body composition was assessed by dual-energy X-ray absorptiometry (DXA; QDR 4500 with Discovery upgrade; Hologic, Bedford, MA). Children (≤24 months) were asleep throughout the scan on a receiving blanket wearing only a clean diaper. Older children were scanned while lying still, in a hospital gown. Only scans without movement artifact were used for analysis (QDR software for Windows XP, version 12.3; Hologic). FM and fat-free mass (FFM) were calculated using the QDR software. Fat Mass Index (FMI) and Fat-Free Mass Index (FFMI) were calculated using the following equation: Index = FM (kg) or FFM (kg)/height (m)2.
Child dietary intake
Dietary information was collected using 3-day food records at each study visit. The food records were reviewed by a trained nutritionist with the parent to obtain detailed information for each meal. For BF infants, mothers recorded the number of feeds per day and estimated average volume intake. Human milk macronutrients were assumed to be 0.044 g/ml of fat, 0.069 g/ml of carbohydrates, and 0.010 g/ml of protein. This was based upon the nutrient analysis performed with the Nutrition Data System for Research (Nutrition Coordinating Center (NCC), Minneapolis, MN) on all of the food records provided according to NCC recommendations.
Child neurocognitive and language skills
Psychological examiners, supervised by a licensed psychologist and blinded to the study groups, administered all measures. All measures were administered at the 72-month visit. The Wechsler Preschool and Primary Scale for Children—Third Edition (WPPSI-III)9 provided a measure cognitive development. Full-Scale Intelligence Quotient (FSIQ) is reported. The Preschool Language Scale-3 (PLS-3)10 provided comprehensive information about language development. Total Language Score (TLS), Auditory Comprehension (AC), and Expressive Communication (EC) results are reported. The Children’s Memory Scale (CMS)11 evaluated learning and memory functioning. General Memory, Delayed Recognition, Attention/Concentration, and Learning Index scores are reported.
Maternal intellectual functioning
The Wechsler Abbreviated Scale of Intelligence (WASI) was administered by psychological examiners to participants’ mothers at the 3-month visit. This assessment provided an estimate of general intellectual ability and yielded an FSIQ score.
Statistical analysis
Summary statistics for study cohort continuous measurements are expressed as means ± standard deviations and as counts and percentages for categorical measurements. Differences of continuous subject characteristics were compared among the feeding groups using an analysis of variance (ANOVA) followed by Tukey multiple comparison methodology. Categorical study group characteristics were compared using Pearson’s Chi-square test. Two different variants of linear models were fit to the data. The measures head circumference, height, mid arm circumference, triceps circumference, weight, energy kcal, FFM, FFMI, percentage of calories from carbohydrate, percentage of calories from fat, and percentage of calories from protein were modeled with a feeding group-by-time repeated-measures ANOVA. The covariates in this ANOVA model were child gender, child race, gestational age, birth weight, birth length, and weeks strictly on feeding diet. These covariates were selected prior to analysis based on previous research and for comparability to earlier work. The primary effects of interest are the comparisons among the feeding groups. In our repeated-measures model, if the group-by-time interaction effect was significant at the 5% level, we reviewed the estimated pairwise feeding group (“simple effects”) at each of the study time points using the Tukey procedure to adjust for multiplicity. If the group-by-time interaction effect was not significant, we compared the feeding group main-effect means using the Tukey procedure.
The CMS, PLS-3, and WPPSI-III study outcomes were measured at 72 months and were modeled with an ANOVA model with a feeding group effect and covariates of child sex, child race, birth weight, weeks strictly on feeding group, mother’s education level, father’s education level, and mother’s IQ. Again, these covariates were selected prior to analysis based on previous research and for comparability to earlier work. The primary statistical interest is the comparison among the feeding groups controlling for covariates via significance of the model’s group effect. If the group effect was significant at the 5% level of significance, the Tukey procedure was employed to perform the pairwise contrasts among the feeding groups. All analyses were performed by the SAS 9.4 Mixed Procedure.
Results
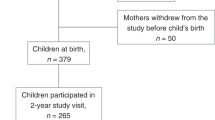
Of the 600 eligible participants, 74 did not meet inclusion criteria, were lost to follow-up, or withdrew from the study; a further 3 participants were excluded because of a medical diagnosis (neurological disorder, developmental disorder, and growth disorder, see Supplementary Fig. 1). The final cohort analysis consisted of 178 BF participants (89 females), 179 MF participants (89 females), and 169 SF participants (76 females). Gestational age, although similar between groups (39 weeks), was significantly greater (+2.8 days) in BF infants when compared to MF and SF infants (Table 2). Maternal and paternal education was higher in the BF than in the MF and SF groups. There was no difference in birth weight, birth length, or child’s sex between groups.
Of the infants who were fed formula from birth, 43% (N = 77) were fed MF and 16.6% (N = 28) were fed SF (Table 3). Infants who switched to formula feeding at age ≥2 weeks were more likely to be given SF (22.5%) than MF (13.4%). Infants who were switched from BF to SF were switched secondary to perceived intolerance. All FF infants remained on their assigned formula until 12 months of age and 52.2% of the BF infants continued to BF until 12 months of age (N = 93).
BMI was significantly lower in BF children compared to SF children between 24 and 72 months (−0.58 and −0.78, respectively). There were no significant differences in BMI between SF and MF or between BF and MF (Fig. 1a). At 9 and 12 months, BF children’s weight (8.6345 and 9.4481 kg, respectively) was significantly lower compared to MF (9.0632 and 9.9552 kg, respectively) or SF children (9.1495 and 10.0831 kg, respectively); however, between 36 and 72 months, weight was significantly lower only in BF children (14.4064, 16.5257, 18.8522, 21.5488 kg, respectively) compared to SF children (15.0619, 17.3835, 19.9108, 22.9609 kg, respectively, p < 0.001; Fig. 1d). There was a significant difference in height at 6, 9, and 12 months, with the BF group being slightly shorter (by 86.8297 cm on average) compared to MF or SF.
At 3 and 6 months, BF infants had a significantly higher FMI (4.8410 and 5.8641 kg fat mass/m2, respectively) than SF infants (3.7085 and 4.6409 kg fat mass/m2, respectively), whereas BF children had significantly lower FMI at 36 and 48 months (3.9714 and 3.7076 kg fat mass/m2, respectively) than SF children (4.4252 and 4.2117 kg fat mass/m2, respectively; Fig. 1b). By 60 and 72 months, BF children had significantly lower FMI (3.5952 and 3.4993 kg fat mass/m2, respectively) compared to both SF (4.2474 and 4.2268 kg fat mass/m2, respectively) and MF children (4.0806 and 4.1099 kg fat mass/m2, respectively). BF infants had lower FFMI (13.0232 kg fat free mass/m2) than SF infants (13.7692 kg fat free mass/m2) at 3 months and lower FFMI (12.5559 kg fat free mass/m2) than SF (13.5786 kg fat free mass/m2) and MF (13.1942 kg fat free mass/m2) infants at 6 months. FFMI was also significantly higher in the SF group at 36 and 72 months (12.3287 and 12.4684 kg fat free mass/m2, respectively) compared to MF children (12.0072 and 12.0768 kg fat free mass/m2, respectively; Fig. 1c).
At 3 months, BF infants had significantly lower energy intake (588.15 kcal/day) compared to MF infants (−85.8924, p < 0.001) and SF infants (−32.4575, p < 0.05), whereas MF infants had significantly higher energy intake 674.04 kcal/day) compared to SF infants (53.4350, p < 0.005; Fig. 2a). At ages 6, 9, and 12 months, average energy intake (kcal) was significantly lower in BF infants (6 months: −229.79, 218.86; p < 0.0001), (9 months: −229.09, −211.72; p < 0.0001), (12 months: −80.1255, −110.27; p < 0.05) compared to MF and SF infants, respectively, with no differences between the formula groups. There was no difference in energy and macronutrient intake (fat, carbohydrate, and protein) between the three groups from 24 to 72 months (Fig. 2b–d). See Table 4 for details on significant interactions between feeding groups over time.
There were no significant differences at 72 months between the feeding groups in intelligence as measured by the WPPSI-III (FSIQ) or in language scores (TLS, AC and EC scores), and all scores were in the average range (Table 5). The Delayed Recognition Index of the CMS, was higher in SF children (104.4) than in MF children (97.9) at 72 months. When Delayed Recognition scores were stratified by sex, the significant effect between SF and MF children was found to be driven by the boys (SF boys mean standard score (SS) = 104.25 vs. MF boys mean SS = 95.21, p value = 0.0029). Other CMS results, including General Memory, Attention & Concentration, and Learning, were not significantly different between the feeding groups.
Discussion
This is one of the largest longitudinal studies simultaneously comparing growth, body composition, dietary intake, and developmental outcomes during the first 6 years of life in children who were BF, MF, or SF as infants. We used standardized tests and procedures to measure neurodevelopmental outcomes as well as state-of-the-art approaches to assess body composition and dietary intake to compare the three diet groups. The use of DXA provided a non-invasive state-of-the-art measurement for body composition, which is the best instrumentation to provide accurate and precise measure of bone mineral content, FM, and FFM in children compared to quantitative nuclear magnetic resonance or air displacement plethysmography.12,13 The main limitations of the instrument are its cost, the low dose radiation exposure, and challenges with child stillness. Dietary intake was assessed using the 3-day food records, which have been shown to have higher accuracy than food recalls or food frequency questionnaires. While there were significant differences between all the feeding groups, most differences were between the BF group compared to the MF or the SF, with even more significance seen when comparing the BF and SF groups. As predicted, growth outcomes between SF and MF infants were very similar throughout childhood.
BF children had a lower BMI between 24 and 72 months of age compared to SF infants. This is consistent with systematic reviews in the literature showing that BF is associated with a reduced risk of obesity in early childhood compared to FF.14,15,16 With childhood obesity becoming an epidemic in the United States, these results support a role for early infant feeding practices in the development of obesity in childhood.17
The advanced body composition measures used in this study demonstrate that BF infants have higher FMI and concomitantly lower FFMI at 3 and 6 months compared to FF infants. These results may be a consequence of higher dietary fat (%kcal) intake or a lower protein intake (%kcal)18 in BF infants at 3 months of age compared to MF and SF infants, although further studies are needed. The differences observed in FMI and FFMI during infancy between the three diet groups are consistent with data showing that FF infants have a higher FFM throughout the first year of life when compared to BF infants.19,20 Despite a greater FMI during infancy, BF infants had lower FMI compared to SF and MF children between 36 and 72 months. This is consistent with previous studies demonstrating that high intake of fat in infancy does not alter later preferences of intake in childhood.21 SF children’s body composition was mostly similar to MF children. These results are aligned with previous studies including a large meta-analysis22 demonstrating that healthy full-term SF infants display normal anthropometric growth compared to MF and BF infants.
There were differences in dietary intake in the first 9 months of life that could have driven variations in body composition later in life. Indeed, children who were BF had lower energy intake during the first year of life as well as lower carbohydrates, protein, and fat intake between 6 and 12 months of age compared to children who were fed MF or SF as infants. As reported in the Etude Longitudinale Alimentation Nutrition et Croissance des Enfants (ELANCE) study, higher protein intake in early life can be associated with an earlier adiposity rebound during childhood.23 Many studies have confirmed an association between high early protein intake and increased growth later in life leading to an increased risk of obesity in childhood.23,24,25 Further studies are needed to investigate differences in the effects of all macronutrients and energy intake between the groups.26 Importantly, assessing human milk energy and macronutrient intake using laboratory techniques would enhance the validity of future comparisons.
Overall, mean index scores at 72 months on assessments of intelligence, memory, and language across all early infant feeding groups were within normal limits. As measured by the WPPSI-III, no significant differences were found between the feeding groups in FSIQ. Studies of the effects of BF on intelligence in later childhood, adolescence, and adulthood often reveal a positive association but not necessarily clinical relevance with scores well within the average range.27 In fact, when adjusting for major confounders, as done in this study, differences in IQ scores of only one or two points on average are observed, indicating less benefit from BF.28 This current data also revealed no significant differences among the feeding groups on language development as noted by the TLS, AC, or EC. Colen and Ramey studied 11 children’s health outcomes, including IQ, achievement, and language, and suggested that while BF children did perform better than FF children on most indicators, the effect is typically overrated in studies and may be attributed mostly to individual factors resulting in the self-selection of BF as well as confounding variables, such as socioeconomic status and education.29
Delayed Recognition Index of the CMS was significantly lower in children fed MF compared to children fed SF, which was not hypothesized and is unexpected. When the analysis was stratified by sex, it was revealed that this effect response is driven by the performance of SF males. While this finding is relatively large compared to the non-significant findings in the primary learning and memory measures, it is likely not as meaningful as if other memory measures were significant. There is some evidence in animal models that memory may actually be improved with soy supplementation, but this predominantly occurs in females, not in males, as well as inconsistent findings in older, postmenopausal women using soy to protect against cognitive decline.30 Further analysis of diet and memory is recommended, particularly when examining additional cognitive processes that may be involved in the efficiency of long-term recognition memory, such as verbal skills and executive functioning.
The strengths of this study include the large sample size of participants, the longitudinal characterization of the participants at 9 different time points from infancy into early childhood, and the precision and accuracy of the technology used to measure body composition throughout the first 6 years of life.31 It is also strengthened by the participants maintaining a strict diet during the first year of life. While bias cannot be avoided in clinical research, it can be minimized, which is why children were enrolled only after the parents made a decision on their feeding intention and all groups were provided with similar compensation (free formula or free diapers for the first year of life). The results are limited by the lack of randomization, however, that methodology could not be used due to ethical concerns, as well as the lack of adjustment for maternal pre-pregnancy BMI, which was not available in this cohort.
The results of this study suggest minor yet statistically significant differences in body composition between BF, MF, and SF infants during the first year of life and early childhood. Most striking is the finding that children who were BF the first 12 months of life have a lower BMI from 12 months of age until 6 years when compared to FF infants. In addition, the current data further support that SF and MF infants perform within normal limits and similarly to BF infants in areas of intelligence, memory, and language development in infancy and into early childhood. In conclusion, this study supports a growing body of evidence that early feeding practices can play a role in growth outcomes of infants and children, while these feeding practices show minimal differences in neurodevelopmental outcomes in children up to 6 years of age.
Data availability
Deidentified individual participant data will not be made available.
References
Bhatia, J., Greer, F. & Committee on Nutrition. Use of soy protein-based formulas in infant feeding. Pediatrics 121, 1062–1068 (2008).
Andres, A., Casey, P. H., Cleves, M. A. & Badger, T. M. Body fat and bone mineral content of infants fed breast milk, cow’s milk formula, or soy formula during the first year of life. J. Pediatr. 163, 49–54 (2013).
American Academy of Pediatrics. Infant feeding timeline, AAP Health initiatives. https://www.aap.org/en-us/advocacy-and-policy/aap-health-initiatives/HALF-Implementation-Guide/Age-Specific-Content/Pages/Infant-Timeline.aspx (2019).
Badger, T. M. et al. The health implications of soy infant formula. Am. J. Clin. Nutr. 89, 1668S–1672S (2009).
Merritt, R. J. & Henks, B. H. Safety of soy-based infant formulas containing isoflavones: the clinical evidence. J. Nutr. 134, 1220S–1224S (2004).
Rossen, L. M., Simon, A. E. & Herrick, K. A. Types of infant formulas consumed in the United States. Clin. Pediatr. 55, 278–285 (2016).
Messina, M., Rogero, M. M., Fisberg, M. & Waitzberg, D. Health impact of childhood and adolescent soy consumption. Nutr. Rev. 75, 500–515 (2017).
Andres, A. et al. Developmental status of 1-year-old infants fed breast milk, cow’s milk formula, or soy formula. Pediatrics 129, 1134–1140 (2012).
Wechlsler, D. Wechsler Preschool and Primary Scale of Intelligence - Third Edition (The Psychological Corporation, San Antonio, TX, 2002).
Zimmerman, I. L., Steiner, V. G. & Pond, R. E. Preschool Language Scale 3rd Edition (The Psychological Corporation, San Antonio, TX, 1992).
Cohen, M. J. Children’s Memory Scale (The Psychological Corporation, San Antonio, TX, 1997).
Heard-Lipsmeyer, M. E. et al. Evaluating body composition in infancy and childhood: a comparison between 4C, QMR, DXA, and ADP. Pediatr. Obes. 15, e12617 (2020).
Kelly, T. L., Wilson, K. E. & Heymsfield, S. B. Dual energy X-ray absorptiometry body composition reference values from NHANES. PLoS ONE 4, e7038 (2009).
Rzehak, P. et al. Infant feeding and growth trajectory patterns in childhood and body composition in young adulthood. Am. J. Clin. Nutr. 106, 568–580 (2017).
Owen, C. G. et al. Effect of infant feeding on the risk of obesity across the life course: a quantitative review of published evidence. Pediatrics 115, 1367–1377 (2005).
Owen, C. G. et al. The effect of breastfeeding on mean body mass index throughout life: a quantitative review of published and unpublished observational evidence. Am. J. Clin. Nutr. 82, 1298–1307 (2005).
Butte, N. F. Impact of infant feeding practices on childhood obesity. J. Nutr. 139, 412S–416S (2009).
Rolland-Cachera, M. F., Akrout, M. & Peneau, S. Nutrient intakes in early life and risk of obesity. Int. J. Environ. Res. Public Health 13, 564 (2016).
Gale, C. et al. Effect of breastfeeding compared with formula feeding on infant body composition: a systematic review and meta-analysis. Am. J. Clin. Nutr. 95, 656–669 (2012).
Ntalla, I. et al. Body composition and eating behaviours in relation to dieting involvement in a sample of urban Greek adolescents from the TEENAGE (TEENs of Attica: Genes & Environment) study. Public Health Nutr. 17, 561–568 (2014).
Deheeger, M. et al. Individual patterns of food intake development in children: a 10 months to 8 years of age follow-up study of nutrition and growth. Physiol. Behav. 59, 403–407 (1996).
Vandenplas, Y. et al. Safety of soya-based infant formulas in children. Br. J. Nutr. 111, 1340–1360 (2014).
Rolland-Cachera M. F. & Scaglioni, S. Role of Nutrients in Promoting Adiposity Development (ECOG, 2016).
Michaelsen, K. F. & Greer, F. R. Protein needs early in life and long-term health. Am. J. Clin. Nutr. 99, 718S–722S (2014).
Hornell, A., Lagstrom, H., Lande, B. & Thorsdottir, I. Protein intake from 0 to 18 years of age and its relation to health: a systematic literature review for the 5th Nordic Nutrition Recommendations. Food Nutr. Res. 57 (2013).
Haisma, H. et al. Breast milk and energy intake in exclusively, predominantly, and partially breast-fed infants. Eur. J. Clin. Nutr. 57, 1633–1642 (2003).
Mortensen, E. L., Michaelsen, K. F., Sanders, S. A. & Reinisch, J. M. The association between duration of breastfeeding and adult intelligence. JAMA 287, 2365–2371 (2002).
Horta, B. L., Loret de Mola, C. & Victora, C. G. Breastfeeding and intelligence: a systematic review and meta-analysis. Acta Paediatr. 104, 14–19 (2015).
Colen, C. G. & Ramey, D. M. Is breast truly best? Estimating the effects of breastfeeding on long-term child health and wellbeing in the United States using sibling comparisons. Soc. Sci. Med. 109, 55–65 (2014).
Lund, T. D. et al. Visual spatial memory is enhanced in female rats (but inhibited in males) by dietary soy phytoestrogens. BMC Neurosci. 2, 20 (2001).
Wells, J. C. & Fewtrell, M. S. Measuring body composition. Arch. Dis. Child 91, 612–617 (2006).
Acknowledgements
The authors are grateful to the children and families who participated in this study and to the clinical core team for their assistance in data collection. All phases of this study were supported by USDA-ARS Project 6026-51000-010-05S.
Author information
Authors and Affiliations
Contributions
S.S. drafted the initial manuscript, interpreted the data, and reviewed and revised the manuscript. A.A. contributed to the conceptualization and design of study, coordinated and supervised data collection, analyzed and interpreted data, and contributed to the writing and review of the manuscript. T.M.B. and P.H.C. conceptualized, designed the study, and reviewed manuscript. C.R.S. and D.K.W. analyzed and interpreted the data and reviewed manuscript. J.B. coordinated and supervised data collection and reviewed manuscript. G.M. performed data collection, analyzed and interpreted data, and contributed to the writing of the manuscript. S.T.S. supervised data collection and reviewed the manuscript. All authors reviewed the manuscript critically for important intellectual content and approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent
Consent was obtained prior to study procedures. The study was approved by the Institutional Review Board of the University of Arkansas for Medical Sciences.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Sobik, S., Sims, C.R., McCorkle, G. et al. Early infant feeding effect on growth and body composition during the first 6 years and neurodevelopment at age 72 months. Pediatr Res 90, 140–147 (2021). https://doi.org/10.1038/s41390-020-01157-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-020-01157-z
This article is cited by
-
Differential associations between body composition indices and neurodevelopment during early life in term-born infants: findings from the Pakistan cohort: Multi-Center Body Composition Reference Study
European Journal of Clinical Nutrition (2023)
-
Alternativen zu Säuglingsnahrungen auf Kuhmilchproteinbasis
Monatsschrift Kinderheilkunde (2023)
-
Unexpected content of kynurenine in mother’s milk and infant formulas
Scientific Reports (2022)