Abstract
Background
Infants born preterm are at increased risk of pulmonary morbidity. The contribution of antenatal factors to impairments in lung structure/function has not been fully elucidated. This study aimed to compare standardized lung volumes from foetuses that were delivered <32 weeks’ gestation with foetuses that were delivered >37 weeks.
Methods
Fourteen women who delivered <32 weeks gestation and 56 women who delivered >37 underwent a foetal MRI. Slice-volume reconstruction was then used and the foetal lungs were then segmented using multi-atlas approaches. Body volumes were calculated by manual segmentation and lung:body volume ratios generated.
Results
Mean gestation at MRI of the preterm group was 27+2 weeks (SD 2.9, range 20+6–31+3) and control group 25+3 weeks (SD 4.7 range 20+5–31+6). Mean gestation at delivery of the preterm group was 29+2 weeks (SD 2.6, range 22+0–32+0). Lung:body volume ratios and foetal lung volumes were smaller in foetuses that were delivered preterm both with and without preterm premature rupture of membranes compared to those born at term (p < 0.001 in all cases).
Conclusions
Foetuses that were delivered very preterm had reduced lung volumes when standardized for foetal size, irrespective of ruptured membranes. These are novel findings and suggest an antenatal aetiology of insult and possible focus for future preventative therapies.
Similar content being viewed by others
Introduction
Preterm birth (PTB) is the most important single determinant of adverse infant outcome with regard to survival and quality of life.1 Morbidity is inversely correlated to gestational age (GA), and the most significant adverse outcomes are associated with delivery before 32 weeks.
Although the advent of antenatal steroids has significantly improved respiratory morbidity of infants born preterm, specifically respiratory distress syndrome (RDS), it remains a significant complication associated with PTB.2 RDS results from failure of adequate lung expansion post-delivery due to surfactant deficiency. Many of these infants progress to prolonged supplementary oxygen requirement with additional effects from ventilator and hyperoxic-induced acute lung injury.3 This chronic evolving condition is referred to as bronchopulmonary dysplasia (BPD) and complications can persist into adulthood.4 The aetiology is complex and multifactorial: genetic predisposition and infection have been implicated in its development.2 Various factors are thought to culminate in inflammatory cytokine and chemokine release, ultimately disrupting alveolar and microvascular development of the peripheral lung.5 The understanding of BPD associated with PTB is hampered by a lack of antenatally derived assessments that can be utilized to predict long-term outcomes and mechanisms underlying the disease pathogenesis.2
Infection/inflammation has been implicated in the aetiology of PTB and approximately 80% of cases of PTB prior to 28 weeks have evidence of significant microbial colonization within placental parenchyma.6 It is therefore hypothesized that infection/inflammation associated with events triggering PTB may additionally affect antenatal lung development. No studies to date have explored lung volumes standardized for the overall size of the foetus in pregnancies that subsequently deliver preterm. This study therefore has two aims: (1) to establish magnetic resonance imaging (MRI)-derived normative ranges for foetal lung volumes standardizing for foetal size and (2) to compare standardized lung volumes in foetuses that were subsequently delivered <32 weeks gestation with a group of control foetuses that were delivered at term.
Methods
Mothers of foetuses at high risk of PTB were prospectively recruited from the antenatal ward and the Preterm Surveillance Clinic at St Thomas’ Hospital London between December 2015 and October 2017.
Inclusion criteria were: GA 20–32 weeks, high risk of PTB encompassing either asymptomatic women with a history of previous PTB, late miscarriage >16 weeks or cervical surgery with a >50% risk of PTB in the next 2 weeks (based on an algorithm derived from quantitative cervico-vaginal fibronectin and cervical length7) or preterm premature rupture of membranes (PPROM). Exclusion criteria were: foetuses known to have structural or chromosomal abnormalities, multiple pregnancies, active labour, inability to give informed consent, pregnancy complications such as pre-eclampsia or gestational diabetes, contraindications to MRI such as claustrophobia or a recently sited metallic implant.
Following assessment of eligibility, women were invited to participate and written consent obtained. Foetal MRI was performed using a 1.5-T Philips Ingenia MRI System (Philips Medical systems, Best, the Netherlands) with a 32-channel cardiac coil placed around the mother’s abdomen. Imaging of the foetal trunk was performed using T2-weighted single-shot turbo spin echo (ssTSE), acquired in three orthogonal planes using the following scanning parameters: TR = 25,991 ms, TE = 80 ms, slice thickness 2.5 mm, slice overlap 1.25 mm, and flip angle = 90o. In addition, T2-weighted ssTSE images were acquired (using a larger slice thickness/lower resolution) to provide coverage of the whole uterus, again in three orthogonal planes. Two subjects were imaged at 3 T on a Philips Achieva system using otherwise similar procedures.
To obtain volumetric data of the lungs, for each subject, all available T2-weighted ssTSE stacks covering the foetal thorax, which were typically corrupted by foetal movement between individual slices, were motion corrected and combined to produce a single three-dimensional volumetric image, using slice to volume reconstruction (SVR).8,9
Normal anatomical atlases were derived from the volumetric reconstructions of 25 previously imaged normal subjects (20+1–31+6 weeks’ GA). A mask was initially placed over the foetal thorax and then lung volumes were manually segmented for each of these normal subjects from the SVR image using ITK SNAP (version 2.2.0).10
Lung segmentation was then performed automatically on the study foetuses using the age-appropriate atlases as follows: eight age-matched atlas subjects were selected and each was first affine registered to the study foetus segmented target image to achieve a global pose alignment.10 Free form deformation-based non-linear registration with control points spaced at 3 mm intervals was used to estimate the local deformations. After registration, the atlas segments were transformed to the target subject using linear interpolation and a final segmentation was determined by ‘majority vote’.10
Segmentation of the foetal body and amniotic fluid was performed using the uterine single-shot fast spin echo images using ITK-SNAP (version 2.2.0) in a two-step process. In each case, a rough automatic segmentation of the body was obtained based on image contrast while utilizing user-defined thresholds. Editing was then performed manually. Intra- and inter-observer variability had previously been confirmed (intraclass correlation co-efficient (ICC) intra-observer variability 0.85 and inter-observer ICC 0.971). Lung:body volumes were then calculated.
A control group was identified from the intelligent foetal imaging and diagnosis project (www.iFINDproject.com). This is a study conducted at King’s College London, which aims to improve foetal ultrasound imaging through automated image processing. Part of this study combines conventional ultrasound imaging with more detailed MRI scans to build a comprehensive map of foetal anatomy to use for computer-assisted diagnosis of foetal anomalies. Cases were selected from low-risk pregnancies where the MRI was performed between 20+0 and 32+0 weeks’ gestation and delivery occurred >37 weeks. All foetuses underwent the same imaging sequences and reconstruction as described above.
Details of maternal demographics, timing of steroid administration, delivery and neonatal parameters were collected, including gestation at delivery, sex of infant, birth weight, birth weight centile, neonatal unit admission, number of days of invasive ventilation, continuous positive airway pressure and need for supplemental oxygen.
Data were assessed for normality using distributional plots of standardized residuals. Demographic and neonatal outcome data were analysed using Student’s t test where data were continuous and Chi-squared when categorical. For the low-risk control cases delivering at term, obtained from the iFIND study, the normal growth trajectory of lung volume and lung:body volume ratios between 20 and 32 weeks’ gestation was estimated by maximum likelihood regression, using the Stata command xriml.11 Gestation-adjusted centiles were then calculated for all lung volumes and lung:body volume ratios. Receiver operating characteristic curves were generated for low lung:body volume ratio and low gestation-adjusted lung volume centiles as predictors of PTB. Adjusting for the effects of gestation, multiple regression analysis was used to produce estimates between foetuses that were delivered preterm and those that were delivered at term for lung and body volumes and lung:body volume ratios. Multiple regression analysis was also used to compare amniotic fluid volumes between foetuses that were delivered preterm with and without ruptured membranes and the control group, accounting for the effects of gestation.
For neonatal outcome data (not normally distributed), the Spearman Rank Correlation was used. Statistical analysis was performed using the SPSS software package (version 25, SPSS IBM) and Stata version 14.0 (StataCorp, College Station, Texas, USA).
This study was conducting under the ethics numbers: 07/H0707/105 and 14/LO/1806.
Results
Thirty-eight women were identified as eligible to participate in the study group: 35 agreed to undergo MRI imaging: 17 delivered prior to MRI (median 2 days from agreeing to participate, range 0–11 days). Eighteen patients had an MRI: three delivered >32 weeks. Lung reconstruction was not possible in one case leaving 14 suitable for analysis. Six women had ruptured and eight intact membranes at the time of imaging. Twelve were performed on a 1.5-T and two on a 3-T scanner. Eleven of the 14 cases had antenatal steroids prior to imaging. Fifty-six controls were identified from the iFIND study (all healthy volunteers) as a control group.
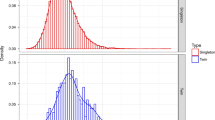
Clinical characteristics of the cohorts can be seen in Table 1 and lung volume, body volume and lung:body volume ratios in Table 2. Distribution plots for lung volumes and lung:body volume ratios from 20–32 weeks’ gestation derived from the control group of foetuses are shown in Fig. 1. In foetuses that were delivered at term, the lung volume comprised 4.2% of the total body volume (standard deviation 0.6%) compared to 3.4% in the preterm group (standard deviation 0.6%).
Normal ranges and 3, 10, 50, 90 and 97th centiles for MRI-derived a lung volumes and b lung:body volume ratios between 20 and 32 weeks’ gestation obtained from a 1.5-T MRI scanner. The expected mean lung volume (cm3), Z score and gestation-adjusted centile at a given gestational age (GA) were estimated: m = −447.4 × ga + 233.5 × log_e(ga) + 576. SD = 0.1821 × expected mean. Z score for any individual foetus = (measured lung volume − m)/SD. The expected lung:body volume ratio was estimated, with no detectable variation of healthy lung:body volume ratios with gestational age: m = 0.0419881, SD = 0.0062148.
The gestation-adjusted lung volume centiles and the lung:body volume ratio were tested as predictors of PTB (Fig. 2). Foetuses that were delivered preterm had significantly lower lung volumes, body volumes and lung:body volume ratios at the time of scan than those that were delivered at term, allowing for gestational change (Table 2 and Fig. 3). Excluding the two foetuses scanned at 3 T did not affect this finding (accounting for different magnetic field strengths potentially causing contrast differences and measurement discrepancies). These results were observed in both groups: foetuses with ruptured (mean 33,900 mm3, SD 13,500) and intact membranes (mean 27,200 mm3, SD 13,200; p < 0.001 in both cases). Body volumes were also significantly smaller in foetuses that were subsequently delivered preterm (both with (mean 981,000 mm3, SD 397,000, p = 0.007) and without (mean 861,000 mm3, SD 421,000; p = 0.009) ruptured membranes) when adjusted for GA at MRI. When standardized for foetal size, foetuses that were delivered preterm had significantly lower lung:body volume ratios. These effects were again observed in both foetuses that had intact membranes (mean 0.035, SD 0.006) and those with ruptured membranes (mean 0.032, SD 0.007) at the time of MRI (p < 0.001 in both cases).
Graphs illustrating the relationship between gestation at MRI and a lung volume, b body volume and c lung:body volume ratio in foetuses who were delivered <32 weeks’ gestation and those who were delivered >37 weeks’ gestation. The line of best fit and 95% confidence interval is given for the control group.
Low values of both lung volume and lung:body volume ratio appeared predictive of preterm delivery with the gestation-adjusted centile being superior.
There was no significant difference in the amniotic fluid volume between the control group and foetuses that were delivered <32 weeks with intact membranes.
There was one intrapartum death at 22+0 weeks’ gestation. The mean number of days from MRI to delivery in the preterm group was 10.5 (range 0–48). The median number of days of rupture of membranes to MRI in the group that had PPROM was 4 (range 3–18). Two foetuses that were delivered >37 weeks were admitted to the neonatal unit, one for a few hours with suspected RDS and the second for 2 days with hypoglycaemia not requiring respiratory support.
There was no correlation between respiratory outcomes (length of ventilation and supplemental oxygen use) and antenatal lung:body volume ratios (Supplementary Table S1). Placental histology was available for 12 of the cases: 10 had confirmed chorioamnionitis.
Discussion
We have reported normal ranges for MRI-derived foetal lung volumes and lung:body volume ratios of foetuses 20–32 weeks’ gestation. We found a significant reduction in lung volumes in both foetuses with and without ruptured membranes that were delivered <32 weeks when standardized for body volume compared to foetuses that were delivered >37 weeks. Foetuses that were delivered preterm were globally smaller, when antenatal MRI-derived body volumes were compared with a control population that were delivered at term.
Absolute lung volumes
Previous studies have reported a reduction in absolute MRI-derived lung volumes in foetuses with PPROM in comparison with control cohorts.12,13 This finding may partly be explained by the fact that lung volume in utero also comprises of some amniotic fluid. However, our finding that a reduction in lung volume also occurs in foetuses with intact membranes is a novel one. Unlike the previous studies in foetuses with PPROM, this study is also the first to standardize the lung volume for overall foetal size.
Body volumes
Our study has demonstrated that foetuses that were delivered preterm were globally smaller on antenatal MRI imaging; however, there was no statistical difference in the birth weight centile between foetuses that were delivered preterm and those that were delivered at term.
There has been discussion as to whether reference centiles for preterm babies should be based on ‘typical’ preterm infants or on healthy growing foetuses that will be delivered at term. The World Health Organization birth weight centiles14,15 (used to evaluate birth weight centiles in this study) and the INTERGROWTH centiles16 both use information from ‘typical’ preterm deliveries. Gardosi’s customized birth weight centiles, as well as adjusting for maternal and neonatal features, use healthy term deliveries for their reference sample and then extrapolate backwards for earlier gestations.17
This study, and other studies using MRI scanning for foetal volume, allows direct measurement of body volume in foetuses that later were delivered both term and preterm. Using volume as a proxy for weight, our results contribute to the discussion on foetal reference standards noted above. Unfortunately, owing to the limitations of data capture in this study it was not possible to calculate customized birth weight centiles; however, if our results are confirmed, in a larger more ethnically representative sample, they would imply that the factors that drive spontaneous PTB may have a detrimental effect on foetal growth antenatally. This is supported by the findings of Partap et al., who undertook a prospective cohort study of nulliparous women with a singleton pregnancy assessing 3892 women at 20 and 28 weeks’ gestation using ultrasound. In all, 2.5% of women underwent a spontaneous PTB. It was noted that slow growth velocity of the femur was associated with an increased risk of spontaneous PTB.18
Lung:body volume ratios
Lung:body volumes were created in order to standardize for foetal size. The lung:body volume ratio was consistent across gestation in the control group. In foetuses that were delivered <32 weeks, lung:body volume ratios were significantly lower than in foetuses that were delivered >37 weeks gestation. This finding was consistent in foetuses with both ruptured and intact membranes. Low values of both lung volume and lung:body volume ratio appeared predictive of preterm delivery with the gestation-adjusted centile being superior.
Lung:body volume ratios have also been derived using MRI to investigate foetuses with congenital diaphragmatic hernia.19 Although 44 normal foetuses were assessed, the normative data were not explicitly reported in their paper. No studies have previously used lung:body volume ratios to investigate foetuses that were delivered preterm.
Although a major determinant of lung volume will be amniotic fluid volume, we have also demonstrated that foetuses which have intact membranes that were delivered prior to 32 weeks of gestation also have a reduction in lung:body volume ratios. We hypothesize that this may be due to cytokines implicated in the aetiology of PTB also affecting pulmonary development. Infection/inflammation is reported to be associated in pathways mediating PTB,20 common even with intact membranes. Foetal membranes express immune sensors and mount a response to microbial products with inflammatory activation,21 which can include cytokines that promote neutrophil and monocyte infiltration.22 Elevated levels of interleukin 1 (IL)-1β and IL-8 have been reported in umbilical cord blood of neonates delivered with evidence of the foetal inflammatory response.23 Eighty-three percent of cases that were delivered preterm in this study had histological evidence of chorioamnionitis, where histology was available, post-delivery.
Although a number of studies have demonstrated that exposure to intra-amniotic infection is protective for the development of RDS,24 the relationship between BPD and antenatal infection/inflammation is less well defined. In PTB, normal pulmonary development is disrupted.25 Pathology specimens of infants with BPD demonstrate a decreased number of alveoli, which are larger and more simplified in structure, and blunted pulmonary microvascular growth.26 Intrauterine infection has been shown to spread to the lung causing injury and remodelling in a sheep model27 resulting in persistent changes in lung morphometry28 and vascular development.29,30 It is therefore possible that these pathological processes are affecting foetal lung development.
Numbers in this study are currently small; however, no strong correlations were observed between antenatal lung:body volume ratios and short-term neonatal respiratory outcomes. Further studies will therefore be warranted to explore this association, increasing the sample size to confirm the findings and taking into account the multiple confounding factors perinatally as well as the varying times between the antenatal MRI and delivery. The association between markers of infection/inflammation in maternal samples, umbilical cord blood and neonatal samples with imaging findings will also need further exploration.
Conclusions
Although it should be noted that numbers in this study are small to date, it does appear that antenatal lung and body volumes are smaller in foetuses that were subsequently delivered preterm. In the future, more work is warranted to see if these findings are reproducible in a larger group of foetuses. If this is the case, the findings are novel and may suggest an antenatal aetiology of insult. This knowledge may facilitate individualization of postnatal treatment plans and provide a possible focus for future preventive therapies.
References
Saigal, S. & Doyle, L. W. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet 371, 261–269 (2008).
Maitre, N. L. et al. Respiratory consequences of prematurity: evolution of a diagnosis and development of a comprehensive approach. J. Perinatol. 35, 313–321 (2015).
Bolton, C. E., Bush, A., Hurst, J. R., Kotecha, S. & McGarvey, L. Lung consequences in adults born prematurely. Thorax 70, 574–580 (2015).
Hilgendorff, A. & O'Reilly, M. A. Bronchopulmonary dysplasia early changes leading to long-term consequences. Front. Med. (Lausanne) 2, 2 (2015).
Jobe, A. J. The new BPD: an arrest of lung development. Pediatr. Res. 46, 641–643 (1999).
Onderdonk, A. B. et al. Colonization of second-trimester placenta parenchyma. Am. J. Obstet. Gynecol. 199, 52 e51–52 e10 (2008).
Kuhrt, K. et al. Development and validation of a predictive tool for spontaneous preterm birth, incorporating quantitative fetal fibronectin, in symptomatic women. Ultrasound Obstet. Gynecol. 47, 104–109 (2015).
Kuklisova-Murgasova, M., Quaghebeur, G., Rutherford, M. A., Hajnal, J. V. & Schnabel, J. A. Reconstruction of fetal brain MRI with intensity matching and complete outlier removal. Med. Image Anal. 16, 1550–1564 (2012).
Kainz, B. et al. Fast volume reconstruction from motion corrupted stacks of 2D slices. IEEE Trans. Med. Imaging 34, 1901–1913 (2015).
Rueckert, D. et al. Nonrigid registration using free-form deformations: application to breast MR images. IEEE Trans. Med. Imaging 18, 712–721 (1999).
Royston, P. & Wright, E. M. How to construct ‘normal ranges’ for fetal variables. Ultrasound Obstet. Gynecol. 11, 30–38 (1998).
Kasprian, G. et al. [Fetal lung development on MRT. Normal course and impairment due to premature rupture of membranes]. Radiologe 46, 120–127 (2006).
Messerschmidt, A. et al. Fetal MRI for prediction of neonatal mortality following preterm premature rupture of the fetal membranes. Pediatr. Radiol. 41, 1416–1420 (2011).
Freeman, J. V. et al. Cross sectional stature and weight reference curves for the UK, 1990. Arch. Dis. Child. 73, 17–24 (1995).
Norris, T. et al. Updated birth weight centiles for England and Wales. Arch. Dis. Child. Fetal Neonatal Ed. 103, F577–F582 (2018).
Villar, J. et al. The likeness of fetal growth and newborn size across non-isolated populations in the INTERGROWTH-21st Project: the Fetal Growth Longitudinal Study and Newborn Cross-Sectional Study. Lancet Diabetes Endocrinol. 2, 781–792 (2014).
Gardosi, J., Francis, A., Turner, S. & Williams, M. Customized growth charts: rationale, validation and clinical benefits. Am. J. Obstet. Gynecol. 218, S609–S618 (2018).
Partap, U., Sovio, U. & Smith, G. C. Fetal growth and the risk of spontaneous preterm birth in a prospective cohort study of nulliparous women. Am. J. Epidemiol. 184, 110–119 (2016).
Weidner, M. et al. MRI-based ratio of fetal lung volume to fetal body volume as a new prognostic marker in congenital diaphragmatic hernia. AJR Am. J. Roentgenol. 202, 1330–1336 (2014).
Keelan, J. A. Intrauterine inflammatory activation, functional progesterone withdrawal, and the timing of term and preterm birth. J. Reprod. Immunol. 125, 89–99 (2018).
Hoang, M. et al. Human fetal membranes generate distinct cytokine profiles in response to bacterial Toll-like receptor and nod-like receptor agonists. Biol. Reprod. 90, 39 (2014).
Kim, C. J. et al. Acute chorioamnionitis and funisitis: definition, pathologic features, and clinical significance. Am. J. Obstet. Gynecol. 213, S29–S52 (2015).
Mestan, K. et al. Cord blood biomarkers of the fetal inflammatory response. J. Matern. Fetal Neonatal Med. 22, 379–387 (2009).
Kunzmann, S., Collins, J. J., Kuypers, E. & Kramer, B. W. Thrown off balance: the effect of antenatal inflammation on the developing lung and immune system. Am. J. Obstet. Gynecol. 208, 429–437 (2013).
Balany, J. & Bhandari, V. Understanding the impact of infection, inflammation, and their persistence in the pathogenesis of bronchopulmonary dysplasia. Front. Med. (Lausanne) 2, 90 (2015).
Husain, A. N., Siddiqui, N. H. & Stocker, J. T. Pathology of arrested acinar development in postsurfactant bronchopulmonary dysplasia. Hum. Pathol. 29, 710–717 (1998).
Kramer, B. W. et al. Intravenous lipopolysaccharide-induced pulmonary maturation and structural changes in fetal sheep. Am. J. Obstet. Gynecol. 200, 195 e191–110 (2009).
Willet, K. E. et al. Antenatal endotoxin and glucocorticoid effects on lung morphometry in preterm lambs. Pediatr. Res. 48, 782–788 (2000).
Kallapur, S. G. et al. Vascular changes after intra-amniotic endotoxin in preterm lamb lungs. Am. J. Physiol. Lung Cell. Mol. Physiol. 287, L1178–L1185 (2004).
Thomas, W. et al. Airway angiopoietin-2 in ventilated very preterm infants: association with prenatal factors and neonatal outcome. Pediatr. Pulmonol. 46, 777–784 (2011).
Acknowledgements
This work was undertaken with the support of patients and staff at St Thomas’s Hospital London and was supported by the Wellcome Trust IEH Award (102431), the iFIND project, Engineering and Physical Sciences Research Council, the National Institute for Health Research (Dr Lisa Story is funded by the NIHR as a Clinical Lecturer for this project. The views expressed are those of the author and not necessarily those of the NIHR or the Department of Health and Social Care), Medical Research Council, Tommy’s and the Biomedical Research Centre at Guy’s and St Thomas’ NHS Foundation Trust. Miss Amelie Spangenberg (medical student at King’s College London) assisted with analysis of the amniotic fluid volume data.
Author information
Authors and Affiliations
Contributions
L.S. and M.R. devised the study. L.S. recruited patients, analysed the data with assistance from P.T.S. and wrote the manuscript. J.A. was the research radiographer who acquired the MRI images of the preterm cohort. T.Z. developed the techniques used for lung segmentation and images and protocols were optimized by J.H. and J.V.H. J.M. assisted with the calculation of body volumes. D.P., T.D., A.G. and A.H.S. assisted with data interpretation and writing of the manuscript. J.K.S. assisted in the collection of neonatal outcome data.
Corresponding author
Ethics declarations
Competing interests
A.H.S. is the chief investigator on a number of trials funded by NIHR and charity sources related to preterm birth prediction and prevention. Hologic, Biomedical and Qiagen have provided samples for these studies. Hologic have provided funding (paid to the institution) to evaluate technical performance of their samples. No other disclosures.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Story, L., Zhang, T., Steinweg, J.K. et al. Foetal lung volumes in pregnant women who deliver very preterm: a pilot study. Pediatr Res 87, 1066–1071 (2020). https://doi.org/10.1038/s41390-019-0717-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-019-0717-9