Abstract
Background
Intraventricular hemorrhage (IVH) and post-hemorrhagic hydrocephalus (PHHC) remain major problems among premature infants. The need, timing and type of ventricular drainage are based on sonographic ventricular measures, without assessment of the dimensions of the frontal lobe. The aim of our study was to establish new reference values for sonographic frontal lobe cortico-ventricular thickness (FL-CVT) in a large cohort of infants.
Methods
All normal head ultrasound scans that were performed in our center during the first 4 days of life between January 2014 and December 2016 were retrospectively evaluated.
Results
Scans were evaluated and plotted to create a reference range for the thickness of the frontal lobe in normal infants of 24–40 weeks’ gestation. The FL-CVT increased significantly during gestation. Calculating the area under the curve of the FL-CVT in 9 infants with post-hemorrhagic-hydrocephalus (PHHC) reveals a 20% mean loss of FL-CVT. The impact of increasing ventricular dilatation and of the various ventricular drainage procedures on the frontal lobe growth were described in two infants demonstrating the potential clinical value of this tool.
Conclusions
Head ultrasound provides a simple, non-invasive method for measuring the thickness of the frontal lobe, which grows significantly between 24 and 40 weeks’ gestation. In premature infants with PHHC, we suggest the use of the FL-CVT measure, in addition to ventricular size measures, as a direct assessment of the impact of the enlarged ventricles on the surrounding brain parenchyma. This could assist in the management of PHHC and determine the need and optimal timing for intervention.
Similar content being viewed by others
Introduction
Intraventricular hemorrhage (IVH) and post-hemorrhagic hydrocephalus (PHHC) are still major problems among premature infants. IVH occurs in 33% of premature infants with birth weight <1000 g and is associated with neurodevelopmental impairment. Compared to infants without IVH, those with IVH grades 3–4 have a twofold higher rate of neurodevelopmental impairment. About a fourth of infants with high-grade IVH require a shunt insertion, with a markedly higher risk for adverse neurodevelopmental outcome compared to infants with IVH who did not require a shunt insertion.1
In the late 1990s, Hüppi and colleagues2 reported a magnetic resonance imaging (MRI) volumetric brain segmentation technique to document maturational changes in brain tissue volumes among premature infants. The results showed a fourfold increase in cortical gray matter and a fivefold increase in myelinated white matter from 29 to 41 weeks of postconceptional age. Subsequently, several studies have demonstrated regional volume alterations at term equivalent age following a premature birth.3,4,5,6 Volumetric MRI studies among premature infants with PHHC showed impaired total cerebral, thalamic, and particularly cerebellar growth and revealed a correlation between these brain volumes and the severity of mental and motor development.7
Although MRI can provide three-dimensional volumetric data of the frontal lobe (FL), head ultrasound (HUS) is still the modality of choice for the diagnosis and follow-up of infants with IVH accompanied with PHHC. Levene8 in 1981 was the first to report reference sonographic values of the neonatal ventricular dimensions, termed the ventricular index (VI). VI and other ventricular measures are currently used for the diagnosis of PHHC and evaluation of the need for intervention.9,10,11 Sonographic follow-up of the FL growth was first described in the fetus by Persutte et al.12 who assessed its correlation with fetal aneuploidy.
We hypothesized that monitoring frontal lobe cortico-ventricular thickness (FL-CVT) in infants with PHHC may provide crucial information regarding the impact of the enlarged ventricles on the surrounding brain parenchyma and might potentially be helpful in assessing the need and the optimal timing for intervention. The aims of the present study were: (a) to establish reference values for sonographic FL-CVT measurements in a large cohort of infants born at 24–40 weeks’ gestation and (b) to use the reference values among infants with PHHC and assess the length of time and extent of reduction of FV-CVL size due to surrounding pressure.
Patients and methods
Subjects
This is a retrospective study performed in the Neonatal Intensive Care Unit (NICU), Rambam Health Care Campus, Haifa, Israel. The study was approved by the Institutional Helsinki Committee of our center.
All infants admitted to our NICU between January 2014 and December 2016 and had a HUS done on the first 4 days of life were considered for inclusion in the study. Excluded were infants with congenital central nervous system (CNS) or cranial malformations, chromosomal abnormalities, intraventricular or intracranial hemorrhage, severe perinatal asphyxia, CNS infection, or head circumference (HC) <3rd percentile or >97th percentile. Growth percentiles were determined according to Fenton’s Fetal–Infant Growth Chart for premature infants.13
US scans and measurements
In our NICU, HUS scans are performed by an US technician and evaluated by a single senior pediatric radiologist. All scans were undertaken with the infant supine and the head in a mid-line position. Scans were performed with a Philips HD11 scanner (Philips, Bothell, WA, USA) with a 5–8 MHz curvilinear sector probe. The charts of nine infants who were born during the study period and developed PHHC (defined in infants with IVH who developed clinical signs of increased intracranial pressure and progressively enlarged ventricles on HUS) were reviewed for demographic data and HC measurements.
Ventricular parameters
Ventricular measurements were done in the coronal view via the anterior fontanelle at the level of the foramen of Monro.
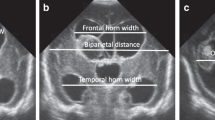
FL-CVT was measured from the frontal horn of the lateral ventricle up to the cortex. A straight line, parallel to the anterior falx, was drawn, starting at the point where the lateral border of the corpus collosum is adjacent to the lateral ventricle, ending at the cortex border on the same side (Fig. 1). VI, defined as the distance between the falx and the lateral wall of the anterior horn in the coronal plane, was also measured.8
Statistical analysis
SPSS (Statistics Products Solutions Services) version 25.0 software for Windows (IBM Corp. Armonk, NY, USA) was used to tabulate detailed summary statistics, such as means and standard deviations, and plot a nomogram of the 3rd, 50th, and 97th percentiles for the FL-CVT measurements by gestational age (GA). Pearson correlation was used to determine the relationship between GA and FL-CVT. For the nine infants with PHHC, we calculated both the AUC (area under the curve) of the deviated curve and that of the 50th nomogram percentile and compared them using t test. As for the error estimation, we used the root mean square error (RMSE) measure that gives the RMSEs of FL-CVT from the normal.
Intra- and inter-observer reliability
The intra- and inter-observer reliability was assessed in 15 infants. To evaluate the intra-observer variability, the FL-CVT measurements were repeated three times by the same observer. The inter-observer reliability was evaluated by repeating the measurements for each infant by a second senior pediatric radiologist, who was unaware of the first observer’s data.
The inter- and intra-observer variability was assessed using intra-class correlation coefficient (ICC). The scale of Brennan and Silman was used for interpretation.
Results
The HUS scans of 418 healthy infants were plotted. The infants’ enrollment and GAs are described in Fig. 2. Mean (±SD) age at HUS was (2.5 ± 1.1) days. The nomogram of the 5th, 50th, and 95th percentiles of the FL-CVT measurements by GA is shown in Fig. 3. FL thickness increased significantly with GA (y = 0.62x, R2 = 0.996, p < 0.0001). The ICC was calculated and the strength of agreement scale of Brennan and Silman was used for interpretation. The ICC was very good (0.988) suggesting that all these measurements are readily reproducible with minimal inter- and intra-observer variability.
Frontal lobe cortico-ventricular thickness (FL-CVT) reference ranges. Reference curve for the FL-CVT in infants between 24 and 40 weeks of gestation (n = 418). Presented are the 3rd, 50th, and 97th percentiles (y = 0.62x, R2 = 0.966). FL-CVT frontal lobe cortico-ventricular thickness, GA gestational age
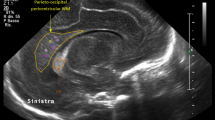
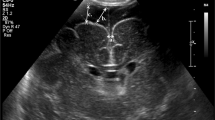
Figure 4a, b demonstrates the use of the FL-CVT nomogram in two infants who developed IVH with PHHC. Their FL-CVT and VI measurements are plotted on the nomogram, as well as their HC and ventricular drainage interventions. In both infants, the change of FL-CVT (initially decreasing and then increasing) is in general a mirror image of the VI (increasing and then decreasing) with a few noteworthy exceptions. In infant A, the second lumbar puncture (LP), which did not influence the VI, resulted in an increase in the FL thickness. In infant B, the VI decreased after the ventricular tap while the FL-CVT increased only later, after the insertion of a ventriculoperitoneal shunt.
Examples. Two examples of infants with post-hemorrhagic hydrocephalus- a and b. 3rd, 50th, and 97th percentiles of head circumference (HC), frontal lobe cortico-ventricular thickness (FL-CVT), and ventricular index (VI) are plotted as well as timing of different drainage procedures. AAUC and BAUC area under the FL-CVT 3rd percentile curve (hatched area), representing the period in which the frontal lobe thickness was below the 3rd percentile for gestational age. LP lumbar puncture, VP shunt ventriculoperitoneal shunt
AAUC and BAUC demonstrate the area under the FL-CVT curve in these two infants. The AUC provides an estimation of growth deceleration, namely, its extent and duration during the period of time in which the FL-CVT is below the third percentile.
Nine infants with IVH developed PHHC (mean GA at birth 27.5 ± 1.3 weeks), which led to deceleration of FL-CVT growth until drainage of ventricles was undertaken (Supplementary Table 1).
For each infant with PHHC, we determined the closest fitting equation that describes the FL-CVT curve (third-degree polynomial equations) and then calculated the AUC of the FL-CVT measurements of both the patient and the 50th percentile of our nomogram for the same period. Subtraction of these two AUCs showed an approximately 20% reduction of mean FL-CVT AUC from 176.3 ± 55 to 143 ± 40.6 mm2 in the infants with PHHC (p = 0.14). The AUC difference takes into consideration both the magnitude and duration of FL-CVT deviation from normal. Three infants displayed bi-phasic curves: short deceleration period followed by rapid recovery and thus their FL-CVT AUC values were smaller. The RMSE measure of FL-CVT from the normal was 4.34 ± 2.07 (range 1.09–7.80).
We expected that, in PHHC, the HC initially enlarges in order to absorb the increasing pressure within the brain. In five of the nine PHHC, the HC percentile did not change.
Discussion
IVH and PHHC remain major problems among premature infants. The need, timing, and type of ventricular drainage are based on sonographic ventricular measures, without assessment of the dimensions of the FL. We provide new sonographic dimension of FL-CVT in 418 healthy infants born at 24–40 weeks’ gestation. This allows assessing the impact of PHHC on FL growth.
The gradual increase in the FL thickness shown in our nomogram is consistent with the results from Mewes et al.3 using MRIs and showing a 1.5-fold increase of FL cerebral parenchyma growth between 32 and 42 weeks’ gestation.
While MRI might be more accurate, HUS remains a useful modality for follow-up and can be performed in three ways: uni-dimensional, such as for VI and FL-CVT measurements; two-dimensional as reported by Makhoul et al.14 who used pre-defined triangular area containing most of the FL and showed that the triangular area increased significantly during gestation and was strongly correlated with HC; two studies have shown promising results using three-dimensional HUS for evaluation and follow-up of the ventricular size in infants with IVH.15,16
Severe IVH is a risk factor for poor neurodevelopmental outcome, and accompanying PHHC that requires shunt insertion further increases the risk for impaired neurodevelopmental outcome.1 Suggested mechanisms for such a worse outcome include the delayed formation of myelin,17 reduced cerebral perfusion and oxygenation,18 and inflammatory process.19 Ventricular drainage has been shown to improve cerebral hemodynamics and oxygenation and to allow volume expansion adjacent to the lateral ventricles and in the temporal and frontal regions.18,20,21 The optimal timing and method of intervention are still both unsettled. A survey among 32 neonatal centers in 17 European countries revealed considerable variation in the diagnostic and therapeutic approaches to infants with PHHC.22 In the ELVIS Trial,23 ventricular measures were used to compare low versus high thresholds for intervention in premature infants with PHHC. No significant differences in the composite main outcome of VP shunt insertion or death were found between the groups. Data regarding the neurodevelopmental outcomes have not yet been reported.
Adding the FL thickness as a direct measure of the periventricular parenchymal status to the current routine assessment of ventricular size might be more informative and could better predict outcome. Plotting a FL-CVT curve on our nomogram in infants who developed PHHC, we demonstrated two phases: first, a concomitant increase of VI and a decrease of FL-CVT, reflecting the extra pressure on the FL by the enlarging ventricles. Second, a nadir turning point, occurring after various drainage procedures, and including a decrease in VI and increase in the FL thickness. Though the FL-CVT was mostly a mirror image of the VI, the different response to the drainage procedures demonstrates the added value of FL-CVT monitoring. We speculate that the use of the FL-CVT nomogram in infants with PHHC may be helpful in determining the optimal timing for intervention so that additional weeks of excess pressure on the FLs may be avoided.
During the study period, our policy regarding the timing of intervention was based on the increase in HC, anterior fontanelle tension, and VI. When intervention was indicated, LP was the first step, followed by a reservoir insertion (an insertion of reservoir) in cases of a “dry” tap suggesting a non-communicating hydrocephalus, or the need for frequent LPs. Prior to conducting this study, we assumed that the first sign of the mounting ventricular pressure would be an excessive increase in HC. Surprisingly in the two examples shown, despite marked changes in the VI and FL-CVT, the HC increased on the same percentile (example A) or even decreased (example B). Owing to the retrospective nature of this study and the lack of meticulous documentation of daily HC measurements, we mention this observation solely as a demonstration of the potential utility of the offered tool.
Summary of sonographic findings of nine infants with PHHC showed acquired FL growth impairment starting around 28 weeks’ corrected age. In the presence of PHHC, the FL growth decelerates, and this deserves a new clinical entity: “acquired FL growth impairment” caused by enlarged ventricles. Instead of continued growth, the FL is compressed and therefore impaired growth is noted, without adequate timely response from caregivers. We also show that cerebrospinal fluid drainage (any method) will reduce pressure on the FL and allows its regrowth.
To this end, the innovative thinking of this study is our ability to assess the magnitude and duration of over-pressure on the FL by the adjacent enlarging ventricle, as demonstrated by the AUC in Fig. 4. It is well known that the FL is the largest in humans and includes the cognitive abilities and other high functions. Hence, FL assessment might become of utmost importance for setting the optimal timing for drainage and the extent of future neurodevelopmental adverse outcome.
The study limitations are: (1) retrospective design and (2) there is yet no available evidence presented that management of PHHC on the basis of FL-CVT gives better short- or long-term outcome than using conventional ventricular measurements.
Conclusion
Our study provides a nomogram for the FL-CVT measurements in newborn infants and demonstrates the growth profile of the thickness of the FL. Using our nomogram in infants with PHHC, we suggest a new clinical entity—“acquired FL growth impairment.” Naming the problem might increase awareness regarding early monitoring of the FL thickness and timely intervention. This way, the FL could have more chances for renewed growth and potentially avoidance of future neurodevelopmental impairment.
Future studies are needed to examine the impact of FL-CVT monitoring on the timing of drainage procedures and on the neurodevelopmental outcome of infants with PHHC.
References
Adams-Chapman, I., Hansen, N. I., Stoll, B. J. & Higgins, R. NICHD Research Network. Neurodevelopmental outcome of extremely low birth weight infants with post hemorrhagic hydrocephalus requiring shunt insertion. Pediatrics 121, e1167–e1177 (2008).
Hüppi, P. S. et al. Quantitative magnetic resonance imaging of brain development in premature and mature newborns. Ann. Neurol. 43, 224–235 (1998).
Mewes, A. U. et al. Regional brain development in serial magnetic resonance imaging of low-risk preterm infants. Pediatrics 118, 23–33 (2006).
Peterson, B. S. et al. Regional brain volumes and their later neurodevelopmental correlates in term and preterm infants. Pediatrics 111, 939–948 (2003).
Srinivasan, L. et al. Quantification of deep gray matter in preterm infants at term-equivalent age using manual volumetry of 3-tesla magnetic resonance images. Pediatrics 119, 759–765 (2007).
Thompson, D. K. et al. Perinatal risk factors altering regional brain structure in the preterm infant. Brain 130, 667–677 (2007).
Jary, S., De Carli, A., Ramenghi, L. A. & Whitelaw, A. Impaired brain growth and neurodevelopment in preterm infants with posthaemorrhagic ventricular dilatation. Acta Paediatr. 101, 743–748 (2012).
Levene, M. I. Measurement of the growth of the lateral ventricles in preterm infants with real-time ultrasound. Arch. Dis. Child. 56, 900–904 (1981).
Davies, M. W., Swaminathan, M., Chuang, S. L. & Betheras, F. R. Reference ranges for the linear dimensions of the intracranial ventricles in preterm neonates. Arch. Dis. Child. Fetal Neonatal Ed. 82, F218–F223 (2000).
Sondhi, V., Gupta, G., Gupta, P. K., Patnaik, S. K. & Tshering, K. Establishment of nomograms and reference ranges for intra-cranial ventricular dimensions and ventriculo-hemispheric ratio in newborns by ultrasonography. Acta Paediatr. 97, 738–744 (2008).
Brouwer, M. J. et al. New reference values for the neonatal cerebral ventricles. Radiology 262, 224–233 (2012).
Persutte, W. H., Coury, A. & Hobbins, J. C. Correlation of fetal frontal lobe and transcerebellar diameter measurements: the utility of a new prenatal sonographic technique. Ultrasound Obstet. Gynecol. 10, 94–97 (1997).
Fenton, T. R. A new growth chart for preterm babies: Babson and Benda’s chart updated with recent data and a new format. BMC Pediatr. 3, 13 (2003).
Makhoul, I. R. et al. Sonographic biometry of the frontal lobe in normal and growth-restricted neonates. Pediatr. Res. 55, 877–883 (2004).
Gilmore, J. H. et al. Infant cerebral ventricle volume: a comparison of 3-D ultrasound and magnetic resonance imaging. Ultrasound Med. Biol. 27, 1143–1146 (2001).
Kishimoto, J. et al. 3D ultrasound system to investigate intraventricular hemorrhage in preterm neonates. Phys. Med. Biol. 58, 7513–7526 (2013).
Del Bigio, M. R., Kanfer, J. N. & Zhang, Y. W. Myelination delay in the cerebral white matter of immature rats with kaolin-induced hydrocephalus is reversible. J. Neuropathol. Exp. Neurol. 56, 1053–1066 (1997).
Van Alfen-van der Velden, A. A. et al. Cerebral hemodynamics and oxygenation after serial CSF drainage in infants with PHVMD. Brain Dev. 29, 623–629 (2007).
Savman, K., Nilsson, U. A., Blennow, M., Kjellmer, I. & Whitelaw, A. Non-protein-bound iron is elevated in cerebrospinal fluid from preterm infants with posthemorrhagic ventricular dilatation. Pediatr. Res. 49, 208–212 (2001).
Soul, J. S., Eichenwald, E., Walter, G., Volpe, J. J. & du Plessis, A. J. CSF removal in infantile posthemorrhagic hydrocephalus results in significant improvement in cerebral hemodynamics. Pediatr. Res. 55, 872–876 (2004).
Singer, O. C. et al. MR volumetric changes after diagnostic CSF removal in normal pressure hydrocephalus. J. Neurol. 259, 2440–2446 (2012).
Brouwer, A. J. et al. European perspective on the diagnosis and treatment of posthaemorrhagic ventricular dilatation. Arch. Dis. Child. Fetal Neonatal Ed. 97, F50–F55 (2012).
de Vries, L. S. et al. Treatment thresholds for intervention in posthaemorrhagic ventricular dilation: a randomised controlled trial. Arch. Dis. Child. Fetal Neonatal Ed. 104, F70–F75 (2019).
Author information
Authors and Affiliations
Contributions
All authors take responsibility for the reported findings and have participated in the concept and design, analysis and interpretation of data, drafting or revising, and approval of this manuscript as submitted.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
About this article
Cite this article
Borenstein-Levin, L., Makhoul, S., Ilivitzki, A. et al. Neonatal frontal lobe: sonographic reference values and suggested clinical use. Pediatr Res 87, 536–540 (2020). https://doi.org/10.1038/s41390-019-0605-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-019-0605-3