Abstract
Introduction
Acetaminophen is the only analgesic recommended for use during pregnancy. This use has recently been linked to childhood developmental disorders, a finding that requires further investigation. Adverse birth outcomes—preterm birth, low birthweight, and small for gestational age—are associated with increased risk of developmental disorders and can serve as intermediate outcomes when examining the impact of maternal acetaminophen use.
Methods
Clinical and lifestyle-factor data were gathered from 1200 women within the Ontario Birth Study who delivered between January 2013 and June 2017. Poisson regression with robust error variance was used to estimate the relationship between acetaminophen use before and during pregnancy and low birthweight, preterm birth, and small for gestational age.
Results
Offspring of mothers who used acetaminophen before pregnancy had a higher risk of low birthweight and small for gestational age. Acetaminophen use <once/week was associated with small for gestational age, adjusted relative risk (aRR) = 1.46 (95% CI 1.02, 2.11). Acetaminophen use ≥once/week was associated with both small for gestational age, RR = 1.82 (95% CI 1.12, 2.94), and low birthweight, aRR = 2.16 (95% CI 1.02, 4.54). Acetaminophen use during pregnancy was not associated with the examined outcomes.
Conclusions
Prepregnancy acetaminophen use may be associated with higher risk of adverse birth outcomes.
Similar content being viewed by others
Introduction
Nonsteroidal anti-inflammatory drugs (NSAIDs) (e.g., aspirin) are used to treat pain, fever, and inflammation, but have been proven harmful for the fetus, especially when used in late pregnancy.1,2,3 Acetaminophen (also known as paracetamol) is the only analgesic recommended by doctors for use during pregnancy,4 making it the most commonly used over-the-counter (OTC) pain medication taken during pregnancy, with 56−70% of mothers reporting use during this time.5,6,7 Given this widespread use, the potential impact on birth and developmental outcomes requires examination.
Recent studies have found maternal acetaminophen use during pregnancy to be associated with an increased risk of neurodevelopmental disorders,8 including attention deficit hyperactivity disorder (ADHD),6,9 and other behavioral problems.10 Studies conducted using data from the Danish National Birth Cohort found associations between prenatal acetaminophen use and a higher risk of cerebral palsy,11 hyperkinetic disorders, and ADHD-like behaviors in children,6,12 as well as lower performance IQ among 5-year olds.7 However, findings across the literature are inconclusive. A systematic review concluded that current data do not provide sufficient evidence of the existence of a causal relationship between maternal acetaminophen use and the development of ADHD among children later in life.13 A recently published Canadian cohort study of acetaminophen measured in fetal meconium also found no association with children’s neurocognitive development at age 6−8.14
Adverse birth outcomes, including low birthweight, preterm birth and small for gestational age, have consistently been related to an increased risk for developmental disorders including ADHD,15 and are also leading causes of perinatal morbidity, mortality, and disability among neonates worldwide.16 Previous literature suggests that third-trimester acetaminophen use is associated with preterm birth.17 The objective of this study was to evaluate the association between maternal acetaminophen use in the 3 months prior to pregnancy, as well as during early and mid–late pregnancy, and adverse birth outcomes: low birthweight, preterm birth and small for gestational age.
Methods
Study design and participants
The Ontario Birth Study (OBS) is an ongoing prospective pregnancy and birth cohort study established in Ontario, Canada in 2013.18 The goal of OBS is to provide a platform for research on pregnancy complications and maternal and infant health, and support studies on the developmental origins of health and disease. The study population is composed of pregnant women receiving prenatal care at obstetrical clinics affiliated with Mount Sinai Hospital in Toronto. Eligible participants were women 18 years of age or older, in the first or early second trimester of pregnancy (<17 weeks gestation), delivering and having antenatal care at Mount Sinai Hospital. Women were recruited at the time of their ultrasound or first antenatal visit, usually at 11–14 weeks of gestational age.
OBS participants completed three self-administered comprehensive questionnaires at 12–16 weeks of gestation, 24–28 weeks of gestation, and 6–10 weeks postpartum. These questionnaires were designed to capture personal and family medical history, demographic and lifestyle factors as well as environmental exposures. Details of the study cohort and recruitment have been published previously.18 The study was approved by the Research Ethics Board of Mount Sinai Hospital, and all participants provided informed consent.
Acetaminophen exposure
Use of OTC analgesics, including acetaminophen, was identified from the questionnaires. Exposure was categorized by timing of use: (i) prepregnancy: in the 3 months before pregnancy, (ii) early pregnancy: in the first 12–16 weeks of pregnancy, and (iii) mid–late pregnancy: between completion of the first questionnaire and 28–32 weeks of gestation. Acetaminophen use was also categorized by frequency into: never, less than once per week, and one or more times per week. Acetaminophen exposure was analyzed using a second classification method, where participants were classified as never users (ref), early users only (ever-use in prepregnancy or early pregnancy), late users only (ever-use in mid−late pregnancy) and continuous users (ever-use during each of the three periods: prepregnancy, early pregnancy, mid−late pregnancy).
Pregnancy outcomes
Information on the baby’s sex, birthweight, and gestational age was derived from clinical data collected from hospital medical charts.
Preterm birth is defined as live birth before 37 weeks of pregnancy are complete. It can be further categorized, based on gestational age, into extremely preterm (birth before 28 weeks gestation), very preterm (birth after 28 and before 32 weeks gestation), and moderate to late preterm (birth after 32 and before 37 weeks gestation).19 Babies born at 37 weeks of gestation or later were categorized as having a term birth.
Low birthweight is defined as a birthweight of less than 2500 g.20 Subcategories of low birthweight include very low birthweight (1000 g to less than 1500 g), and extremely low birthweight (less than 1000 g).21 Birthweight greater than or equal to 2500 g (and less than 4000 g) was categorized as normal. Newborns with a birthweight greater than or equal to 4000 g were categorized as having a high birthweight.
Small for gestational age was defined as having a birthweight below the sex-specific and gestational age-specific tenth centile of a Canadian population-based reference group.22
Sample size
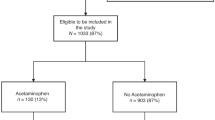
Figure 1 provides a detailed overview of recruitment and inclusion/exclusion criteria. Women were included in the current analysis if they were enrolled in the OBS, delivered a liveborn singleton offspring between January 2013 and June 2017, and completed the first two lifestyle questionnaires (N = 1200). For women with more than one pregnancy (N = 38), only data from the first pregnancy were included. Women with missing data for both the infant’s birthweight and gestational age (N = 196) were excluded from all analyses. Following this, women missing only either gestational age (N = 2) or birthweight (N = 1) were excluded from the relevant analyses. Women with offspring who had high birthweight (birthweight >4000 g, N = 66) were removed from the analysis of low birthweight, in order to compare low birthweight infants to those with normal birthweight. We then removed missing exposure data from each analysis, as depicted in Fig. 1.
Statistical analysis
Poisson regression with robust error variance was used to estimate risk ratios (RR) for preterm birth, low birthweight, and small for gestational age. Three separate analyses were conducted for the three main exposures of interest: (i) prepregnancy acetaminophen use, (ii) early pregnancy acetaminophen use, and (iii) mid−late pregnancy acetaminophen use. To assess the impact of cumulative acetaminophen use, a sensitivity analysis was conducted to estimate RRs for the same outcomes but classifying women as never users (ref)/early users only/late users only/continuous users.
Covariates included a priori defined variables based on literature review and hypothesized relationships between acetaminophen use and birth outcomes. Information on covariates was obtained from lifestyle questionnaires. Multivariable adjusted models included: age (continuous), smoking in the 3 months before pregnancy (ever, never, missing),23,24 body mass index (BMI) at baseline (continuous),24 maternal ethnicity (white vs. non-white), education (less than versus at least a bachelor’s degree), fever during pregnancy (yes, no, missing), and paternal smoking in the 3 months before pregnancy (never [reference category], occasionally, daily, missing).25 Missing BMI values (N = 56) were replaced with the median BMI value of women in our sample. Further, to adjust for baseline health status, a comorbidity index was created as a sum of the number of pre-existing conditions reported by each individual. The following conditions, as captured in the self-administered lifestyle questionnaires, were included in the comorbidity index: diabetes, hypertension, cardiovascular disease, arthritis, epilepsy, cancer, multiple sclerosis, chronic obstructive pulmonary disorder, celiac disease, Crohn’s disease, and kidney disease. Lastly, we also adjusted for participants’ use of other pain medications including OTC nonsteroidal anti-inflammatory drugs, and prescription pain medications such as diclofenac, morphine, oxycodone, and codeine, during the respective time periods (ever, never, missing).
All analyses were conducted using SAS version 9.4 (Cary, NC).
Results
Mothers included in our study had a mean age of 33.8 years at baseline, with a mean BMI of 23.2 kg/m2. The cohort was highly educated, with 83.8% of mothers having at least a bachelor’s degree. Our population was comprised of generally healthy women, with only 79 individuals (6.6%) reporting one comorbidity, and 7 individuals (0.6%) reporting two comorbidities. Other demographic characteristics are presented in Table 1.
Pregnancy outcomes are summarized in Table 2. Both preterm birth and low birthweight were rare in the population. Sixty-three (5.2%) were born preterm, out of which 5 were born very preterm (28 to <32 weeks), and 58 were born moderate to late preterm (32 to <37 weeks). Fifty-five (4.6%) had low birthweight (<2500 g), out of which two had very low birthweight (1000 to <1500 g). The prevalence of offspring born small for gestational age was higher (N = 136, 11.3%).
In the study population, 726 women (60.5%) reported using acetaminophen at any point during the study period and 156 women (13.0%) used acetaminophen across all three time periods. Acetaminophen use was strongly correlated between pre- and early pregnancy (Cramer’s V = 0.39), and between early and mid−late pregnancy (Cramer’s V = 0.32). The correlation between prepregnancy and mid−late pregnancy was quite low (Cramer’s V = 0.14) (Supplemental Table S3 (online)). The correlation between acetaminophen use and the use of other pain medications was strong for all three time periods; Cramer’s V = 0.25, 0.29, 0.41, respectively, for the pre-, early, and mid−late pregnancy periods (Supplemental Table S4 (online)). However, the prevalence of other pain medication use (<5%) was much lower than acetaminophen use, especially in the mid−late pregnancy period.
In the study population, very few women experienced hypertensive disorders of pregnancy (N = 62, 5.2%). The prevalence of preeclampsia specifically was very low (N = 22, 1.8%). There was no relationship between these outcomes and the use of acetaminophen at any of the examined timepoints.
The relationships between maternal acetaminophen use and preterm birth, low birthweight, and small for gestational age are presented in Table 3. Offspring of women who used acetaminophen <once per week or ≥once per week in the 3 months prior to pregnancy had a higher risk of being born small for gestational age (RR = 1.46, 95% CI 1.02, 2.11 and RR = 1.82, 95% CI 1.12, 2.94, respectively). A trend test also indicated that with higher frequency of acetaminophen use, there was an increasingly strong association with the risk of the offspring being born small for gestational age. Specifically, there was a 38% increase in the relative risk of small for gestational age per category increase in acetaminophen use, p = 0.004, providing evidence of dose−response specific to that outcome. Offspring of women who used acetaminophen ≥once per week were also at a higher risk of having a low birthweight (RR = 2.16, 95% CI 1.02, 4.54).
In addition, we found that for women who took acetaminophen ≥once per week in the 3 months prior to pregnancy, the association with preterm birth approached statistical significance, RR 1.86 95% CI (0.96, 3.63). No associations were found between maternal acetaminophen use in the early and mid−late pregnancy periods and risk of adverse birth outcomes.
Early users of acetaminophen (women who used acetaminophen in the prepregnancy or early pregnancy periods only) had an increased risk of having an infant born small for gestational age, RR = 1.54 95% CI (1.02, 2.34). Notably, we did not find an increased risk of any of the examined outcomes among offspring of continuous users of acetaminophen (women who used acetaminophen across all three examined time periods), preterm birth RR = 1.29, 95% CI (0.58, 2.88), low birthweight: RR = 1.18 (0.47, 2.95), small for gestational age: RR = 0.80, 95% CI (0.41, 1.53) (Table 4). We also modeled three mutually exclusive outcome groups: term-not-small for gestational age (reference), preterm birth, and term-small for gestational age, using multinomial logistic regression, and did not see any difference in the results (not shown).
Discussion
Among women enrolled in the OBS, we found that children born to mothers who used acetaminophen in the 3 months before pregnancy had a higher risk of having low birthweight and being born small for gestational age, with a positive, but nonstatistically significant association seen with preterm birth. Further, our results present evidence of dose response with more frequent use of acetaminophen being associated with increased risk of the offspring being born small for gestational age. During pregnancy, however, maternal use of acetaminophen was not associated with the examined adverse birth outcomes: preterm birth, low birthweight, and small for gestational age. In addition, continuous use of acetaminophen (relative to never use) was not associated with any of the examined birth outcomes.
While third-trimester acetaminophen use has been shown to be associated with increased risk of preterm birth,17 maternal acetaminophen use in the prepregnancy period has not been previously studied. The periconceptional period is an important window for human development, and there are gaps in research on preconception exposures.26 Our study provides insight into maternal use of acetaminophen in the 3 months prior to pregnancy, and its associations with adverse birth outcomes. In addition, preconception exposure to acetaminophen may also capture periconception exposure, which includes very early development.
Prior studies have suggested that maternal use of acetaminophen is associated with neurodevelopmental outcomes. A recent review suggests that prenatal acetaminophen exposure may be associated with negative neurodevelopmental outcomes including ADHD, autism spectrum disorder, and lower IQ.27 In a Norwegian cohort, long-term (≥28 days) acetaminophen exposure in utero was associated with poorer gross motor skills, and internalizing and externalizing behavior at age 3 in a sibling-controlled study.28 In the same study population, maternal acetaminophen use was found to be associated with gross motor milestone delay and impaired communication skills among children at 18 months of age.8 In a Swedish pregnancy and birth cohort, acetaminophen use during pregnancy was associated with language delay—an early marker of neurodevelopment—among girls at 30 months.29 A recent meta-analysis of seven observational studies found maternal use of acetaminophen during pregnancy to be associated with a 20–30% increase in the risk of neurodevelopmental disorders.30 While our study examined the association between maternal acetaminophen use and three adverse birth outcomes, we aimed for this to be a stepping-stone to further explore the link between acetaminophen and negative neurodevelopmental outcomes.
To our knowledge, no explicit biological mechanism exists to explain the relationship between acetaminophen use before or during pregnancy and risk of small for gestational age offspring. However, acetaminophen can cross the placental barrier during pregnancy and can also cross the fetal brain barrier, thus potentially altering fetal growth and development.31 Acetaminophen primarily acts by inhibiting prostaglandin synthesis,32 although unlike NSAIDs, acetaminophen does not inhibit prostaglandin synthesis in peripheral tissues or reproductive organs.33 Acetaminophen is hypothesized to interfere with prostaglandin-regulated fetal development, including male reproductive development34 as well as a decline in female offspring fertility.35 Preconception use of acetaminophen has not been explored in the context of reproduction, except in two human studies that did not find an association between maternal preconception use of acetaminophen and fecundability or time-to-pregnancy in mothers.36,37 Further research is needed to determine the impact of acetaminophen use in the pre- and periconception period on the risk of adverse birth outcomes.
Women taking acetaminophen ≥once per week during the prepregnancy period had a slightly higher median BMI, BMI = 23.4, IQR (21.4–26.6) relative to women who did not take acetaminophen as frequently, BMI = 22.1, IQR, (20.5–24.5). These women did not differ in age and number of comorbidities. Because study participants were generally healthy and very few women reported having multiple comorbid conditions (N = 7), detailed examination of comorbidities was not possible; however, the comorbid conditions that we were able to examine do not appear to explain our findings. We hypothesize that the underlying mechanism behind the increased risk of low birthweight and small for gestational age involves the indication for acetaminophen use, which may vary between women.
A limitation of our study is the lack of information on the indication for acetaminophen use. We were able to consider maternal fever during pregnancy; however, other potential indicators such as inflammation and pain were not addressed due to a lack of available data. As such, we cannot preclude the possibility of confounding by indication. Further, there is a potential for limited generalizability as the women studied are highly educated, and slightly older than the average pregnant Canadian woman. While 50.4% of Canadians aged 25−64 had at least a bachelor’s degree in 2016,38 in our sample, 83.8% of women had a bachelor’s degree or higher. In 2011, the average maternal age at childbirth was 30.2,39 while in our cohort, the mean age was 33.8 years. While approximately 10% of women had missing exposure data, women with missing acetaminophen use information were nearly identical to those with available data in terms of their demographic characteristics; we found no significant differences in the two groups’ age, BMI, ethnicity, or education. Characteristics between participants missing versus not-missing exposure and outcome data are presented in Supplemental Tables S1 and S2 (online). Finally, since the prevalence of adverse birth outcomes was relatively low (preterm birth: 5.2%, low birthweight: 4.6%, and small for gestational age, 11.3%), it is possible that the study was inadequately powered. As a result, the possibility of findings due to chance cannot be eliminated.
Notably, we did not adjust for alcohol consumption in our model. According to the literature, the examined birth outcomes have only been shown to be affected by moderate to high alcohol consumption during pregnancy (defined as ≥10 g pure alcohol per day i.e. one drink per day or more);40 however, it is important to note that no safe level of alcohol consumption exists in the context of other outcomes, such as fetal alcohol syndrome disorder. Our study population had only two people who consumed more than three drinks per week at any point during their pregnancy; these two participants did not have any of the examined negative birth outcomes. To be certain, we conducted a sensitivity analysis where we adjusted for time-period-specific alcohol use as well as ever-use, with no meaningful impact on the results (results not shown).
Strengths of our study include the prospective design of the cohort, which avoids differential recall bias. Another strength is the use of detailed questionnaire data on acetaminophen use and other lifestyle factors covering three time periods: the 3 months before pregnancy, early pregnancy, and mid−late pregnancy. Further, questionnaire data were integrated with clinical information from medical records and included information related to antenatal exposures, delivery details and birth outcomes. We were also able to incorporate information on frequency of acetaminophen use, to detect a dose−response relationship between maternal acetaminophen use during the prepregnancy period and the offspring being born small for gestational age. In addition, we limited the possibility of confounding by other pain medications by adjusting for the use of other commonly used pain medications including NSAIDs.
As data on longer-term outcomes in the OBS cohort are not yet available, preterm birth, low birthweight, and small for gestational age serve as intermediary outcomes that have been implicated in later negative developmental outcomes. Follow-up of offspring in the OBS cohort is ongoing and future studies to investigate child developmental outcomes are planned. Here we found that maternal acetaminophen use in the 3 months prior to pregnancy is associated with an increased risk of adverse birth outcomes.
The results of our study should not be overstated so as to not unnecessarily burden pregnant women and those planning for pregnancy. To our knowledge, acetaminophen remains the safest analgesic for use during pregnancy as per the current literature. However, further research is warranted to evaluate and validate our results, and to investigate the effects of preconception acetaminophen use.
References
Hernandez, R. K. et al. Nonsteroidal antiinflammatory drug use among women and the risk of birth defects. Am. J. Obset. Gynecol. 206, 228.e221–228 (2012).
Nezvalova-Henriksen, K., Spigset, O. & Nordeng, H. Effects of ibuprofen, diclofenac, naproxen, and piroxicam on the course of pregnancy and pregnancy outcome: a prospective cohort study. BJOG 120, 948–959 (2013).
Schoenfeld, A., Bar, Y., Merlob, P. & Ovadia, Y. Nsaids: maternal and fetal considerations. Am. J. Reprod. Immunol. 28, 141–147 (1992).
Babb, M., Koren, G. & Einarson, A. Treating pain during pregnancy. Can. Fam. Physician 56, 25–27 (2010).
Werler, M. M., Mitchell, A. A., Hernandez-Diaz, S. & Honein, M. A. Use of over-the-counter medications during pregnancy. Am. J. Obset. Gynecol. 193, 771–777 (2005).
Liew, Z., Ritz, B., Rebordosa, C., Lee, P. C. & Olsen, J. Acetaminophen use during pregnancy, behavioral problems, and hyperkinetic disorders. JAMA Pediatr. 168, 313–320 (2014).
Liew, Z., Ritz, B., Virk, J., Arah, O. A. & Olsen, J. Prenatal use of acetaminophen and child iq a danish cohort study. Epidemiology 27, 912–918 (2016).
Vlenterie, R. et al. Neurodevelopmental problems at 18 months among children exposed to paracetamol in utero: a propensity score matched cohort study. Int. J. Epidemiol. 45, 1998–2008 (2016).
Thompson, J. M. et al. Associations between acetaminophen use during pregnancy and adhd symptoms measured at ages 7 and 11 years. PLoS ONE 9, e108210 (2014).
Stergiakouli, E., Thapar, A. & Davey Smith, G. Association of acetaminophen use during pregnancy with behavioral problems in childhood: Evidence against confounding. JAMA Pediatr. 170, 964–970 (2016).
Petersen, T. G. et al. Use of paracetamol, ibuprofen or aspirin in pregnancy and risk of cerebral palsy in the child. Int. J. Epidemiol. 47, 121–130 (2018).
Liew, Z., Bach, C. C., Asarnow, R. F., Ritz, B. & Olsen, J. Paracetamol use during pregnancy and attention and executive function in offspring at age 5 years. Int. J. Epidemiol. 45, 2009–2017 (2016).
Hoover, R. M., Hayes, V. A. & Erramouspe, J. Association between prenatal acetaminophen exposure and future risk of attention deficit/hyperactivity disorder in children. Ann. Pharmacother. 49, 1357–1361 (2015).
Laue, H. E. et al. Association between meconium acetaminophen and childhood neurocognitive development in geste, a canadian cohort study. Toxicol. Sci. 167, 138–144 (2019).
Momany, A. M., Kamradt, J. M. & Nikolas, M. A. A meta-analysis of the association between birth weight and attention deficit hyperactivity disorder. J. Abnorm. Child Psych. 46, 1409–1426 (2018).
Liu, L. et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet 379, 2151–2161 (2012).
Rebordosa, C., Kogevinas, M., Bech, B. H., Sorensen, H. T. & Olsen, J. Use of acetaminophen during pregnancy and risk of adverse pregnancy outcomes. Int. J. Epidemiol. 38, 706–714 (2009).
Anderson, L. N. et al. The ontario birth study: a prospective pregnancy cohort study integrating perinatal research into clinical care. Paediatr. Perinat. Epidemiol. 32, 290–301 (2018).
World Health Organization. Preterm Birth (World Health Organization, 2018).
United Nations Children's Fund, World Health Organization. Low Birthweight: Country, Regional and Global Estimates (UNICEF, New York, 2004).
Ballot, D. E., Potterton, J., Chirwa, T., Hilburn, N. & Cooper, P. A. Developmental outcome of very low birth weight infants in a developing country. BMC Pediatr. 12, 11 (2012).
Kramer, M. S. et al. A new and improved population-based canadian reference for birth weight for gestational age. Pediatrics 108, E35 (2001).
Nomura, Y., Marks, D. J. & Halperin, J. M. Prenatal exposure to maternal and paternal smoking on attention deficit hyperactivity disorders symptoms and diagnosis in offspring. J. Nerv. Ment. Dis. 198, 672–678 (2010).
Rosenberg, T. J., Garbers, S., Lipkind, H. & Chiasson, M. A. Maternal obesity and diabetes as risk factors for adverse pregnancy outcomes: Differences among 4 racial/ethnic groups. Am. J. Public Health 95, 1545–1551 (2005).
Shapiro, G. D. et al. Paternal education and adverse birth outcomes in canada. J. Epidemiol. Community Health 71, 67–72 (2017).
Mumford, S. L., Michels, K. A., Salaria, N., Valanzasca, P. & Belizan, J. M. Preconception care: it's never too early. Reprod. Health 11, 73 (2014).
Bauer, A. Z., Kriebel, D., Herbert, M. R., Bornehag, C. G. & Swan, S. H. Prenatal paracetamol exposure and child neurodevelopment: a review. Horm. Behav. 101, 125–147 (2018).
Brandlistuen, R. E., Ystrom, E., Nulman, I., Koren, G. & Nordeng, H. Prenatal paracetamol exposure and child neurodevelopment: a sibling-controlled cohort study. Int. J. Epidemiol. 42, 1702–1713 (2013).
Bornehag, C. G. et al. Prenatal exposure to acetaminophen and children's language development at 30 months. Eur. Psychiat. 51, 98–103 (2018).
Masarwa, R. et al. Prenatal exposure to acetaminophen and risk for attention deficit hyperactivity disorder and autistic spectrum disorder: a systematic review, meta-analysis, and meta-regression analysis of cohort studies. Am. J. Epidemiol. 187, 1817–1827 (2018).
Thiele, K., Kessler, T., Arck, P., Erhardt, A. & Tiegs, G. Acetaminophen and pregnancy: short- and long-term consequences for mother and child. Am. J. Reprod. Immunol. 97, 128–139 (2013).
Anderson, B. J. Paracetamol (acetaminophen): mechanisms of action. Paediatr. Anaesth. 18, 915–921 (2008).
Li, D., Ferber, J. R., Odouli, R. & Quesenberry, C. Use of nonsteroidal antiinflammatory drugs during pregnancy and the risk of miscarriage. Am. J. Obstet. Gynecol. 219, 275.e1–275.e8 (2018).
Lind, D. V. et al. Maternal use of mild analgesics during pregnancy associated with reduced anogenital distance in sons: a cohort study of 1027 mother-child pairs. Hum. Reprod. 32, 223–231 (2017).
Dean, A. et al. Analgesic exposure in pregnant rats affects fetal germ cell development with inter-generational reproductive consequences. Sci. Rep. 6, 19789 (2016).
McInerney, K. A. et al. Preconception use of pain-relievers and time-to-pregnancy: a prospective cohort study. Hum. Reprod. 32, 103–111 (2017).
Smarr, M. M. et al. Urinary paracetamol and time-to-pregnancy. Hum. Reprod. 31, 2119–2127 (2016).
Statistics Canada. Education in Canada: Key Results from the 2016 Census (Statistics Canada, 2017).
Statistics Canada. Fertility: Fewer Children, Older Moms (Statistics Canada, 2014).
Patra, J. et al. Dose−response relationship between alcohol consumption before and during pregnancy and the risks of low birthweight, preterm birth and small for gestational age (SGA)—a systematic review and meta-analyses. BJOG 118, 1411–1421 (2011).
Acknowledgements
The authors would like to thank the participants of the Ontario Birth Study. This work was supported by funding from the Dalla Lana School of Public Health at the University of Toronto, the Lunenfeld−Tanenbaum Research Institute, Mount Sinai Hospital Women’s Auxiliary, through a grant to S.J.L., and the Department of Obstetrics and Gynecology at Mount Sinai Hospital. Additional funding was provided by the Canadian Institute of Health Research through a grant to S.J.L. (FDN‐143262).
Author information
Authors and Affiliations
Contributions
All authors (J.A., R.J.H., R.A.S., J.A.K., S.L.H., A.B., S.J.L., and J.D.B.) have met the Pediatric Research requirements for authorship. All authors (J.A., R.J.H., R.A.S., J.A.K., S.L.H., A.B., S.J.L., and J.D.B.) made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; drafted the article or revised it critically for important intellectual content; and provided final approval of the version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Arneja, J., Hung, R.J., Seeto, R.A. et al. Association between maternal acetaminophen use and adverse birth outcomes in a pregnancy and birth cohort. Pediatr Res 87, 1263–1269 (2020). https://doi.org/10.1038/s41390-019-0726-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-019-0726-8
This article is cited by
-
Association of acetaminophen use with perinatal outcomes among pregnant women: a retrospective cohort study with propensity score matching
BMC Pregnancy and Childbirth (2024)