Abstract
Background
Mercury, lead, and cadmium are developmental neurotoxicants. We predict that preterm newborns requiring packed red blood cell (PRBC) transfusions may be exposed to neurotoxic doses. We explored the relationship between donor concentration, number of donors, number of transfusions and mercury, lead and cadmium exposure.
Methods
Single-donor PRBCs were analyzed for mercury, lead and cadmium concentration. Dose per transfusion was calculated and compared to intravenous reference doses (IVRfDs). Linear regression analyses were performed to correlate donor and infant exposure.
Results
Thirty-six infants received 268 transfusions from 94 donors. Number of donors and transfusions were significantly correlated with birthweight and gestational age. All three metals were detected in ≥95% of donor PRBCs. Number of donors was significantly associated with cumulative dose, and there was a significant correlation between mercury and lead doses/transfusion. IVRfDs were exceeded for mercury and lead in 8.6% and 38% of transfusions, respectively. None exceeded the IVRfD for cadmium. For lead, infants exposed to three donors had more transfusions exceeding IVRfD than those exposed to 1–2 donors.
Conclusions
Preterm infants are exposed to heavy metals via transfusions. Doses exceeded the IVRfDs for mercury and lead. Cadmium did not pose a risk. Prescreening donor blood could reduce exposure risk.
Similar content being viewed by others
Introduction
Anemia of prematurity occurs frequently in preterm infants. Packed red blood cell (PRBC) transfusion may be a life-saving therapy, especially in cases of severe anemia and hemorrhage.1,2 It is estimated that approximately 90% of extremely low birthweight infants (EBLW, ≤1000 g at birth) and 60% of very low birthweight infants (VLBW, ≤1500 g at birth) require at least one transfusion while hospitalized in the neonatal intensive care unit (NICU); many require multiple transfusions.1,2,3 Both the number of transfusions and the number of donors from whom these infants receive blood are inversely proportional to birthweight (BW) and gestational age (GA).
The heavy metals mercury (Hg), lead (Pb), and cadmium (Cd) are known developmental neurotoxicants.4,5,6,7,8,9,10,11 Hg and Pb are bound to hemoglobin and accumulate in red blood cells12,13 while greater than 90% of Cd is bound to albumin in plasma.14 Hg, Pb, and Cd can readily penetrate the blood−brain barrier in the fetus and preterm neonate and bioaccumulate in the developing brain.4,11,13,15 Since 1999, the annual Center for Disease Control and Prevention (CDC) National Health and Nutrition Examination Survey (NHANES 1999–2014) has documented the presence of Hg, Pb, and Cd in variable amounts in blood samples from U.S. adults.4 Therefore, preterm infants receiving PRBC transfusions utilizing adult donor blood may be exposed concurrently to variable doses to these neurotoxic metals, based on donor blood concentration.
In order to limit exposure to multiple donors, it is routine practice for VLBW infants to be assigned a dedicated single donor unit of PRBCs in the blood bank. This unit is utilized for multiple consecutive transfusions until it expires after 42 days.16,17,18 However, if a dedicated unit contains a high concentration of heavy metal, a potential risk of this practice is unintentional repetitive exposure. Thus, we hypothesize that (1) VLBW infants requiring PRBC transfusions are exposed concurrently to potentially neurotoxic doses of heavy metals, (2) Hg and Pb doses via PRBC transfusion are correlated, and (3) use of single, dedicated donors impacts exposure risk. To address these hypotheses, we measured metal concentrations in donor PRBC units, and calculated an infant dose of Hg, Pb, and Cd across multiple transfusions during their hospitalization in the NICU. While the presence of heavy metals in donor blood used for transfusions in the NICU has been reported previously, this is the first time that concomitant exposures are reported and correlated. Moreover, this is the first report of heavy metal exposure in preterm infants via PRBC transfusion in Maryland; previous studies have evaluated cohorts in Ohio, Massachusetts, and Tennessee.3,19,20 Our findings highlight the risk of heavy metal exposure from PRBC transfusion in the NICU and support prescreening of donor blood.
Methods
Subjects
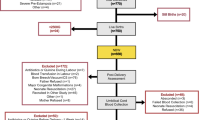
This study was conducted from October 2011 to February 2013 at two affiliated institutions, the University of Maryland Medical Center (UMMC) and Mercy Medical Center (MMC), Baltimore. The Institutional Review Board at both centers approved the study. All VLBW infants admitted to the NICU who survived to discharge were eligible. Patient charts were reviewed prospectively for demographics and transfusion data including date(s) of each transfusion, age in days, current weight, transfusion volume, and donor number linking the donor to the PRBC recipient. Per UMMC and MMC blood bank policy, infants less than 4 months of age were issued a single, dedicated donor unit of PRBCs once a transfusion was requested. Each dedicated unit was maintained specifically for that infant until it expired after 42 days per blood bank policy.
Measurements
After a transfusion was ordered for a study subject, 1 mL of donor blood was collected as a segment from the PRBC unit in the blood bank and stored at −20 °C. Following discharge, personal identifiers were destroyed, and donor blood was linked to study subject by an assigned donor number and study subject number. Donor PRBC samples were analyzed for total concentration of Hg (µg/L), Pb (µg/dL), and Cd (µg/L) at the Maryland Department of Health by inductively coupled plasma mass spectrometry (ICP-MS, Elan DRC-II, Perkin-Elmer, Waltham, MA). The lower limit of detection for all three metals was 0.1 µg/L. Accuracy and precision of metal analysis was tested using the external quality control program of the CDC (Wisconsin State Laboratory of Hygiene). Three quality control samples obtained from the CDC containing different metal concentrations were run with each batch of study samples from donor segments. For every ten study samples, one sample was spiked with Laboratory Fortified Sample Matrix as an internal standard and analyzed in duplicate.
Protocol
In order to quantify neonatal Hg, Pb, and Cd exposure from PRBC transfusions based on donor PRBC concentration, each transfusion given over less than or equal to 6 h (or per day) was considered as an intravenous (IV) dose of Hg, Pb, and Cd. Metal dose was determined by concentration in donor PRBC of Hg (µg/L), Pb (µg/dL), and Cd (µg/L) and volume transfused, and infant weight. Donor PRBC metal level was converted to a standard concentration (µg/mL), and metal exposure from each transfusion was calculated as follows: Volume transfused (mL) × PRBC metal level (µg/mL)/weight (kg) = µg/kg/day.
Calculated IV metal doses were compared to previously published reference doses (RfDs). RfD is defined as the maximum estimated daily oral dose of metal likely to be without adverse effects for adults over a lifetime.19 As intravenous reference doses (IVRfDs) for Hg, Pb, and Cd have not been characterized, we estimated IVRfDs based on oral RfDs previously cited in the literature13,20,21 and percent gastrointestinal absorption of each metal. The U.S. Environmental Protection Agency has established an oral RfD of 0.1 µg/kg/day for Hg and 1 µg/kg/day for Cd.21 There is no oral RfD for Pb, as no amount of Pb exposure is considered to be safe, especially for the developing brain. An oral RfD for Pb of 1.9 µg/kg/day was used for this study based on previous publications,20 and representing approximately half of the provisional tolerable weekly intake (PTWI) previously published by the World Health Organization.13 As 95% of oral Hg,22 10% of Pb,14 and 10% of Cd23 are absorbed from an ingested dose, IVRfDs were estimated at 0.095 µg/kg/day for Hg, 0.19 µg/kg/day for Pb, and 0.1 µg/kg/day for Cd.
Data analysis
Demographic factors, donor metal concentration, and infant doses of Hg, Pb, and Cd were compared using descriptive statistics. Linear regression analyses were performed to correlate variables such as donor metal concentration, concurrent exposure, and infant dose. Because the number of transfusions above the IVRfD for Hg, Pb, and Cd were not normally distributed, we used a nonparametric analysis of variance (Kruskall−Wallis test) followed by post-hoc Mann−Whitney tests to determine the effect of donor exposure on number of transfusions above the IVRfD. Significance was considered as P ≤ 0.05.
Results
We enrolled 45 VLBW infants from October 2011 to February 2013; 41/45 survived to discharge. While in the NICU, 36/41 (88%, 24 males, 17 females) received 268 transfusions from 94 donors. Five infants (12%) did not receive a transfusion. Transfused infants had significantly lower mean BW (963 ± 285 g vs. 1424 ± 78 g, P < 0.001) and were younger in GA (mean GA 26 ± 2.2 weeks vs. 31 ± 1 week, P < 0.001) than nontransfused infants. Both the number of transfusions and donor exposures were inversely correlated with BW and GA; these findings were significant (Fig. 1). Transfusion volume was variable, ranging from 10 to 25 mL/kg. Number of transfusions and donor exposures are further characterized in Table 1. Number of transfusions and donor exposures was greatest in 10/41 ELBW infants who were born between 23 and 29 weeks GA. These ten subjects each received more than ten transfusions during their NICU stay; four of these infants received the most transfusions in the study population, with a total of 65 transfusions from six donors per infant.
Number of transfusions and donors based on birthweight and gestational age. Linear regression analysis showed an inverse correlation between birth weight and both number of transfusions (a; R2 = 0.42, P < 0.001) and number of donors (b; R2 = 0.36, P = <0.001). Similarly, gestational age was inversely correlated with both number of transfusions (c; R2 = 0.5, P = <0.001) and number of donors (d, R2 = 0.49, P = <0.001)
Metal concentration in donor PRBCs
Metal concentrations in donor PRBCs were analyzed for 86/94 (91%) of the donor PRBC units (donor PRBC hematocrit range 55−60%). Hg and Pb were detected in all 86 donor units; Cd was detected in 82/86 donor units. Cumulative distribution of donor PRBC concentration of Hg, Pb, and Cd is shown in Fig. 2. Donor concentration was greater than or equal to the 50 percentile for Hg, Pb, and Cd reported in whole blood samples from NHANES 2011 to 2012.4 One donor unit contained 15.2 µg/L of Hg, which is the reportable level of occupational exposure for Hg in the State of Maryland.24 In this same donor unit, Pb concentration 5.5 µg/dL of Pb, exceeding the CDC reference level of concern for children.25
Median dose per transfusion and cumulative metal dose
Median doses per transfusion and cumulative dose received during NICU hospitalization for Hg, Pb, and Cd are shown in Table 2. Median dose of Pb was 0.15 µg/kg/transfusion, approaching the IVRfD of 0.19 µg/kg/dose. Median dose of Hg and Cd were 0.03 and 0.01 µg/kg/transfusion, respectively, less than the IVRfD for both metals. Median cumulative doses of Hg, Pb, and Cd across all transfusions in the NICU (μg/kg) were 0.25 (range 0.01–1.15) for Hg, 0.9 (range 0.01–4.24) for Pb, and 0.03 (range 0–0.21) for Cd.
Number of donors and metal dose
Linear regression analysis was performed to test the effect of number of donors on mean and total dose of Hg, Pb, and Cd received through transfusions. There was a significant correlation between increasing number of donors and the total dose of Hg (R2 = 0.16, P = 0.015), Pb (R2 = 0.12, P = 0.04) and Cd (R2 = 0.20, P = 0.006).
Exposure above IV reference doses
Twelve infants received transfusions that exceeded the IVRfD for Hg. Each of these infants received a median of 2 (range 1–5) transfusions greater than the IVRfD for Hg. These transfusions were from 1 of their dedicated donor units. Overall, doses were greater than the IVRfD for Hg in 29/268 transfusions (10.6%, median dose 0.12 µg/kg/day, range 0.095–0.3 µg/kg/day) from 12 donors. In addition, 5/268 (1.9%) of transfusions contained greater than twice the estimated IVRfD for Hg, and 25/268 (9%) contained greater than twice the IVRfD for Pb. For Pb, 6, 2, and 3 transfusions contained greater than 3, 4, and 5 times the IVRfD, respectively (0.6–1.11 µg/kg/day).
Twenty-six infants received transfusions with doses greater than the IVRfD for Pb. Each of the 26 infants received a median of three (range 1–11) transfusions greater than the IVRfD for Pb from a median of 2 (range 1–4) donors per subject. One infant received 11 transfusions from three dedicated single donors (5, 4, and 2 transfusions per donor) with all doses greater than the IVRfD. Overall, doses were greater than the IVRfD for Pb in 110/268 transfusions (41 percent, median 0.27, range 0.19–1.11 µg/kg/day) from 41 donors.
Concurrent exposure to Hg and Pb above the IVRfD occurred in 19/268 (7%) of transfusions from eight donors. None of the transfusions exceeded the estimated IVRfD for Cd. Figure 3 depicts a linear regression comparing concurrent doses of Hg and Pb (µg/kg/day) from each PRBC transfusion. There was a significant correlation between Hg and Pb doses/transfusion (F = 78, P = <0.0001, R2 = 0.23). Transfusions containing a higher dose of Hg were correlated with concurrent exposure to Pb.
Concurrent Hg and Pb dose in a single transfusion. Each dot represents Hg and Pb dose (µg/kg/day) in a single (n = 268) PRBC transfusion. Linear regression analysis demonstrates a significant correlation between Hg and Pb dose per transfusion (F = 78, P = <0.0001, R2 = 0.23). Transfusions containing a higher dose of Hg were positively correlated with Pb dose
Figure 4 depicts the number of transfusions above the RfD for Pb in infants exposed to 1, 2, 3 or ≥4 donors. Kruskall−Wallis test demonstrated a significant effect of donor number on the number of transfusions greater than the RfD for Pb (c2 = 10.8, d.f. = 3, P = 0.013). Post-hoc Mann−Whitney tests showed that infants exposed to three donors had a significantly greater number of transfusions above the IVRfD than the group receiving transfusions from either one (MW-U = 8.5, Z = 2.6, P = 0.009) or two donors (MW-U = 9.5, Z = 2.7, P = 0.008). There was no significant difference between infants with 3 or ≥4 donors (MW-U = 16.5, Z = 1.3, P = 0.18). While a trend was observed between infants who received transfusions from ≥4 donors and those receiving transfusions from one donor (MW-U = 21, Z = 1.7, P = 0.09) or two donors (MW-U = 23.5, Z = 1.7, P = 0.085), the difference in number of transfusions above the RfD for Pb was not significant. Of note, a limitation with analysis of the effect of donor exposure is the direct correlation with number of transfusions (R2 = 0.52, P < 0.0001), making it difficult to separate these two variables.
Number of transfusions > IV RfD for Pb in infants exposed to 1, 2, 3, or ≥4 donors. An individual dot represents the number of transfusions above the RfD each infant received. The gray bar represents the group median number of donors. Kruskall−Wallis test demonstrated a significant effect of donor number on the number of transfusions greater than the RfD for Pb (c2 = 10.8, d.f. = 3, P = 0.013). Post-hoc Mann−Whitney tests showed that infants exposed to three donors had a significantly greater number of transfusions above the RfD than the group receiving transfusions from either one (MW-U = 8.5, Z = 2.6, P = 0.009) or two donors (MW-U = 9.5, Z = 2.7, P = 0.008)
Discussion
Our paper is the first to characterize concurrent exposure to Hg, Pb, and Cd via PRBC transfusion in an urban Maryland Level IV NICU and to describe the relationship between Hg, Pb, and Cd exposure in preterm infants via dedicated donor transfusions. We present three novel findings: (1) Hg and Pb doses from donor PRBCs are highly and positively correlated; (2) Cd exposure via red blood cell transfusion is negligible and (3) increasing number of donors is positively associated with Hg and Pb dose via PRBC transfusion.
Hg, Pb, and Cd are potent teratogens and neurotoxins. While Hg and Pb are lipid-soluble and readily cross the placenta, placental permeability to Cd is variable.11,26,27 Despite reduced placental transport of Cd, studies have reported the presence of all three metals in cord blood.11,26,27 Organic Hg (methyl Hg) comprises 70–95% of Hg in blood.28 The fetus is highly vulnerable to the neurotoxic effects of methyl Hg; fetal exposure is associated with microcephaly, seizures, and cognitive and sensory impairment.5,11,12 Pb is especially neurotoxic; any exposure is potentially detrimental to the developing nervous system of the fetus and young child.4,5,7,29 Known effects include impaired cognition, intelligence, and executive functioning; poor school performance; diagnosis with ADHD; and other social and neurobehavioral issues.6,7,8,26,29 Recent evidence links Pb exposure early in life to CNS dysfunction in adulthood, including risk of Alzheimer’s disease.30 Cd bioaccumulates in air, soil, and water, and is found in cigarette smoke.27 Perinatal exposure has been associated with cognitive impairment, learning disabilities, and increased anxiety.10,26,31
PRBC transfusions represent a potential source of exposure to Hg, Pb, and Cd for VLBW infants. The preterm infant is susceptible to the effects of these neurotoxicants during this critical stage of brain growth and neurodevelopment.5,13 While reference doses and population-based means have been established for older children and adults, safe levels of exposure in the VLBW infant are unknown. Premature infants have impaired capacity to metabolize and excrete toxicants due to immature and developing metabolic processes.32,33 Therefore, reference doses deemed safe for older children and adults likely underestimate risks of neurotoxicity in this population.
Donor blood represents a potential source of exposure to other toxicants that could be detrimental to preterm infants. Nicotine is a known fetal neurotoxicant; active smoking during pregnancy has been linked to postnatal cognitive impairment, anxiety, depression, ADHD, and conduct disorders.34 Blood banks are not required to screen potential donors for tobacco use. The presence of nicotine and metabolites such as cotinine has been documented in donor blood.35 Additionally, drugs of abuse have also been found in donor blood; the presence of opiates, benzodiazepines, stimulants, barbiturates, and marijuana in banked donor blood has been reported.36,37
In our study, the number of RBC transfusions and donor exposures were substantial, inversely proportional to BW and GA, and similar to previously reported transfusion rates.2 As seen in our cohort, most PRBC transfusions occur in the first weeks of life, a critical time for neurodevelopment in the fetus and VLBW infant.38 All 86 donor units contained Hg and Pb, while 95% contained detectable levels of Cd. Donor PRBC utilized in this study were obtained from the local American Red Cross. The donors represented in this study were residents of watershed regions of the Chesapeake Bay, a region in which fish consumption is high and could impact Hg levels in donor blood. To our knowledge, regional background heavy metal blood levels in adults are not documented. However, blood Pb levels for children are monitored and reported annually by the Maryland Department of the Environment.39 In 2017, blood lead levels from 131,832 children 0–72 months of age were ≥10 µg/dL in 388 children. Levels of 5–9 µg/dL and ≥5 µg/dL were found in 1661 and 2049 children, respectively.39
Both Hg and Pb bind to hemoglobin and accumulate in PRBCs.12,13,18 Cd, however, is bound to albumin and is found in higher concentration in plasma.14 Thus, it is anticipated that Hg and Pb concentrations would typically be the same or greater in PRBC samples than whole blood, while Cd concentration would be lower. Gehrie et al. tested PRBC Pb concentration as compared to Pb found in whole blood and plasma, and found that 90% of premeasured whole blood Pb remained compartmentalized in PRBC, while 10% had equilibrated with plasma supernatant.3,18 In our study, 9% and 37% of transfusions exceeded estimated IVRfDs for Hg and Pb, respectively; 2% and 9% of transfusions contained at least twice the estimated IVRfD for Hg and Pb. None of the 268 transfusions were greater than the estimated IVRfD for Cd, suggesting that PRBC transfusion may not represent a significant source of exposure to Cd.
Doses concurrently exceeding the IVRfD for both Hg and Pb occurred in 7% of transfusions from eight donors. One donor unit contained substantial levels of both Hg (15.2 µg/L) and Pb (5.5 µg/dL). This unit was used for a total of two transfusions to a single infant (29 weeks GA, 980 g at birth). A growing body of evidence supports the deleterious effect of simultaneous exposure to neurotoxicants, even at concentrations lower than known to cause toxicity individually.26,40 Data regarding co-exposure to neurotoxic heavy metals is limited, but preliminary evidence supports enhanced neurotoxicity in infants and children when prenatal Pb exposure occurs simultaneously with both Hg and Cd, and other neurotoxic metals such as manganese and arsenic.26,40
The benefits of dedicated, single-donor units for preterm infants include reduced incidence of transfusion-transmitted infections and antibody formation that could lead to hemolytic transfusion reactions.16,18 The use of dedicated donor units is advocated by guidelines describing best practice in neonatal transfusion.16,18 However, donor blood is not currently screened for environmental toxicants such as heavy metals. As transfusion rates remain high in ELBW and VLBW infants, multiple transfusions from a dedicated donor unit represents a potential source of recurrent exposure to neurotoxic heavy metals. Gehrie et al. recommended a Pb threshold of 1 μg/dL in donor PRBC units targeted for pediatric transfusions and suggested that testing for metals in whole blood could occur via sensitive methods such as ICP-MS in reference laboratories, concurrent with testing for infectious diseases, prior to distribution to hospitals.18 Our results suggest that donor units should also be screened for Hg concentration.
Preterm infants are exposed to Hg, Pb, and Cd via PRBC transfusions. These exposures may impact the neurodevelopment preterm infants who require transfusions in the NICU. We found that donor concentration correlated with infant doses that exceeded the estimated IVRfD for Hg and Pb, heavy metals that are known to accumulate in the RBC. The common practice of dedicated donor units for VLBW infants may lead to repetitive and frequent doses of Hg, Pb, and Cd at variable concentration, depending on number of transfusions per donor and donor concentration.
Safe levels of exposure to neurotoxic heavy metals in the VLBW infant are unknown but are likely much lower than reference doses established for older children and adults. Simultaneous exposure to Hg, Pb, and Cd may decrease thresholds for neurotoxicity in this population, and further investigation of the role of coexposure is warranted. Prescreening of donor blood for heavy metals could eliminate those units with excessive concentrations of neurotoxic metals that may impact neurodevelopment.
A limitation to our study is the lack of post-transfusion data from study subjects to correlate the estimated exposure from donor blood with infant serum and/or urinary heavy metal level. In addition, we did not follow this cohort of VLBW infants to determine long-term neurodevelopmental outcome. Investigation of the short- and long-term impact of exposure to Hg and Pb via donor blood in this vulnerable population will be the focus of future research.
References
Colombatti, R., Sainati, L. & Trevisanuto, D. Anemia and transfusion in the neonate. Semin. Fetal Neonatal Med. 21, 2–9 (2016).
Carroll, P. D. & Widness, J. A. Nonpharmacological, blood conservation techniques for preventing neonatal anemia- effective and promising strategies for reducing transfusion. Semin. Perinatol. 36, 232–243 (2012).
Bearer, C. F., O’Riordan, M. A. & Powers, R. Lead exposure from blood transfusion to premature infants. J. Pediatr. 137, 549–554 (2000).
U.S. EPA Report on the Environment: Blood mercury, lead and cadmium level, NHANES 1999−2014 (Mercury found at https://cfpub.epa.gov/roe/indicator.cfm?i=64, Lead found at https://cfpub.epa.gov/roe/indicator.cfm?i=63. Cadmium found at https://cfpub.epa.gov/roe/indicator.cfm?i=61).
Davidson, P. W., Myers, G. J. & Weiss, B. Mercury exposure and child development outcomes. Pediatrics 113(Suppl 3), 1023–1029 (2004).
Lanphear, B. P., Dietrich, K., Auinger, P. & Cox, C. Cognitive deficits associated with blood lead concentrations< 10 micrograms/dL in U.S. children and adolescents. Public Health Rep. 115, 521–529 (2000).
Bellinger, D. C. Very low lead exposures and children's neurodevelopment. Curr. Opin. Pediatr. 20, 172–177 (2008).
Canfield, R. L. et al. Intellectual Impairment in Children with Blood Lead Concentrations below 10 µg per Deciliter. N. Engl. J. Med. 348, 1517–1526 (2003).
Malin, A. J. & Wright, R. O. The developmental neurotoxicity of cadmium. In Handbook of Developmental Neurotoxicology 2nd edn (eds Slikker, W., Paule, M. G., Wang, C.) 407−412 (Elsevier, Philadelphia, PA, 2018).
Ciesielski, T. et al. Cadmium exposure and neurodevelopmental outcomes in US children. Environ. Health Perspecs. 120, 758 (2012).
Karagas, M. R. et al. Evidence on the human health effects of low-level methylmercury exposure. Environ. Health Perspecs. 120, 799–806 (2012).
Shih, G., Quilliam, D. N., Morton, J. & Magee, S. R. Mercury, lead, and cadmium in umbilical cord blood. J. Environ. Health 75, 38–43 (2013).
World Health Organization. Lead in drinking water. http://www.who.int/water_sanitation_health/dwq/chemicals/lead.pdf (2011).
Liu, Y., Chen, M., Jiang, L. & Song, L. New insight into molecular interaction of heavy metal pollutant cadmium (II) with human serum albumin. Environ. Sci. Polutl. Res 21, 6994–7005 (2014).
Rai, A., Maurya, S. K., Khare, P., Shrivastava, A. & Bandyopadhyay, S. Characterization of developmental neurotoxicity of As, Cd and Pb mixture: synergistic action of metal mixture in glial and neuronal functions. Toxicol. Sci. 118, 586–601 (2010).
Fergusson, D. A. et al. Effect of fresh red blood cell transfusions on clinical outcomes in premature, very low-birth-weight infants: the ARIPI randomized trial. JAMA 308, 1443–1451 (2012).
Cushing, M. M. et al. Transfusion practices and infections at Four Level III Neonatal Intensive Care Units. Blood 122, 3657 (2013).
Gehrie, E. et al. Primary prevention of pediatric lead exposure requires new approaches to transfusion screening. J. Pediatr. 163, 855–859 (2013).
Zubairi, H., Visintainer, P., Fleming, J., Richardson, M. & Singh, R. Lead exposure in preterm infants receiving red blood cell transfusions. Pediatr. Res. 77, 814 (2015).
Elabiad, M. T. & Hook, R. E. Lead content of blood transfusions for extremely low-birth-weight infants. Am. J. Perinatol. 30, 765–770 (2013).
U.S. EPA National Center for Environmental Assessment. Chemical Assessment Summary Integrated Risk Information System (IRIS) (Methyl Hg found at https://cfpub.epa.gov/ncea/iris/iris_documents/documents/subst/0073_summary.pdf. Pb found at https://cfpub.epa.gov/ncea/iris/iris_documents/documents/subst/0277_summary.pdf, Cd at https://cfpub.epa.gov/ncea/iris/iris_documents/documents/subst/0141_summary.pdf (2001).
CDC National Biomonitoring Program: Monitoring Summary, Mercury http://www.cdc.gov/biomonitoring/Mercury_BiomonitoringSummary.html (2013).
Health Risk Assessment Guide for Metals Gastrointestinal Uptake and Absorption, and Catalogue of Toxicokinetic Models https://www.icmm.com/document/264 (2007).
Maryland Department of Mental Health and Hygiene. Confidential report of occupational disease and injury. https://phpa.health.maryland.gov/OEHFP/EH/Shared%20Documents/Occupational%20Reporting%20Form%20MD_2016.pdf (2017).
Center for Disease Control. Lead, update on blood lead levels in children http://www.cdc.gov/nceh/lead/ACCLPP/blood_lead_levels.htm. (2016).
Kim, Y. et al. Prenatal lead and cadmium co-exposure and infant neurodevelopment at 6 months of age: The Mothers and Children's Environmental Health (MOCEH) study. Neurotoxicology 35, 15–22 (2013).
García-Esquinas, E. et al. Lead, mercury and cadmium in umbilical cord blood and its association with parental epidemiological variables and birth factors. BMC Public Health 13, 1 (2013).
Mortensen, M. E., Caudill, S. P., Caldwell, K. L., Ward, C. D. & Jones, R. L. Total and methyl mercury in whole blood measured for the first time in the U.S. population: NHANES 2011-2012. Environ. Res. 134, 257–264 (2014).
Chiodo, L. M., Jacobson, S. W. & Jacobson, J. L. Neurodevelopmental effects of postnatal lead exposure at very low levels. Neurotoxicol. Teratol. 26, 359–371 (2004).
Bellinger, D. C. Prenatal exposures to environmental chemicals and children’s neurodevelopment: an update. Saf. Health Work 4, 1−11 (2013).
Kippler, M., et al. Impact of prenatal exposure to cadmium on cognitive development at preschool age and the importance of selenium and iodine. Eur. J. Epidemiol. 31, 1123−1134 (2016).
Milsap, R. L. & Jusko, W. J. Pharmacokinetics in the infant. Environ. Health Perspect. 102(Suppl. 11), 107–110 (1994).
McClary, J. D. Principles of drug use in the fetus and neonate. In Fanaroff and Martin’s Neonatal Perinatal Medicine 10th edn (eds Martin, R. J. et al.) 654−659 (Elsevier Saunders, Philadelphia, PA, 2015).
Falck, A. J., Mooney, S. & Bearer, C. F. Adverse exposures to the fetus. In Fanaroff and Martin’s Neonatal Perinatal Medicine 11 edn (eds Martin, R. J. et al.) 246−247 (Elsevier Saunders, Philadelphia, PA, 2019).
Wiencek, J. R. et al. Detection of nicotine and nicotine metabolites in units of banked blood. Am. J. Clin. Pathol. 151, 516–521 (2019).
Gehrie, E. A., Keiser, A., Haglock-Adler, C. J., Strathmann, F. & Booth, G. S. Detecting pharmaceuticals in the red blood cell inventory of a hospital blood bank. J. Pediatr. 189, 227–231 (2017).
Booth, G. S. & Gehrie, E. A. Implications of legalized recreational marijuana on the United States blood supply. Transfusion 54, 1903–1904 (2014).
Faustman, E. M., Sibernagel, S. M., Fenske, R. A., Burbacher, T. M. & Ponce, R. A. Mechanisms underlying children’s susceptibility to environmental toxicants. Environ. Health Perspect. 108(Suppl 1), 13–21 (2000).
Maryland Department of the Environment Lead Poisoning Prevention Program. Maryland Childhood Blood Lead Surveillance Calendar Year 2017, Annual Report. mde.maryland.gov/programs/LAND/Documents/LeadReports/LeadReportsAnnualChildhoodLeadRegistry/LeadReportCLR2017.pdf (2018).
Henn, B. C., Coull, B. A. & Wright, R. O. Chemical mixtures and children’s health. Curr. Opin. Pediatr. 26, 223 (2014).
Acknowledgements
We gratefully acknowledge the contribution of the late Dr. Prince A.K. Kassim for assistance with sample analysis. This study was supported by a grant from the Gerber Foundation.
Author information
Authors and Affiliations
Contributions
A.J.F.: data analysis and interpretation, drafting the article, critical revisions, final approval. A.E.M.: data analysis and interpretation, drafting the article, critical revisions, final approval. J.C.-O. acquisition of data, critical revisions, final approval. D.E.-M.: acquisition of data, critical revisions, final approval. C.F.B.: conception and design, acquisition of data, analysis and interpretation of data, critical revisions, final approval of the version to be published.
Corresponding author
Ethics declarations
Competing interests
A.J.F., A.E.M., J.C.-O., and D.E.-M. have no competing interests. C.F.B. is Editor-in-Chief of Pediatric Research.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Falck, A.J., Medina, A.E., Cummins-Oman, J. et al. Mercury, lead, and cadmium exposure via red blood cell transfusions in preterm infants. Pediatr Res 87, 677–682 (2020). https://doi.org/10.1038/s41390-019-0635-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-019-0635-x
This article is cited by
-
New frontiers in neonatal red blood cell transfusion research
Journal of Perinatology (2023)
-
Heavy metals from donor blood and breast milk products in the NICU
Pediatric Research (2022)