Abstract
Background
This study evaluates the association between major neonatal morbidities and autism spectrum disorder (ASD) in children and adolescents born of very low birth weight (VLBW).
Methods
Historical cohort study using the Israel national VLBW infant database linked with the Maccabi Healthcare Services (MHS) medical records. The study cohort comprised 4963 VLBW subjects born from 1999 to 2012, >1 year of age. Multivariable logistic regression analyses were used to assess factors associated with ASD.
Results
The diagnosis of ASD was confirmed in 113 children (2.3%). Infants with major neonatal morbidities had higher rates of ASD; however, in the multivariable analyses these were not significantly associated with ASD: severe intraventricular hemorrhage (OR 1.21 [95% CI 0.60–2.45]), post-hemorrhagic hydrocephalus (OR 1.77 [0.73–4.29]), periventricular leukomalacia (OR 1.02 [0.42–2.51]), severe retinopathy of prematurity (OR 1.91 [0.995–3.67]), and bronchopulmonary dysplasia (OR 1.44 [0.84–2.45]). Postnatal steroid therapy when included separately was associated with an OR of 1.97 [1.18–3.29] for ASD. This association remained significant when postnatal steroid therapy was included with each of the neonatal morbidities (ORs ranging from 1.91 to 2.11).
Conclusions
This study suggests a significant association between postnatal steroid therapy and ASD in VLBW infants. This possible association should be considered in future studies evaluating potential risk factors for ASD in preterm infants.
Similar content being viewed by others
Introduction
The prevalence of autism spectrum disorder (ASD) has increased from about 0.7% to 1.5% in recent years.1 Children born preterm have higher risk to be diagnosed with ASD compared with term born children.1,2,3,4 A recent meta-analysis reported that the overall prevalence rate for ASD among preterm infants was 7% (95% confidence interval (CI): 4–9%).4 The highest prevalence is seen among children born preterm after 23–27 weeks’ gestation and for those with severe intrauterine growth restriction.5,6 Very low birth weight (VLBW) infants have a greater than threefold increased odds of ASD compared with the normal BW infants.6 Among very preterm newborns, physiologic derangements present in the first 12 postnatal hours as measured by the Score for Neonatal Acute Physiology were associated with dysfunction in several neurodevelopmental domains, including ASD, at 10 years of age.7 Children with ASD born before 30 weeks’ gestation or with BWs <1250 g were reported to have more cystic lesions in the cortical white matter and smaller cerebellar volumes at full term equivalent age compared with those without ASD.8
A recent meta-analysis of the environmental risk factors for ASDs revealed several conditions strongly associated with ASD including advanced parental age and birth complications associated with trauma, ischemia, or hypoxia and maternal reports of presumed cervical–vaginal infection during pregnancy.5,9 Other pregnancy-related factors such as maternal obesity, maternal diabetes, cesarean delivery, and prenatal exposure to air pollution have also been associated with an increased risk of ASD.9,10 This study, linking the Israel national VLBW infant database with the community healthcare provider’s database, aimed to evaluate the association between major neonatal morbidities and ASD in a population of children and adolescents born of VLBW.
Methods
This cohort study was performed using data collected by the Israel national VLBW infant database.11 linked with the Maccabi Healthcare Services (MHS) health maintenance organization (HMO) electronic patient medical records. Data were collected by the Israel national VLBW infant database on all newborn infants of VLBW (BW ≤1500 g) born in Israel from 1995 through 2012 as previously reported.12 All 28 neonatal units in Israel, comprising the Israel Neonatal Network, were included in data collection (Appendix). The identification numbers of all infants with VLBW born from January 1995 through December 2012 and discharged home were crosslinked to the MHS members’ database. Only infants who were registered with MHS on their discharge from the neonatal intensive care unit (NICU) following the initial birth hospitalization were included. This process created a new linked file comprising the VLBW infant database record and the MHS electronic patient record. This de-identified secure linked file served as the research file for this study. Birth hospital and patient identification subsequently remain confidential by consensus agreement of all participating centers. The study was approved by the institutional review boards of MHS (MHS 0001-2017) and of the Sheba Medical Center (SMC 1587-14).
Study population
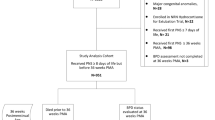
From January 1995 through December 2012, 27,434 infants of VLBW were included in the VLBW infant database, representing >99% of all infants of VLBW born alive in Israel. On discharge from the NICU, 6385 infants were registered with the MHS HMO and formed the study cohort.12 Follow-up data were collected until December 31, 2017 or until leaving MHS or until death, whichever occurred earlier. For the purpose of this study, we excluded 1317 children born prior to January 1999, since the electronic patient medical records for child development in MHS were available from this date. An additional 105 children who left MHS or died before 1 year of age (the age of possible ASD diagnosis) were excluded. The final study cohort comprised 4963 children, born from 1999 through 2012 and >1 year of age. These represent 27.8% of all 17831 VLBW infants born in Israel from 1999 through 2012 and alive at 1 year of age.
ASD diagnosis
Within the framework of the Israeli national health insurance law, children diagnosed with ASD are entitled by directive of the Ministry of Health to receive enhanced treatments.13 To be eligible, the diagnosis of ASD must meet all the following criteria: (1) the diagnosis is based on a physical, neurological, and developmental assessment conducted by a pediatric neurologist, developmental pediatrician, or a pediatric psychiatrist, (2) the diagnosis is based on meeting all required Diagnostic and Statistical Manual (DSM)—DSM-IV14 or DSM-515 criteria; and (3) an independent psychological assessment including communication, cognitive, and adaptive behavior confirms the diagnosis of ASD. Additional evaluation by speech pathologists, occupational therapists, and physiotherapists are performed as needed. Pediatric neurologists or heads of child development centers together with a social worker subsequently review the records and approve the final diagnosis applying a specific administrative code in the MHS medical records. Subsequent to the Ministry of Health directive,13 the MHS electronic patient medical records of members born from 1999 and with a documented diagnosis of ASD, which did not have an administrative code, were reviewed and the diagnosis validated. The MHS electronic patient medical records identified a total of 6734 validated ASD cases among MHS members. Among the study cohort of 4963 VLBW infants born from 1999 to 2012 and members of MHS from 1 year of age, 113 (2.3%) had a confirmed diagnosis of ASD.
Definitions
Definitions have been previously reported in detail.12 The gestational age (GA) in completed weeks was defined as the best estimate of GA based on last menstrual period, obstetric history and examination, prenatal ultrasound, or early postnatal physical examination. Small for gestational age (SGA) was defined as a BW below the 10th percentile for GA according to the sex-specific growth charts of Kramer et al.16 Delivery room resuscitation included endotracheal intubation, chest compressions, or epinephrine administration. Necrotizing enterocolitis (NEC) was diagnosed clinically and confirmed radiographically. Only definite NEC (Bell stages 2 and 3) was included. Late-onset sepsis was defined as positive microbial growth on one or more bloodstream cultures obtained after 72 h of life with accompanying clinical signs of sepsis. Bronchopulmonary dysplasia (BPD) was diagnosed according to the criteria of Bancalari et al.,17 including clinical and radiologic features, together with the requirement for oxygen supplementation at 36 weeks’ post-conceptional age. Postnatal steroid therapy included steroid therapy given only for the prevention or treatment of BPD.18 Retinopathy of prematurity (ROP) was staged according to international standard classification.19 Severe ROP included ROP grades 3 and 4 and/or ROP requiring any treatment including laser or intravitreal Avastin therapy. Intraventricular hemorrhage (IVH) was diagnosed by ultrasound examination and graded according to the classification of Papile et al.20 Periventricular leukomalacia (PVL) was diagnosed by the presence of multiple periventricular cysts identified by cranial ultrasound examination after 28 days of life. Post-hemorrhagic hydrocephalus (PHH) was recorded if severe progressive ventricular dilatation was identified by brain ultrasound examination.21 Composite neurological morbidity comprised one or more of the following severe morbidities: IVH grades 3–4, PVL, PHH, or severe ROP.
Statistical analysis
The demographic and perinatal characteristics and neonatal morbidities in children born of VLBW with and without ASD were compared by chi-square test or by Fisher’s exact test. p Values <0.05 were considered statistically significant. All tests were two sided. Multivariable logistic regression analyses with the generalized estimating equation approach were used to assess the factors independently associated with ASD. First, significant or clinically important demographic and perinatal variables were assessed. The variables included were GA, maternal diabetes mellitus, multiple births, sex, SGA, congenital malformations, delivery room resuscitation, and year of birth. Since BW and GA were closely correlated, only GA was included in the models.
Subsequently, major neonatal morbidities were introduced into the multivariable model in addition to the significant demographic and perinatal variables. These included grades 3–4 IVH, PVL, PHH, severe ROP, composite neurological morbidity, BPD, and postnatal steroid therapy. As the demographic and perinatal characteristics of infants receiving or not receiving postnatal steroid therapy differed significantly, potential confounding was accounted for by including a propensity score for postnatal steroid therapy in the multivariable analysis.22 The propensity score was calculated by logistic regression analysis including significant variables from a univariate analysis comparing the demographic and perinatal characteristics of infants receiving or not receiving postnatal steroid therapy. Results of the logistic regression analyses are presented as odds ratios (ORs) with 95% CIs. Statistical analyses were performed using the SAS statistical software Version 9.4 (SAS Institute, Inc., Cary, NC).
Results
The study population comprised 4963 children and adolescents born of VLBW. The median age of follow-up was 11.6 years (interquartile range 8.1–15.3 years) and only 170 children (3.4%) were followed for <4 years. The diagnosis of ASD was confirmed in 113 (2.3%) of the children. The demographic and perinatal characteristics of children with and without ASD are compared in the univariate analysis shown in Table 1. The children with ASD vs. no ASD were of significantly lower GA (31% vs. 20.8% <28 weeks’ gestation, p < 0.0001) and had higher rates of maternal diabetes mellitus (14.2% vs. 8.5%, p = 0.036), male (78.8% vs. 49.1%, p < 0.0001) and delivery room resuscitation (44.2% vs. 32.6%, p = 0.009)). The multivariable analyses of demographic and perinatal factors associated with ASD are shown in Table 2. The final model (model II) includes only those factors that were significant in the complete model (model I). Each week increase in GA was associated with a decrease in ASD, OR [95% CI] of 0.84 [0.77–0.91]. The perinatal factors significantly associated with an increased odds for ASD were maternal diabetes (OR 1.99 [1.14–3.48]), male (OR 3.49 [2.23–5.46], SGA (OR 2.27 [1.41–3.67]), and 2009–2012 birth epoch (OR 2.08 [1.27–3.41]).
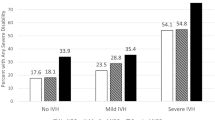
The percentages of major neonatal morbidities in children with and without ASD are compared in the univariate analysis shown in Table 3. Among the children with ASD vs. no ASD, a significantly higher percentage had late-onset sepsis (33.6% vs. 24.4%, p = 0.024), BPD (20.4% vs. 9.4%, p < 0.0001), severe ROP (13.8% vs. 5.1%, p < 0.0001), composite neurological morbidity (23.9% vs. 11.8%, p = 0.0001), and received postnatal steroid therapy (23.9% vs. 5.6%, p < 0.0001). In the multivariable models, which included the significant perinatal factors, each of these morbidities included separately was not significantly associated with ASD: late-onset sepsis OR 1.20 [95% CI, 0.79–1.83], IVH OR 1.21 [0.60–2.45], PHH 1.77 [0.73–4.29], PVL 1.02 [0.42–2.51], severe ROP 1.91 [0.995–3.67], and BPD 1.44 [0.84–2.45]. However, postnatal steroid therapy, when included separately, was associated with an OR of 1.97 [1.18–3.29] for ASD. This association remained statistically significant when postnatal steroid therapy was included with each of the aforementioned morbidities, with ORs ranging from 1.91 to 2.11. The final multivariable model (model III, Table 4), which included the significant perinatal factors, composite neurological morbidity, BPD, and postnatal steroid therapy, showed that postnatal steroid therapy was associated with an OR of 1.98 [1.15-3.43] for ASD.
The demographic and perinatal characteristics of infants receiving or not receiving postnatal steroid therapy differed significantly (Supplementary Table S1). Potential confounding was accounted for by including a propensity score for postnatal steroid therapy in the multivariable analyses. The logistic regression analysis for developing the propensity score is shown in Supplementary Table S2. Inclusion of the propensity score in the multivariable model had a very small effect on the association of postnatal steroid therapy with ASD (OR 1.94 [1.10–3.42]).
Discussion
Preterm birth has been associated with an increased risk for ASD and the incidence of ASD is inversely related to GA.9,23 Much of the increased risk for ASD has been explained by the morbidities associated with preterm birth including SGA, intracranial hemorrhage, perinatal inflammation, respiratory distress, high frequency ventilation, and more.23,24 In a large cohort of children and adolescents of VLBW, the present study identified lower GA, maternal diabetes, males, SGA, the latest birth epoch (2009–2012), and postnatal corticosteroid therapy to be independently associated with ASD. The odds for ASD in 2009–2012 were twofold higher than in 1999–2003. This may reflect changes in diagnostic practice and increasing awareness,1 as well as the earlier diagnosis of ASD. The increasing survival of less mature and sicker preterm infants may also explain the increasing rates of ASD seen in our population. Postnatal corticosteroid therapy, given for the prevention or treatment of BPD, was associated with approximately twofold higher odds for ASD after adjusting for demographic and perinatal variables and major neonatal morbidities. Furthermore, major neonatal morbidities including sepsis, BPD, severe ROP, IVH, PVL, and PHH were not significantly associated with ASD with or without the inclusion of postnatal corticosteroid therapy in the multivariable analyses. Since both the type and number of neonatal morbidities impact long-term neurodevelopmental outcome,25 we included in our models a composite outcome of neurological morbidities comprising severe ROP, IVH, PVL, or PHH. This composite adverse neurological outcome was also not significantly associated with ASD.
Although an independent association of ASD with postnatal steroid therapy has not been previously reported, a number of studies have documented higher rates of ASD among preterm infants receiving postnatal steroid therapy. Among the infants of 23–27 weeks’ gestation in the ELGAN study5 evaluated for ASD at age 10 years, children who received hydrocortisone or dexamethasone during the first postnatal month had moderately higher ASD prevalence than those who did not receive these interventions. In a study assessing early brain growth and ASD among 84 preterm infants born at <27 weeks of gestation who underwent magnetic resonance imaging assessment at term and ASD evaluation at 6.5 years, Padilla et al.26 reported that postnatal steroids were received by 6/23 (26.1%) ASD-positive children compared with 8/61 (13.1%) ASD-negative children. Sriram et al. in a study for the ELGAN investigators reported ASD rates of 13% with severe BPD and 9% with moderate BPD compared to 4% in children who did not develop BPD.27 Although the authors note that steroid therapy for BPD appears to adversely affect long-term development, this was not accounted for in their analyses. In a cohort of extremely preterm, extremely low BW infants, Doyle et al. reported that postnatal corticosteroid treatment was associated with lower cognitive scores at 5 and 18 years, whereas having BPD but not receiving corticosteroids in the newborn period had little effect on outcomes at any age.28 Similarly, extremely preterm neonates who received postnatal dexamethasone had smaller total brain tissue volumes (particularly of white matter, thalami, and basal ganglia) at adolescence than those who did not receive steroid treatment.29
Corticosteroids are well known for their effects on many brain regions and neuronal cells during non-stressful and stressful situations. These effects are mediated through corticosteroid receptors, which differ in distribution and affinity throughout the brain.30 During the perinatal period, the hypothalamic–pituitary–adrenal axis is especially susceptible to programming by glucocorticoids and glucocorticoids are important for normal maturation in the developing brain as well as many other fetal organs. Postnatal administration of glucocorticoids can, however, have harmful effects on brain development. Rat pups, during the first 2 weeks of life, show a markedly reduced adrenocortical response to stress (stress non-responsive period). In this period, low stable corticoid levels appear to be optimal for neuronal development in glucocorticoid-sensitive brain regions.31 High levels of corticoids in this period lead to precocious myelination in the amygdala and decrease brain-derived neurotrophic factor protein levels in the hippocampus and prefrontal cortex.32
Postnatal steroid therapy has been linked to a wide range of adverse neurodevelopmental outcomes in preterm infants including microcephaly, cerebral palsy, poor visual–motor integration, and cognitive impairment,33,34,35,36 and the risks and benefits of postnatal corticosteroids for the prevention or treatment of BPD remains a concern.37 Halliday recently summarized the dilemma for neonatologists and parents in the use of postnatal steroid therapy.38 There is an ongoing dilemma regarding the benefit of postnatal steroids in decreasing the rate of BPD, facilitating extubation,38 and decreasing death rate. Further questions related to the timing, dosage, type, and duration of postnatal steroid therapy as well as potential adverse effects are still to be resolved. The present cohort study adds the possibility of ASD to the range of adverse neurodevelopmental outcomes that have been reportedly associated with postnatal steroid therapy.
Several limitations should be considered when assessing the results of this study. This is an observational study and hence unknown biases and confounders may not have been accounted for. Some of the morbidities considered in this study were relatively rare, and therefore, for these morbidities our analyses might be underpowered to observe significance. In the study protocol, we a priori excluded infants aged <1 year. Although the mean age of diagnosis of ASD is about 3–4 years, it has been reported that ASD can be detected as young as 12 months of age, and earlier assessment is increasingly performed.39 A recent review indicates that screening in children aged 18–24 months can assist in early detection of ASD, consistent with current American Academy of Pediatrics’ recommendations.40 In our study cohort, only 170 children (3.4%) were followed for <4 years, and it is unlikely that the possibility of a later diagnosis of ASD would substantially influence the results of this study. The VLBW infant database only reports steroid therapy given for the treatment or prevention of BPD and does not include steroid therapy that may have been given for other indications, such as hypotension or septic shock. Shinwell et al.18 reported a decline in the use of postnatal steroids in Israel from 1997 to 2003, concomitant with reports of adverse effects associated with the treatment. Over the subsequent decade, rates remained relatively consistent with about 10% of VLBW infants exposed to the treatment. The circumstances influencing the decision to administer steroid therapy, the type of steroids and dosage used, the route of administration, or the length of treatment are not recorded and the severity of the underlying respiratory illness precipitating the initiation of steroid treatment may thus be driving the risk, rather than the treatment itself. As noted by Jobe,37 infants who have BPD tend to have more non-BPD complications of prematurity and one must be careful in attributing outcomes to the treatment rather than to the multiple adverse occurrences experienced by this population of infants. However, adjustment for major neurological morbidities and BPD did not significantly influence the association shown between postnatal steroid therapy and ASD. Furthermore, the inclusion of a propensity score for postnatal steroid therapy in the multivariable analysis also did not substantially alter the results shown. MHS, the second largest HMO in Israel, is responsible for the healthcare of about 30% of the Israeli population and, as previously reported,12 may not be reflective of the whole population of VLBW infants in Israel. The MHS members differ to some degree from the rest of the population with a higher socio-economic status and higher proportions of Jewish infants, older mothers, and infants born after infertility therapy. Despite these limitations, the national VLBW database comprises >99% of all VLBW live born infants from birth to discharge home thereby minimizing any selection bias. The database includes comprehensive information regarding pregnancy complications and antenatal and perinatal care enabling inclusion of many potential confounders in the analyses. In addition, the ASD diagnoses reported in MHS electronic patient records had been carefully validated in order to meet the appropriate diagnostic criteria.
In conclusion, this study suggests that the use of postnatal corticosteroid therapy for the prevention or treatment of BPD may be a potential factor in the reported association of an increased risk for ASD in preterm and VLBW infants. The possible association between postnatal steroids and ASD should be considered in future studies evaluating potential risk factors for ASD in preterm infants.
References
Lyall, K. et al. The changing epidemiology of autism spectrum disorders. Annu. Rev. Public Health 38, 81–102 (2017).
Xie, S. et al. Prevalence of autism spectrum disorders with and without intellectual disability by gestational age at birth in the Stockholm Youth Cohort: a register linkage study. Paediatr. Perinat. Epidemiol. 31, 586–594 (2017).
Darcy-Mahoney, A. et al. Probability of an autism diagnosis by gestational age. Newborn Infant Nurs. Rev. 16, 322–326 (2016).
Agrawal, S., Rao, S. C., Bulsara, M. K. & Patole, S. K. Prevalence of autism spectrum disorder in preterm infants: a meta-analysis. Pediatrics 142, e20180134 (2018).
Joseph, R. M. et al. Extremely low gestational age and very low birthweight for gestational age are risk factors for autism spectrum disorder in a large cohort study of 10-year-old children born at 23-27 weeks' gestation. Am. J. Obstet. Gynecol. 216, 304.e1–304.e16 (2017).
Lampi, K. M. et al. Risk of autism spectrum disorders in low birth weight and small for gestational age infants. J. Pediatr. 161, 830–836 (2012).
Logan, J. W. et al. Early postnatal illness severity scores predict neurodevelopmental impairments at 10 years of age in children born extremely preterm. J. Perinatol. 37, 606–614 (2017).
Ure, A. M. et al. Neonatal brain abnormalities associated with autism spectrum disorder in children born very preterm. Autism Res. 9, 543–552 (2016).
Modabbernia, A., Velthorst, E. & Reichenberg, A. Environmental risk factors for autism: an evidence-based review of systematic reviews and meta-analyses. Mol. Autism 17, 13 (2017).
Pagalan, L. et al. Association of prenatal exposure to air pollution with autism spectrum disorder. JAMA Pediatr. 173, 86–92 (2019).
Riskin-Mashiah, S. et al. Antenatal corticosteroid treatment in singleton, small-for-gestational-age infants born at 24-31 weeks' gestation: a population-based study. BJOG 123, 1779–1786 (2016).
Kuint, J. et al. Rehospitalization through childhood and adolescence: association with neonatal morbidities in infants of very low birth weight. J. Pediatr. 188, 135–141 (2017).
Treatments for Children with Autism, Ministry of Health. Published 2009. https://www.health.gov.il/hozer/mk03_2009.pdf. Accessed 4 Dec 2018.
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (4th Ed., Text Rev.) (APA, 2000).
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (5th Ed.) (APA, Washington, DC, 2013).
Kramer, M. S. et al. A new and improved population-based Canadian reference for birth weight for gestational age. Pediatrics 108, E35 (2001).
Bancalari, E. & Claure, N. Definitions and diagnostic criteria for bronchopulmonary dysplasia. Semin. Perinatol. 30, 164–170 (2006).
Shinwell, E. S., Lerner-Geva, L., Lusky, A. & Reichman, B. Less postnatal steroids, more bronchopulmonary dysplasia: a population-based study in very low birthweight infants. Arch. Dis. Child. Fetal Neonatal Ed. 92, 30–33 (2007).
International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity revisited. Arch. Ophthlamol. 123, 991–999 (2005).
Papile, L. A., Burstein, J., Burstein, R. & Koffler, H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1500 gm. J. Pediatr. 92, 529–534 (1978).
Levene, M. I. Measurement of the growth of the lateral ventricles in preterm infants with real-time ultrasound. Arch. Dis. Child. 56, 900–904 (1981).
Rosenbaum, P. R. & Rubin, D. B. The central role of the propensity score in observational studies for causal effects. Biometrika 70, 41–55 (1983).
Kalish, B. T., Angelidou, A. & Stewart, J. Autism spectrum disorder in preterm children. NeoReviews 18, e431 (2017).
Kuzniewicz, M. W. et al. Prevalence and neonatal factors associated with autism spectrum disorders in preterm infants. J. Pediatr. 164, 20–25 (2014).
Bassler, D. et al. Using a count of neonatal morbidities to predict poor outcome in extremely low birth weight infants: added role of neonatal infection. Pediatrics 123, 313–318 (2009).
Padilla, N. et al. Poor brain growth in extremely preterm neonates long before the onset of autism spectrum disorder symptoms. Cereb. Cortex 27, 1245–1252 (2017).
Sriram, S. et al. Cognitive development and quality of life associated with BPD in 10-year-olds born preterm. Pediatrics 141, e20172719 (2018).
Doyle, L. W. et al. Biological and social influences on outcomes of extreme-preterm/low-birth weight adolescents. Pediatrics 136, e1513–e1520 (2015).
Cheong, J. L. et al. Association between postnatal dexamethasone for treatment of bronchopulmonary dysplasia and brain volumes at adolescence in infants born very preterm. J. Pediatr. 164, 737–743 (2014).
Joëls, M. Corticosteroids and the brain. J. Endocrinol. 238, R121–R130 (2018).
Sapolsky, R. M. & Meaney, M. J. Maturation of the adrenocortical stress response: neuroendocrine control mechanisms and the stress hyporesponsive period. Brain Res. 396, 64–76 (1986).
Kikusui, T. & Mori, Y. Behavioural and neurochemical consequences of early weaning in rodents. J. Neuroendocrinol. 21, 427–431 (2009).
Yeh, T. F. et al. Early dexamethasone therapy in preterm infants: a follow-up study. Pediatrics 101, E7 (1998).
Yeh, T. F. et al. Outcomes at school age after postnatal dexamethasone therapy for lung disease of prematurity. N. Engl. J. Med. 350, 1304–1313 (2004).
Kelly, E. N. et al. Inhaled and systemic steroid exposure and neurodevelopmental outcome of preterm neonates. J. Matern. Fetal Neonatal Med. 31, 2665–2672 (2018).
ter Wolbeek, M. et al. Early life intervention with glucocorticoids has negative effects on motor development and neuropsychological function in 14-17 year-old adolescents. Psychoneuroendocrinology 38, 975–986 (2013).
Jobe, A. H. Postnatal corticosteroids for bronchopulmonary dysplasia. Clin. Perinatol. 36, 177–188 (2009).
Halliday, H. L. Postnatal steroids: still a dilemma for neonatologists and parents? Arch. Dis. Child. Fetal Neonatal Ed. 103, F500–F502 (2018).
Pierce, K., Courchesne, E. & Bacon, E. To screen or not to screen universally for autism is not the question: why the Task Force got it wrong. J. Pediatr. 176, 182–194 (2016).
Zwaigenbaum, L. et al. Early screening of autism spectrum disorder: recommendations for practice and research. Pediatrics 136(Suppl 1), S41–S59 (2015).
Acknowledgements
The Israel national very low birth weight infant database is partially funded by the Israel Centre for Disease Control and the Israel Ministry of Health.
Author contributions
D.M., J.K., and B.R.: (1) Substantial contributions to conception and design, acquisition of data, and analysis and interpretation of data; (2) Drafting the article or revising it critically for important intellectual content; (3) Final approval of the version to be published. L.L.-G.: (1) Substantial contributions to analysis and interpretation of data; (2) Final approval of the version to be published. I.Z.-P.: (1) Substantial contributions to conception and design, acquisition of data, and analysis and interpretation of data; (2) Final approval of the version to be published. R.R.S.: (1) Substantial contributions to analysis and interpretation of data; (2) Drafting the article or revising it critically for important intellectual content; (3) Final approval of the version to be published. G.C. and V.S.: (1) Substantial contributions to conception and design; (2) Final approval of the version to be published.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Members of the Israel Neonatal Network are listed in Supplementary Appendix.
Supplementary information
Rights and permissions
About this article
Cite this article
Davidovitch, M., Kuint, J., Lerner-Geva, L. et al. Postnatal steroid therapy is associated with autism spectrum disorder in children and adolescents of very low birth weight infants. Pediatr Res 87, 1045–1051 (2020). https://doi.org/10.1038/s41390-019-0700-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-019-0700-5