Abstract
Background
Little is known about brain temperature of neonates during MRI. Brain temperature can be estimated non-invasively with proton Magnetic Resonance Spectroscopy (1H-MRS), but the most accurate 1H-MRS method has not yet been determined. The primary aim was to estimate brain temperature using 1H-MRS in infants with neonatal encephalopathy (NE) following perinatal asphyxia. The secondary aim was to compare brain temperature during MRI with rectal temperatures before and after MRI.
Methods
In this retrospective study, brain temperature in 36 (near-)term infants with NE was estimated using short (36 ms) and long (288 ms) echo time (TE) 1H-MRS. Brain temperature was calculated using two different formulas: formula of Wu et al. and a formula based on phantom calibration. The methods were compared. Rectal temperatures were collected <3 hours before and after MRI.
Results
Brain temperatures calculated with the formula of Wu et al. and the calibrated formula were similar as well as brain temperatures derived from short and long TE 1H-MRS. Rectal temperature did not differ before and after MRI.
Conclusions
Brain temperature can be measured using 1H-MRS in daily clinical practice using the formula of Wu et al. with both short and long TE 1H-MRS. Brain temperature remained within physiological range during MRI.
Similar content being viewed by others
Introduction
Over the past decades, magnetic resonance imaging (MRI) has become one of the most important neuro-imaging techniques to assess brain injury in high-risk neonates.1,2 MRI has shown to be more sensitive to diagnose brain injury in neonates compared to computed tomography or cerebral ultrasound.2,3 Furthermore, no radiation is used making it safe to use in neonates.2
Although MRI is safe in neonates, there are some potential risks that should be considered, such as an increase in the temperature of the body and brain.4,5 To conduct an MRI, pulses of radiofrequency (RF) energy are applied to create images.1 This RF energy is partly absorbed by the tissue of the patient, which can potentially lead to an increase in temperature.1 The amount of RF energy in Watt absorbed by 1 kg of tissue of the patient is called the specific absorption rate (SAR). So, a higher SAR increases the risk of a rise in body temperature. Therefore, the SAR level is monitored by the MR scanner and scanning is limited when SAR levels are about to exceed the maximum allowed SAR limits as specified by the FDA guidelines.6
However, little is known about the exact effect of the SAR on the brain temperature of neonates during MRI. The rectal temperatures of term and preterm neonates seems to be similar before and after MRI.4,7 Although body and brain temperatures are correlated, it has not yet been fully elucidated what the exact effect is of MRI on the brain temperature. In addition, the temperature management during MRI is less optimal due to the low temperature in the MR room (18 °C) which might also decrease body temperature if no temperature-controlled MR incubator can be used.5
Recent studies have shown that it is possible to non-invasively measure brain temperature with proton Magnetic Resonance Spectroscopy (1H-MRS).8,9,10 The chemical shift of water is temperature dependent, whereas the chemical shift of some metabolites in the brain tissue such as N-acetyl-aspartate (NAA) is not. This chemical shift difference (ΔH2O-NAA) can be used to determine the temperature with an accuracy of 0.5 °C in 1.5T and 3.0T systems.8,11
The primary aim of this study was to assess the feasibility of measuring brain temperature non-invasively using 1H-MRS in infants with neonatal encephalopathy (NE) following perinatal asphyxia. The clinical feasibility was investigated by determining whether brain temperatures calculated with a previously developed formula were similar to brain temperatures calculated with a formula developed using phantom calibration. Furthermore, brain temperatures measured using short (36 ms) and long (288 ms) echo time (TE) 1H-MRS were compared. The secondary aim was to compare the MRS-derived brain temperature in infants with NE with rectal temperature before and after MRI.
Materials and methods
Design and subjects
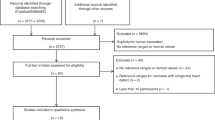
In this single-center retrospective study, (near-)term infants with NE following perinatal asphyxia (referred to as NE) who were admitted to the level III Neonatal Intensive Care Unit (NICU) of the University Medical Center Utrecht (UMCU) between December 2011 and June 2017 were eligible for inclusion. Neonates born with a gestational age of ≥36 weeks, diagnosed with NE following perinatal asphyxia and treated with therapeutic whole-body hypothermia according to international guidelines12 were included. Furthermore, it was essential that 1H-MRS of sufficient quality and rectal temperature data (not more than 3 h) before and after the MRI were available for analyses. Infants with metabolic or genetic abnormalities were excluded. All infants were included in the PharmaCool study (www.trialregister.nl, NL2421)13 or 2-STEP study (www.trialregister.nl, NL5089)14 and informed consent of parents for the use of their infants data was available. These studies were approved by the Ethical Committee of the UMCU.
Clinical parameters
All baseline characteristics and rectal temperature data were retrospectively obtained from electronic medical records. The rectal temperatures closest, and not more than 3 h, before and after MRI were collected. These temperatures were all measured on the NICU using a rectal thermometer or using a rectal temperature probe for continuous temperature monitoring.
Validation of the formula
The local brain temperature was calculated by the formula of the study of Wu et al.:8 T = (−102.89 × ΔH2O−NAA) + 308.64; with T as local brain temperature in degrees Celsius and ΔH2O−NAA being the difference between the spectral positions of water and NAA in parts per million (ppm). This formula is developed in infants with NE using short TE 3T MRI.
With phantom measurement in our 3.0T MR scanner, we also calibrated a new formula specific for our scanner, referred to as the calibrated formula, to validate the formula of Wu et al.7,8 A small, in-house developed, spherical phantom with a diameter of ~3 cm containing a water solution with creatine, GABA, glutamate, glutamine and NAA with a pH of 7.4 was used. Temperature was measured continuously by securing a Neoptix fiber optic sensor on top of the phantom (Neoptix, Qualitrol Company LLC of Fairport, NY, USA). The phantom was heated in a water bath until the temperature was 45 °C. Thereafter, the phantom was wrapped in towels to slow down the cooling process. The phantom was placed in the isocenter of the MRI and during cooling (from 43 to 30 °C), 20 1H-MRS scans were conducted using long and short TE alternating. By analyses of the ΔH2O−NAA and actual measured temperature, a new formula was developed. This formula was compared to the formula of Wu et al. to assess whether the two formulas differed.8
MRI acquisition and brain temperature calculation
MRI examinations were conducted using a 1.5T or 3.0T Achieva scanner (Philips Medical Systems, Best, the Netherlands). As standard of care, all infants with NE who were treated with hypothermia underwent cerebral MRI within the first week after birth with a duration of 30–45 min. All neonates were scanned in a vacuum mattress to prevent movement artifacts (Med Vac Infant Immobilizer Bag, Radstadt, Austria). During the MRI, the heart rate and oxygen saturation was measured with a pulse oximeter (Nonin, Minneapolis, MN) and respiration rate was observed using the standard Philips equipment (Philips Medical Systems, Best, Netherlands).
Scan protocols included, among others, single voxel 1H-MRS (PRESS, repetition time = 2000 ms, TE = 36 ms (short TE) and/or 288 ms (long TE), phase cycles = 16, 64 measurements, voxel sizes varied between 10 × 10 × 10 mm and 20 × 10 × 10 mm, water suppression method = “excitation” (2-water selective pulses followed by spoilers). The region of interest (ROI) for single voxel 1H-MRS was the left deep gray matter, according to Alderliesten et al.15
The quality of the spectra was visually inspected (visual estimation signal to noise ratio (SNR) of NAA peak should be larger than 3 and measurement of line width should be lower than 10 Hz, Supplemental Fig. S1) and spectra with poor quality i.e. low SNR or clear artifacts from water suppression were excluded. If the quality was sufficient, jMRUI software version 5.2 was used to analyze the spectrum.16 The peak of NAA was expected at ∼2.02 ppm. As selective excitation was used for water suppression, the residual water peak is still in phase and can be used to determine its frequency position. The positions of the NAA and H2O peak in the spectrum were determined automatically by using the ‘HLSVDPro’-option of the jMRUI software, which determines the spectral position of the fifteen highest peaks automatically.17
Statistical tests
Statistical analysis was performed using IBM SPSS Statistics for Windows version 25.0 (IBM Corp., Illinois, USA). Linear univariate regression analysis was performed, with the actual temperature of the water bath as independent variable and ΔH2O-NAA as dependent variable, to develop the new, calibrated formula to calculate brain temperature for validation. Paired sample t-tests for normally distributed and Wilcoxon Signed rank tests for non-Gaussian distributions were performed to compare the brain temperatures calculated with the two formulas and with short versus long TEs. The Pearson correlation for normally distributed parameters and Spearman correlation for non-Gaussian distributed parameters were used to test the association between brain temperatures and rectal temperatures. The Kruskal–Wallis test was performed to calculate differences amongst temperature measurements before, during and after MRI, followed by post-hoc comparison with Wilcoxon Signed rank test when differences were statistically significant. Lastly, multivariable linear regression analysis was performed to explore the association between therapeutic hypothermia and head circumference as independent variables and the difference between rectal temperature before MRI and the brain temperature during MRI as dependent variable, because these might be factors that influence the risk of cooling down during MRI. p-values < 0.05 were considered statistically significant. All p-values have been corrected for multiple comparisons by multiplying the p-value with the number of performed tests.
Results
Subjects
Between December 2011 and June 2017, 49 patients with NE, who were treated with therapeutic hypothermia, had 1H-MRS and temperature data available. Of those patients, 13 patients were excluded because of insufficient quality of the 1H-MRS data. So, 36 patients were included in the study. Table 1 shows the baseline characteristics of the included infants. Usually patients were scanned during or after the rewarming process following therapeutic hypothermia (body temperature >35.5 °C). Seven infants had an MRI during therapeutic hypothermia or early rewarming for clinical reasons. In one infant scanned during rewarming, therapeutic hypothermia was stopped before the MRI was conducted and therefore the temperature is higher at the end of the MRI.
Calibration
First of all, the phantom was used to calculate a calibrated formula for our scanner. Figure 1 shows the actual temperatures of the phantom plotted against the ΔH2O−NAA. The following formula is calculated based on these data: T = 263 − (ΔH2O − NAA × 85.76), in which T is the temperature and ΔH2O−NAA is the chemical shift between NAA and H2O in parts per million. The r2 of this curve was 0.96.
Calculation of brain temperature using MRS in neonates: comparing the formulas
The formula of Wu et al. was compared to the calibrated formula to assess whether these were different in neonates, which would imply that calibration is necessary.
The median brain temperature was 35.1 °C (IQR 33.6–36.1) according to the formula of Wu et al. and 35.0 °C (IQR 33.7–35.9) according to the calibrated formula using short TE 1H-MRS (p = 0.68). Using long TE 1H-MRS, the median brain temperature assessed according to Wu et al. was 35.5 °C (IQR 34.4–36.2) and according to the calibrated formula 35.3 °C (IQR 34.4–35.9). This difference was just significant (p = 0.048).
The difference between the brain temperatures calculated with the two formulas varied for short TE 1H-MRS between 0.01 °C and 0.67 °C (mean = 0.06 °C) and for long TE 1H-MRS this varied between 0.01 °C and 0.85 °C (mean = 0.15 °C). See Fig. 2.
Calculation of brain temperature using MRS in neonates: short versus long TE 1H-MRS
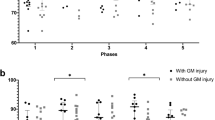
Twenty patients had both short TE and long TE 1H-MRS available and brain temperatures derived from the different TEs were compared within patients. In normothermic and hypothermic patients, differences in brain temperatures derived from short and long TE 1H-MRS according to Wu et al. were not statistically different. The differences in brain temperatures measured with short and long TE 1H-MRS according to the calibrated formula were also comparable for normothermic and hypothermic patients. However, there were individuals in whom there was a difference of >1 °C between short and long TE, see Fig. 3.
Comparison of rectal temperature and brain temperature
Table 2 shows the correlation of the brain temperature, for each method, with rectal temperatures at the NICU. Brain temperatures correlated significantly with temperature before and after MRI when tested in the entire cohort, including both normothermic and hypothermic infants. The rectal temperature before and after MRI were also significantly correlated (r = 0.663, p < 0.001).
Afterward, differences in temperatures before, during and after MRI were compared using the Kruskal–Wallis test and post-hoc tests for normothermic and hypothermic patients for the different methods (see Table 3).
In a multivariable linear regression model the association between therapeutic hypothermia, and head circumference as independent variables and the differences between the temperature before MRI and brain temperature as dependent variable was tested. Head circumference was significantly associated with difference in temperature in which brain temperature was measured using short TE and the formula of Wu et al. (head circumference: β = −0.29, p = 0.024) and in the model using short TE and the calibrated formula (head circumference: β = −0.25, p = 0.019). In the models using the brain temperature measured with long TE, none of the variables were associated with temperature differences.
Discussion
This study showed that it is feasible to estimate brain temperature using 1H-MRS in infants with NE with the formula of Wu et al. and with as well short as long TE 1H-MRS in magnets with field strengths of 1.5 T or 3.0 T. Secondly, brain temperature remained within physiological range and was not higher, but even lower in some infants during MRI compared to rectal temperature at the NICU.
Different methods to determine brain temperature were compared to assess the feasibility in clinical practice. A previously developed formula of Wu et al.8 was compared to a new formula developed based on temperature calibration for the 3.0T MR scanner used in our institute for neonatal MRI. A significant difference between brain temperatures calculated with the two formulas would have implied that calibration is essential. Nonetheless, brain temperatures measured with these two different formulas did not differ for short TE 1H-MRS (mean difference 0.06 °C) and the difference for long TE 1H-MRS was statistically borderline significant. However, the mean difference between the formulas for long TE 1H-MRS was 0.15 °C, which is clinically insignificant. Verius et al. investigated the need for calibration in 30 healthy volunteers and compared this to previously calibrated formulas. The authors concluded, in contradiction to our results, that calibration is essential.18 This contradiction might be explained by the fact that the formula of Wu et al.8 was based on a similar cohort and scan protocol as ours, and Verius et al.18 compared their formula to studies with different methods, such as different age groups and scanners, than theirs.
A few studies have investigated the use of 1H-MRS to measure brain temperature in neonates, all using different formulas and TEs.7,8,9 Bainbridge et al. measured brain temperatures in neonates with NE on a 1.5-T scanner using a TE of 288 ms. The authors compared brain temperatures, calculated using two previously developed formulas from calibrations in animals, with rectal temperatures measured shortly after MRI.11,19 They concluded that both formulas correlated well with the rectal temperature, but were not perfect. The explanation of the authors is the difference in field strength: both formulas were developed at ultra-high field MR scanners in animals and the infants were scanned at 1.5 T.9 Owji et al. measured brain temperatures in infants with NE with and without brain injury using a TE of 288 ms on a 3.0-T MRI scanner.10 They used a previously reported formula calibrated in rabbits.10,20 So, both studies used formulas calibrated in animals, which might not be most representative for neonatal studies. In this study, we therefore used the formula of Wu et al. because they calibrated the temperature in a phantom study using a TE of 35 ms on a 3.0-T MRI scanner and tested this formula in neonates with NE, which is more similar to clinical practice. So, hospitals using 3.0 T MRI can use their standard 1H-MRS scan and no phantom study is needed before starting to measure brain temperature non-invasively. This will improve the feasibility of 1H-MRS brain temperature measurements in clinical practice.
Furthermore, in previous studies either short8 or long9,10 TE 1H-MRS was used, but these two methods were never compared. This study found no statistical differences, but for some individuals there was a difference >1 °C, which is clinically significant. This difference cannot be technically explained. Further research using rectal temperature measurement during MRI in combination with short and long TE 1H-MRS is essential to conclude which TE is more reliable.
Clinical implications of brain temperature measurement
The possibility to measure brain temperature is important in clinical practice for the monitoring of safety, as an additional prognostic tool and for evaluation of the effect of therapeutic hypothermia.
This study showed that brain temperature during MRI was not higher than rectal temperature measured within 3 h before and after MRI in neonates with NE. This suggests that there is no heating of the brain during MRI, which is in accordance with the literature that MRI is safe in different neonatal populations.1,4,5 However, in this study brain temperature was compared to rectal temperature before and after MRI at the NICU. So, we cannot conclude that brain temperature itself did not increase during MRI because of the absence of a baseline measurement of brain temperature.
Furthermore, brain temperature in normothermic infants with NE was even significantly lower during MRI compared to rectal temperature at the NICU, varying between minus 0.6 and 1.4 °C. These findings are in agreement with a study in preterm patients, scanned at 30 weeks of gestation within an MRI incubator, in which 17.3% of the preterm infants became hypothermic with a mean decline in temperature of 0.5 °C during MRI.5 The authors explained the lower temperatures by the cold air that was used for ventilation during MRI instead of the preheated air that is used on the NICU.21 This can also partly explain the decrease in temperature in especially the patients that still received therapeutic hypothermia, because all these infants were ventilated. The decrease in temperature was not statistically significant in infants with therapeutic hypothermia, but this might be due to the small sample size. In addition, infants are placed in a relatively cool MRI scanner environment (temperature 18 °C), which might decrease body temperature. This is supported by the fact that a larger head circumference was associated with a smaller difference between brain temperature and rectal temperature before MRI using short TE. As core temperatures may fluctuate when an infant is exposed to the cooler temperature of the MRI environment, monitoring body temperature during MRI might be recommended to prevent cooling down. An option to prevent body cooling might be the use of a temperature-controlled MRI incubator. A rise in brain temperature in neonates during MRI has never been found, but a decline has also not been described before in NE.4 More research is needed and the results should be interpreted with caution, because the decrease in temperature was not found for all TEs and rectal and brain temperatures were compared.
The lack of information about (brain) temperature during MRI in neonates emphasizes the need for an easy and non-invasive method to measure temperature. This becomes even more important with the use of ultra-high field imaging. Ultra-high field imaging improves the quality of MRI,22 which might also be beneficial in neonates. However, higher field strengths might increase the SAR and thereby the risk of a rise in brain temperature.22 Then, monitoring brain temperature becomes even more important. Additionally, brain temperature measurements can possibly help to assess the severity of brain injury. In adults with stroke, the temperature in injured brain tissue was higher compared to non-injured tissue.23,24 Also in children with epilepsy, the brain temperature in the focal epileptogenic lesions was higher than in controls.25 Therefore, Wu et al. investigated brain temperature in infants with NE during and after therapeutic hypothermia. Both during and after therapeutic hypothermia brain temperatures were significantly higher in infants with severe NE compared to moderate NE.8 Subsequently, Owji et al. confirmed that infants with NE with brain injury have significantly higher brain temperatures compared to healthy controls. This increase in brain temperature of the injured brain might be explained by the combination of the inflammatory response to injury, chemical reaction in ischemic cells such as the production of oxygen radicals and excitatory amino acids and/or reduced cerebral blood flow leading to less release of heat.24 Furthermore, non-invasive brain temperature measurement during therapeutic hypothermia might provide more information about the actual effect on the brain temperature.
Limitations
This study has several limitations. The first limitation is the retrospective design in which no standard protocol was followed for rectal temperature measurements. Consequently, infants had to be excluded, because there was no rectal temperature available within 3 h before or after the MRI, leading to a smaller sample size. With a larger sample size, it would have been more feasible to investigate the effect of brain injury on brain temperature and the effect of more risk factors such as ventilation, sedation and gestational age. Furthermore, it would have been preferable to compare rectal temperature immediately before and after the MRI using a standardized method. Nevertheless, these temperatures do represent the temperature trend of an infant. Another limitation is that the temperature probe could not be placed in the phantom for validation, but only at the surface of the phantom. However, the phantom was small, so it is most likely that the temperature inside the phantom is the same as at the surface. Furthermore, the slope of the calibration curve should remain the same, which is the most relevant.
Conclusions
In conclusion, this study showed that brain temperature can be measured with 1H-MRS in daily clinical practice without calibration using the previously published formula of Wu et al. with both short and long TE 1H-MRS. Furthermore, brain temperature remained in the physiological range during MRI in infants with neonatal encephalopathy following perinatal asphyxia and was in some infants even lower than rectal temperature before and after MRI.
References
Ditchfield, M. 3 T MRI in paediatrics: challenges and clinical applications. Eur. J. Radiol. 68, 309–319 (2008).
Groenendaal, F. & de Vries, L. S. Fifty years of brain imaging in neonatal encephalopathy following perinatal asphyxia. Pediatr. Res. 81, 150–155 (2017).
de Vries, L., Benders, M. & Groenendaal, F. Imaging the premature brain: ultrasound or MRI? Neuroradiology 55, 13–22 (2013).
Fumagalli, M. et al. Clinical safety of 3-T brain magnetic resonance imaging in newborns. Pediatr. Radiol. 48, 992–998 (2018).
Plaisier, A. et al. Safety of routine early MRI in preterm infants. Pediatr. Radiol. 42, 1205–1211 (2012).
U.S. Department of Health and Human Services, Food and Drug Administration C for D and RH. FDA [Internet]. Criteria for Significant Risk Investigations of Magnetic Resonance Diagnostic Devices 2014 (accessed 15 May 2019); http://www.fda.gov/downloads/RegulatoryInformation/Guidances/ucm126418.pdf
Cawley, P. et al. Does magnetic resonance brain scanning at 3.0 tesla pose a hyperthermic challenge to term neonates? J. Pediatr. 175, 228–231 (2016).
Wu, T. W. et al. Brain temperature in neonates with hypoxic-ischemic encephalopathy during therapeutic hypothermia. J. Pediatr. 165, 1129–1134 (2014).
Bainbridge, A. et al. Regional neonatal brain absolute thermometry by 1 H MRS. NMR Biomed. 26, 416–423 (2013).
Owji, Z., Gilbert, G., Saint-Martin, C. & Wintermark, P. Brain temperature is increased during the first days of life in asphyxiated newborns: developing brain injury despite hypothermia treatment. Am. J. Neuroradiol. 38, 2180–2186 (2017).
Cady, E. B., Penrice, J. & Robertson, N. J. Improved reproducibility of MRS regional brain thermometry by “ amplitude-weighted combination. NMR Biomed. 24, 865–872 (2011).
Azzopardi, D. et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N. Engl. J. Med. 361, 1349–1358 (2009).
Haan, T. R. De et al. Pharmacokinetics and pharmacodynamics of medication in asphyxiated newborns during controlled hypothermia. The PharmaCool multicenter study. BMC Pediatr. 12, 45–52 (2012).
Favie, L. & Groenendaal, F. 2-STEP: A Single-centre, Phase II Study To Evaluate The Safety, Tolerability And Pharmacokinetics of 2-Iminobiotin (2-IB) in Neonates With Gestational Age of 36 Weeks With Moderate To Severe Perinatal Asphyxia Treated With Therapeutic Hypothermia. Trialregister, 2015 (accessed 8 April 2019); https://www.trialregister.nl/trial/5089
Alderliesten, T. et al. MRI and spectroscopy in (near) term neonates with perinatal asphyxia and therapeutic hypothermia. Arch. Dis. Child Fetal Neonatal Ed. 102, F147–F152 (2017).
Naressi, A., Couturier, C., Castang, I., de Beer, R. & Graveron-Demilly, D. Java-based graphical user interface for MRUI, a software package for quantitation of in vivo/medical magnetic resonance spectroscopy signals. Comput. Biol. Med. 31, 269–286 (2001).
Pijnappel, W. W. F., van den Boogaart, A., de Beer, R. & van Ormondt, D. SVD-based quantification of magnetic resonance signals. J. Magn. Reson. 97, 122–134 (1992).
Verius, M., Frank, F., Gizewski, E. & Broessner, G. Magnetic resonance spectroscopy thermometry at 3 Tesla: importance of calibration measurements. Ther. Hypothermia Temp. Manag. 0, 1–10 (2018).
Zhu, M., Bashir, A., Ackerman, J. & Yablonskiy, D. Improved calibration technique for in vivo proton MRS thermometry for brain temperature measurement. Magn. Reson. Med. 60, 536–541 (2008).
Kuroda, K. et al. Feasibility of internally referenced brain temperature imaging with a metabolite signal. Magn. Reson. Med. Sci. 2, 17–22 (2003).
Childs, C., Vidyasagar, R. & Kauppinen, R. A. Determination of regional brain temperature using proton magnetic resonance spectroscopy to assess brain—body temperature differences in healthy human subjects. Magn. Reson. Med. 66, 59–66 (2007).
Kraff, O. & Quick, H. H. 7T: physics, safety, and potential clinical applications. J. Magn. Reson. Imaging 46, 1573–1589 (2017).
Marshall, I. et al. Measurement of regional brain temperature using proton spectroscopic imaging: validation and application to acute ischemic stroke. Magn. Reson. Imaging 24, 699–706 (2006).
Karaszewski, B. et al. Measurement of brain temperature with magnetic resonance spectroscopy in acute ischemic stroke. Ann. Neurol. 60, 438–446 (2006).
Sone, D. et al. Noninvasive detection of focal brain hyperthermia related to continuous epileptic activities using proton MR spectroscopy. Epilepsy Res. 138, 1–4 (2017).
Acknowledgements
The authors thank the MR technicians, neonatologists, physician assistants and nurses for their excellent help during the MR examinations. KVA acknowledges support from the European Union's Horizon 2020 research and innovation program (grant agreement No. 667224, ALBINO). The PharmaCool study was funded by ZonMW (40‐41500‐98‐9002). The 2-STEP study was funded in part by a gift of Neurophyxia (www.neurophyxia.com).
Author information
Authors and Affiliations
Contributions
K.V.A. has substantially contributed to conception and design of the study, acquisition, analysis and interpretation of the data and drafting the manuscript. F.G. has substantially contributed to conception and design of the study, acquisition of the MRI, analysis and interpretation of the data and critical revisions of the manuscript for important intellectual content. D.C. has substantially contributed to analysis of the data and drafting the manuscript. N.E.A. has revised the article critically for important intellectual content. T.A. has revised the article critically for important intellectual content. J.D. has contributed to acquisition of the data and critical revisions of the manuscript for important intellectual content. M.J.N.L.B. has contributed to acquisition of the data and critical revisions of the manuscript for important intellectual content. J.P.W. has substantially contributed to conception and design of the study, acquisition of the calibration phantom measurements, data analysis and interpretation and critical revisions of the manuscript for important intellectual content.
Corresponding author
Ethics declarations
Competing interests
Floris Groenendaal is expert witness in cases of perinatal asphyxia. The other authors have no competing interests to declare.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Annink, K.V., Groenendaal, F., Cohen, D. et al. Brain temperature of infants with neonatal encephalopathy following perinatal asphyxia calculated using magnetic resonance spectroscopy. Pediatr Res 88, 279–284 (2020). https://doi.org/10.1038/s41390-019-0739-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-019-0739-3
This article is cited by
-
Brain temperature and free water increases after mild COVID-19 infection
Scientific Reports (2024)