Abstract
Background
Postnatal growth restriction (PNGR) in premature infants increases risk of pulmonary hypertension (PH). In a rodent model, PNGR causes PH, while combining PNGR and hyperoxia increases PH severity. We hypothesized that PNGR causes intestinal dysbiosis and that treatment with a probiotic attenuates PNGR-associated PH.
Method
Pups were randomized at birth to room air or 75% oxygen (hyperoxia), to normal milk intake (10 pups/dam) or PNGR (17 pups/dam), and to probiotic Lactobacillus reuteri DSM 17938 or phosphate-buffered saline. After 14 days, PH was assessed by echocardiography and right ventricular hypertrophy (RVH) was assessed by Fulton’s index (right ventricular weight/left ventricle + septal weight). The small bowel and cecum were analyzed by high-throughput 16S ribosomal RNA gene sequencing.
Results
PNGR with or without hyperoxia (but not hyperoxia alone) altered the microbiota of the distal small bowel and cecum. Treatment with DSM 17938 attenuated PH and RVH in pups with PNGR, but not hyperoxia alone. DSM 17938 treatment decreased α-diversity. The intestinal microbiota differed based on oxygen exposure, litter size, and probiotic treatment.
Conclusion
PNGR causes intestinal dysbiosis and PH. Treatment with DSM 17938 prevents PNGR-associated RVH and PH. Changes in the developing intestine and intestinal microbiota impact the developing lung vasculature and RV.
Similar content being viewed by others
Introduction
Pulmonary hypertension (PH) is an increase in pulmonary vascular resistance typically associated with muscularization and fibrosis of the pulmonary arterioles, decreased pulmonary blood flow, and right ventricular hypertrophy (RVH). The incidence of PH among extremely premature infants is as high as 18% and increases to 25–40% among premature infants with bronchopulmonary dysplasia (BPD).1,2 PH increases the morbidity of preterm neonates with BPD with up to 50% mortality by age 2.3
Poor growth in utero (fetal growth restriction, FGR) is common in premature infants and a significant predictor of PH in this population.2 Postnatal growth restriction (PNGR) is even more common in extremely premature infants, particularly those with BPD (79% of premature infants with gestational age <27 weeks and severe BPD in a recent large cohort study),4 and is also associated with increased risk of PH.5,6,7 While both FGR and PNGR occur at a critical time in pulmonary alveolar and vascular development in premature infants, the distinction between FGR and PNGR is significant. FGR occurs in utero when the mother and placenta are the major source of nutrition, thermoregulation, and gas exchange, while PNGR occurs after birth when the major source of nutrition is parenteral nutrition and milk, and thermoregulation and gas exchange must be supported. Furthermore, the intestinal microbiota is unlikely to play a significant role in FGR, but may be important in PNGR. While there are several animal models of FGR, models of PNGR are limited.
Similar to preterm infants, rats are born in the saccular stage of lung development and do not begin alveolarization until postnatal day 5. Neonatal rats exposed to hyperoxia (75–95% O2) for 14 days develop PH, RVH, pulmonary vascular remodeling plus the alveolar simplification that is characteristic of preterm infants with BPD.8 PNGR, achieved by increasing litter size from 10 pups to 16 pups at birth, results in poor growth (body weight 22% lower at 10 days of life and 24% lower at 20 days).9 We have previously demonstrated that the combination of PNGR and hyperoxia in the rat model results in a more severe PH and BPD phenotype. Increasing litter size to 17 pups at birth significantly augments the increases in pulmonary artery pressure and RVH induced by hyperoxia at 14 days.10 Even more compelling, PNGR alone induces PH and RVH in room air and is associated with impaired signaling pathways common to the hyperoxia model, including decreased lung expression of vascular endothelial growth factor, hypoxia-inducible factors, and endothelial nitric oxide synthase.10
Malnutrition impacts the composition of the community of microbes in the intestinal tract (the microbiota). The association between the intestinal microbiota and poor growth in children with malnutrition and in premature infants has recently been established, with causality confirmed with fecal transplantation studies.11,12,13 Links between nutrition, the intestinal microbiota, and diseases distant from the gut demonstrate systemic effects of gastrointestinal changes as exemplified by the increasing number of chronic inflammatory diseases associated with intestinal dysbiosis.14 We hypothesized that PNGR in neonatal rats alters the intestinal microbiota (i.e., causes dysbiosis) and impacts distal organs including the lung, contributing to PH. The objective of this study was to analyze the intestinal microbiota of neonatal rats exposed to PNGR and/or 75% hyperoxia for 14 days, and to determine if probiotic treatment to reverse any detected dysbiosis attenuates PH. This is the first investigation in a rodent model of the developing gut–lung axis.
Materials and methods
Animals
The animal protocol was approved by the Institutional Animal Care and Use Committee at UC Davis. Timed-pregnant Sprague–Dawley dams at E14–E16 were ordered from Charles River Laboratories (Wilmington, MA). Rats were housed in plastic cages with a 12 h dark:light cycle and allowed to feed ad libitum with a standard diet (2018 Teklad from Harlan). After birth, pups were pooled and randomly assigned to litters of 10 pups (control) or 17 pups (PNGR). Additionally, pups were randomly assigned to cages maintained in room air or exposed to 75% oxygen in a plexiglass chamber (Biospherix, Lacona, NY) continuously, and dams were rotated with the appropriate control or PNGR dam every 24 h. In a second experiment, pups in each group were treated either with 5 × 106 CFU (colony-forming units) Lactobacillus reuteri DSM 17938 (Protectis Biogaia, Sweden) or with phosphate-buffered saline (PBS) daily by gavage at a maximum safe volume of 10 μl/g body weight. Pups treated with L. reuteri were co-housed with PBS-treated pups. At postnatal day 14, the pups were analyzed by echocardiography, weighed, and euthanized for tissue harvest. Hearts, lungs, and intestines were snap-frozen in liquid nitrogen and stored at −80 °C.
Sequencing
Bacterial DNA was extracted from the specimens of proximal small intestine, distal small intestine, and cecum using the ZR Fecal DNA Miniprep Kit (Zymo Research, Irvine, CA) according to the manufacturer’s instructions. DNA library construction was carried out as previously described15 and submitted to the UC Davis Genome Center DNA Technologies Core for sequencing on an Illumina MiSeq instrument (Illumina, San Diego, CA). Raw sequencing data was demultiplexed with Sabre (https://github.com/ucdavis-bioinformatics/sabre) and then imported into the QIIME2 software package (version QIIME2-2018.4).16 Bases prior to base pair 22 and after base pair 230 for the forward read, prior to base pair 20, and after base pair 250 for reverse read were trimmed. Trimmed reads were processed with DADA2. Taxonomy was assigned using the 99% Greengenes naive Bayes classifier in QIIME2-2018.4.
Echocardiography
At day 14, echocardiography was performed using a VisualSonics VIVO 2100 in vivo ultrasound imaging system (VisualSonics, Toronto, ON, Canada) to determine the ratio of the pulmonary acceleration time (PAT) to the total ejection time (ET) a marker of PH as previously described.10
Measurement of RVH
Fulton’s index (the weight of the right ventricle (RV) divided by the weight of the left ventricle (LV) + septum) was determined to assess RVH. Additionally, RV and LV + septum weights were normalized to body weight.17
Statistical analysis
Data are presented as means ± SEM and further expressed as fold change relative to air controls where indicated. “N” represents the number of animals in each group. The effects of PNGR and hyperoxia were tested by two-way analysis of variance (ANOVA) (GraphPad Software, La Jolla, CA). A significant interaction between PNGR and hyperoxia was not detected in any analysis, and the effect of each independent variable was tested from the two-way ANOVA. If the F test was significant, a Newman–Keuls post hoc test was performed. The independent variables were considered significant at P < 0.05.
Statistical analysis for the 16S amplicon sequencing data was carried out with R 3.5.2 statistical software and QIIME2 (versions QIIME2-2018.4 and QIIME2-2018.11). Differences in α- diversity were calculated based on Shannon’s diversity index and significance was determined using the Kruskal–Wallis test followed with a Benjamini and Hochberg correction. Permutational multivariate ANOVA (PERMANOVA),18 based on weighted unifrac distances, as implemented in the R VEGAN package (R package version 2.5-2. https://CRAN.R-project.org/package = vegan) was used to determine if there were significant differences in microbial community structure between treatment groups.
Results
Impact of PNGR and hyperoxia on the intestinal microbiota
In experiment 1, we analyzed specimens of the cecum and the distal and proximal small intestine using high-throughput sequencing on the MiSeq platform to assess the impact of PNGR and/or hyperoxia on the intestinal microbiome. Supplemental Fig. S1 (online) demonstrates non-metric multidimensional scaling (NMDS) based on weighted (a) and unweighted (b) UniFrac distances. PERMANOVA testing of both weighted and unweighted UniFrac distances confirms differences in the microbiota between the three sample types, p = 0.001 for both weighted and unweighted UniFrac measures.
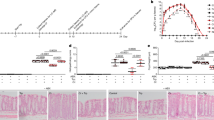
For the cecal specimens, Shannon (α) diversity differed among the four groups (Fig. 1a) as did the relative abundances of the six detected phyla (Fig. 2a, Supplemental File S2) and 37 detected families (Fig. 3a, Supplemental File S2) of bacteria. One animal in the normal litter hyperoxia group (denoted by arrows in Figs. 2a and 3a) had a higher relative abundance of unidentified bacteria (24%) than any of the other samples (<1% unidentified bacteria). As a result, the NMDS plot was not able to resolve given the stress on the model from this outlier (Supplemental Fig. S3a, online). Re-analysis of β-diversity excluding this sample demonstrated differences between groups (p = 0.001 by PERMANOVA testing, Supplemental Fig. S3b, online).
Shannon (α) diversity plots of the cecum (a), distal small intestine (b), and proximal small intestine (c). A = room air, O = hyperoxia, N = normal litter size (10 pups), R = restricted intake (17 pups). Grey circles represent outliers. Asterisks (*) note significant between group differences: cecum: AR/ON, p = 0.04, ON/OR, p = 0.028; distal small intestine: AN/AR, p = 0.008, AN/ON, p = 0.028, AR/OR, p = 0.021; proximal small intestine: none. Differences in α-diversity based on litter size (AN + ON/AR + OR) and hyperoxia (AN + AR/ON + OR) were not significant
Relative bacterial abundance at the phylum level in experiment 1 in the cecum (a), distal small intestine (b), and proximal small intestine (c), and in experiment 2 in the cecum (d) and distal small intestine (e). Blue arrows indicate samples with a high number of unidentified bacteria. Normal litter size = 10 pups, restricted litter size = 17 pups. C = control (PBS treated), LR = L. reuteri treated
Relative bacterial abundance at the family level in experiment 1 in the cecum (a), distal small intestine (b), and proximal small intestine (c), and in experiment 2 in the cecum (d) and distal small intestine (e). Blue arrows indicate samples with a high number of unidentified bacteria. Normal litter size = 10 pups, restricted litter size = 17 pups. C = control (PBS treated), LR = L. reuteri treated
In the distal small bowel, Shannon diversity differed among the four groups (Fig. 1b) as did the relative abundances of the four detected phyla (Fig. 2b, Supplemental File S2) and the 29 detected families (Fig. 3b, Supplemental File S2) of bacteria with differences between groups confirmed by analysis of β-diversity (p = 0.001 by PERMANOVA testing, Supplemental Fig. S4a, online).
In the proximal small intestine there were no significant differences in α-diversity (Fig. 1c), β-diversity (Supplemental Fig. S4b, online), or relative abundance between the four groups, with all dominated by Firmicutes (phylum)/Lactobacillaceae (family) (Figs. 2c and 3c, Supplemental File S2). Two of the proximal small bowel samples had >20% unidentified bacteria (denoted with arrows in Figs. 2c and 3c).
Impact of probiotic Lactobacillus on PNGR and hyperoxia
Given the marked decreases in Lactobacillaceae and the increases in Enterobacteriaceae in the distal small bowel and cecum in the combined PNGR plus hyperoxia group, in experiment 2 we determined the effects of probiotic L. reuteri DSM 17938 on weight gain, PH, RVH, and the intestinal microbiota. PNGR and hyperoxia decreased body weight at 14 days with a further significant decrease when both were combined (Fig. 4a), as we have shown previously.10 Daily gavage of L. reuteri DSM 17938 beginning at day of life 1 had no effects on body weight on day 14 in any of the four groups (Fig. 4a).
In experiment 2, pups in each of the four groups were either treated with probiotic L. reuteri (white bars) or phosphate-buffered saline (black bars). Error bars are standard error of the mean (n = 8–16/group). A = room air, O = hyperoxia, N = normal litter size (10 pups), and R = restricted intake (17 pups). Both PNGR and hyperoxia led to decreased weight gain (a), decreased PAT/ET (increased PH, b), and increased Fulton’s index (increased RVH, c) at 14 days. Daily administration of L. reuteri attenuated PNGR-associated PH and RVH, but not hyperoxia-associated PH and RVH. *P < 0.05 compared to AN, †p < 0.05 compared to AR, and §p < 0.05 compared to no probiotic
We next determined the effects of L. reuteri DSM 17938 on cardiopulmonary changes induced by PNGR and/or hyperoxia. The ratio of the PAT to total ET (PAT/ET) detected by echocardiography decreases with increased pulmonary artery pressures. As we have shown previously,10 PAT/ET ratios were significantly decreased in pups exposed to PNGR or hyperoxia alone, and were decreased further in pups exposed to both (Fig. 4b). Daily gavage with L. reuteri DSM 17938 significantly increased the PAT/ET ratios (i.e., decreased PH) in the PNGR groups, but had no effect on pups exposed to hyperoxia alone (Fig. 4b). RVH was determined by Fulton’s index (the weight of the RV divided by the weight of the LV plus septum). Fulton’s index was significantly increased in pups exposed to PNGR or hyperoxia alone, and was increased further in pups exposed to both (Fig. 4c) in agreement with our previous study.10 Lactobacillus reuteri DSM 17938 significantly decreased Fulton’s index in the PNGR groups, but had no effects on pups exposed to hyperoxia alone (Fig. 4c).
Impact of probiotic Lactobacillus on the intestinal microbiota
We next analyzed the microbiota of the distal small bowel and cecum at 14 days of life in pups from each of the four groups either with or without daily gavage of L. reuteri DSM 17938 (experiment 2). As expected, NMDS based on weighted and unweighted UniFrac distances showed significant differences between distal small bowel and cecum (Supplemental Fig. S5, online). In the cecum, probiotic treatment resulted in decreased α-diversity (Fig. 5a, b). There was significant clustering by group on β-diversity testing (Fig. 5c, d). In this experiment, the increase in Proteobacteria with PNGR was not dramatic (Fig. 1d, Supplemental File S2) as seen in the first experiment (Fig. 1a). There was, however a decrease in Proteobacteria with treatment with L. reuteri in the two groups exposed to hyperoxia (Figs. 1d and 2d). We had fewer samples of distal small bowel available for microbiota analysis. Shannon (α) diversity testing did not indicate significant differences between any of the treatment variables (probiotic treatment, litter size, or oxygen exposure, data not shown), likely due to the small number of specimens. β-Diversity testing demonstrated differences based on probiotic treatment, litter size, and oxygen exposure (Supplemental Fig. S6, online). Relative abundance data are presented in Figs. 2e and 3e and Supplemental File S2. We did not confirm the identity of the lactobacilli at the genus, species, or strain level and so cannot be certain what percentage of the lactobacilli were the administered L. reuteri.
α-Diversity of cecum specimens as determined by Shannon diversity testing indicated a significant difference between treatments (a, probiotic versus control, p = 0.001) and the α-diversity between all eight groups (b, p = 0.008). In a, b, white boxes are PBS-treated controls and shaded boxes are probiotic-treated animals. Pairwise comparison between the groups was significant before adjusting the p value. The adjusted p values (q values) did not show significance. Non-metric multidimensional scaling (NMDS) based on weighted UniFrac distances. Permanova pairwise testing shows a p value of 0.004 for weighted comparisons of microbiota for oxygen exposure (c) and p = 0.001 for probiotic versus control treatments (d)
Discussion
Impaired nutrition during the saccular and alveolar phases of lung development appears to be associated with pulmonary vascular changes predisposing to PH. Growth restriction during late gestation alters pulmonary vascular growth in fetal sheep.19 The effects of PNGR led to changes in pulmonary vascular endothelial function and persistence of PH in adult rats at 9 weeks.20 PNGR is associated with increased risk of PH2,21 and BPD,5 plus non-pulmonary diseases, including necrotizing enterocolitis, sepsis, retinopathy of prematurity,6 and neurodevelopmental delays.7 Whether this association is causal remains uncertain and the potential mechanisms linking PNGR to PH in preterm infants are unknown. More aggressive nutritional approaches have led to improved growth in this population; however, PNGR remains very common in extremely preterm infants regardless of the degree of illness.22,23 An intervention during early postnatal age capable of reversing PH may have long-lasting benefit.
Neonatal rat pups exposed to 14 days of hyperoxia (60% oxygen and greater) display PH, RVH, pulmonary vascular remodeling, and alveolar simplification,17 similar to human infants with PH and BPD. We have shown previously that PNGR in neonatal rats, achieved by increasing litter size at birth, amplifies the adverse effects of 75% oxygen.10,24 Furthermore PNGR is sufficient to induce PH and RVH in neonatal rats maintained in room air for 14 days.10 This age is roughly equivalent to a human infant at 6–12 months,25 a common time of death for premature infants with PH.
Malnutrition impacts the composition of the community of microbes in the intestinal tract (the microbiota) and alters gut barrier function in infants, children, and adults.26,27 However, these associations are poorly understood in the premature infant with poor growth relative to normal growth. Recent studies in germ-free and mono-colonized mice demonstrate the importance of gut microbes to weight gain and longitudinal growth.28 In the distal small bowel Lactobacillus, the dominant commensal organism in rat pups decreased significantly with PNGR but not hyperoxia. Most remarkable was the dramatic increase in Enterobacteriaceae in the combined PNGR with hyperoxia group. Blooms of Enterobacteriaceae (phylum Proteobacteria) have been identified just prior to the onset of necrotizing enterocolitis in premature infants29 and are a signature of dysbiosis in many disease processes.30 Lactobacillus strains were also significantly decreased in the cecum, although we found no significant changes in the composition of the microbiota in the proximal small intestine. These data suggest that the differences seen in the more distal bowel were not directly related to differences in ingested microbes or nutrients, acid production in the stomach, or bile acids in the duodenum and proximal small bowel.
We hypothesized that administration of probiotic L. reuteri DSM 17938 would “correct” the observed dysbiosis in the distal small bowel and cecum, specifically an increase in lactobacilli and a decrease in Enterobacteriaceae. While we did see significant changes in the distal small bowel and cecum with probiotic administration, there was individual variation among rat pups and between experiments. It is noteworthy that the microbiota of the animals in the four treatment groups (AN, AR, ON, OR) that did not receive probiotic L. reuteri DSM 17938 in the second experiment (Figs. 2d, e, 3d, e) differed from that seen in the first experiment (Figs. 2a, b, 3a, b). We obtained the animals from the same supplier, housed the rats in the same facility with the same diet (though at different times), and used the same methods for DNA extraction for each experiment. To avoid variation due to factors intrinsic to the process of bacterial DNA extraction and analysis, all samples for the first experiment were included in a single run and all samples from the second experiment (with and without probiotic) were included in a separate single run. It is possible that co-housing altered the microbiota of the PBS-treated pups; rat pups are generally not co-prophagic at this young age (prior to day 14); however, licking and grooming behavior may have impacted the oral or intestinal microbiota of the dams, potentially altering the intestinal microbiota of the PBS-treated pups. In future studies, analysis of the oral and fecal microbiota of the dams would be valuable. It is noteworthy that in spite of the less obvious changes in the intestinal microbiota in the PBS-treated pups in experiment 2, the impact on PH and RVH (Fig. 4b, c) was significant, suggesting either that the probiotic treatment alters lung development even in the absence of large shifts in the microbiota or that early changes in the microbiota had an effect on the developing lung days before the microbiota analysis (at day 14). Serial analyses of the microbiota of the cecum and distal small intestine in L. reuteri and PBS-treated pups would be valuable.
In the PNGR model the dam provides nutrition to 16–17 pups instead of the usual 10, and previous studies have shown that the milk provided by the dam increases in volume, maintains protein content, but decreases in fat content.31 The pups in larger litters demonstrate altered body composition (25% decrease in body fat at 22 days of life), marked decreases in IGF-1 and leptin, and poor neurodevelopment.9,32 The decreased growth in this model is not purely related to decreased nutrient and energy intake, but likely includes factors such as poor thermoregulation and increased energy expenditure31 that have particular relevance to premature infants. Further research is required to identify the mechanisms that trigger dysbiosis in the PNGR model.
Studies in mice demonstrate the capacity of probiotic Lactobacillus strains to improve growth in chronic undernutrition.28 Lactobacillus reuteri DSM 17938 is commonly administered to premature infants with beneficial effects.33 In premature infants, probiotic administration decreases the risks of death, necrotizing enterocolitis, and sepsis, but does not generally improve weight gain.34 Although L. reuteri DSM 17938 had no effect on body weight in any of the four groups, it attenuated elevated pulmonary artery pressure and RVH induced by PNGR but not by hyperoxia. This is consistent with our sequencing data demonstrating decreased Lactobacillaceae in the distal small bowel and cecum of PNGR pups. Together, these data demonstrate an association between changes in the gut microbiota resulting from PNGR and the development of PH. Evidence for causality using germ-free or mono-colonized animals would be valuable; however, recreating this PNGR model in the mouse has been challenging given the tendency of many mouse strains to consume their young when stressed.
Studies investigating the gut–lung axis have prompted the hypothesis that intestinal dysbiosis is an important driver of systemic inflammation.35 These associations have particular importance in neonates in whom the immune response of the gut and lung are still maturing. The combined increase in Enterobacteriaceae and decrease in Lactobacillaceae in the distal small bowel of PNGR pups seen in experiment 1 (Fig. 3b) is noteworthy, given strong evidence for the pro-inflammatory effects of the former and the protective effects against inflammation of the latter. Previous investigators have demonstrated that interactions between the intestinal microbiota and the developing innate and adaptive immune systems influence long-term risk for inflammatory diseases.36 Toll-like receptors (TLRs) are important in recognition of pathogen-associated molecular patterns and triggering of innate immune responses in both the gut and the lung. TLR4 recognizes lipopolysaccharide that is abundant in the outer membrane of Gram-negative organisms such as Enterobacteriaceae, and this receptor has been implicated in the pathogenesis of such diverse inflammatory disease processes as necrotizing enterocolitis, cancer progression, and acute lung injury.37 Observations in adult TLR4-deficient mice suggest a role in pulmonary vasculogenesis: these mice do not develop PH when exposed to hypoxia.38 Future studies in this model to confirm an increase in Enterobacteriaceae in PNGR pups exposed to hyperoxia and to analyze the potential roles of TLR4 activation in the intestine and/or the lung and of alterations in plasma, lung, and intestinal cytokines would be valuable. An alternative mechanism that would explain the observed attenuation of PH with administration of L. reuteri would be a relative decrease in bacteria that produce short-chain fatty acids, which have important systemic effects, including anti-inflammatory properties. Measurement of short-chain fatty acids and other bacterial metabolites in this model would be of value.
In summary, we show for the first time that PNGR alters the intestinal microbiota and that the probiotic L. reuteri DSM 17938 reverses PNGR-induced PH, suggesting that PH is in part driven by dysbiosis in the developing gut. PNGR combined with hyperoxia causes further dysbiosis, which may worsen PH via several potential mechanisms. Administration of probiotic L. reuteri DSM 17938 altered the intestinal microbiota, but these changes were not universal, suggesting that the probiotic effect is likely broader than just an alteration in the composition of the microbiota. Alternative mechanisms supported by previous studies include inhibition of the TLR4-NFκB pathway by L. reuteri,39 alterations in intestinal motility and/or permeability by Lactobacillus strains,40 and production of anti-microbial bacteriocins.41 The possibility that intestinal dysbiosis influences development at a distant site like the pulmonary vasculature represents a dramatic paradigm shift in understanding of the pathogenesis of PH in the preterm infant. Further studies are needed to determine whether probiotics in combination with other established treatments provide a more effective strategy for prevention of PH in the most vulnerable premature infants with PNGR.
References
Berkelhamer, S. K., Mestan, K. K. & Steinhorn, R. H. Pulmonary hypertension in bronchopulmonary dysplasia. Semin. Perinatol. 37, 124–131 (2013).
Check, J. et al. Fetal growth restriction and pulmonary hypertension in premature infants with bronchopulmonary dysplasia. J. Perinatol. 33, 553–557 (2013).
Khemani, E. et al. Pulmonary artery hypertension in formerly premature infants with bronchopulmonary dysplasia: clinical features and outcomes in the surfactant era. Pediatrics 120, 1260–1269 (2007).
Natarajan, G. et al. Postnatal weight gain in preterm infants with severe bronchopulmonary dysplasia. Am. J. Perinatol. 31, 223–230 (2014).
Nyp, M. F., Taylor, J. B., Norberg, M. & Truog, W. E. Impaired growth at birth and bronchopulmonary dysplasia classification: beyond small for gestational age. Am. J. Perinatol. 32, 75–82 (2015).
Yamakawa, T., Itabashi, K. & Kusuda, S., Neonatal Research Network of J. Mortality and morbidity risks vary with birth weight standard deviation score in growth restricted extremely preterm infants. Early Hum. Dev. 92, 7–11 (2016).
Arcangeli, T., Thilaganathan, B., Hooper, R., Khan, K. S. & Bhide, A. Neurodevelopmental delay in small babies at term: a systematic review. Ultrasound Obstet. Gynecol. 40, 267–275 (2012).
Koppel, R., Han, R. N., Cox, D., Tanswell, A. K. & Rabinovitch, M. Alpha 1-antitrypsin protects neonatal rats from pulmonary vascular and parenchymal effects of oxygen toxicity. Pediatr. Res. 36, 763–770 (1994).
Jou, M. Y., Lonnerdal, B. & Griffin, I. J. Effects of early postnatal growth restriction and subsequent catch-up growth on body composition, insulin sensitivity, and behavior in neonatal rats. Pediatr. Res. 73, 596–601 (2013).
Wedgwood, S. et al. Postnatal growth restriction augments oxygen-induced pulmonary hypertension in a neonatal rat model of bronchopulmonary dysplasia. Pediatr. Res. 80, 894–902 (2016).
Blanton, L. V. et al. Gut bacteria that prevent growth impairments transmitted by microbiota from malnourished children. Science 351, https://doi.org/10.1126/science.aad3311 (2016).
Yu, Y., Lu, L., Sun, J., Petrof, E. O. & Claud, E. C. Preterm infant gut microbiota affects intestinal epithelial development in a humanized microbiome gnotobiotic mouse model. Am. J. Physiol. Gastrointest. Liver Physiol. 311, G521–532 (2016).
Lu, L. et al. Transcriptional modulation of intestinal innate defense/inflammation genes by preterm infant microbiota in a humanized gnotobiotic mouse model. PLoS ONE 10, e0124504 (2015).
Clemente, J. C., Manasson, J. & Scher, J. U. The role of the gut microbiome in systemic inflammatory disease. BMJ 360, j5145 (2018).
Bokulich, N. A. & Mills, D. A. Facility-specific “house” microbiome drives microbial landscapes of artisan cheesemaking plants. Appl. Environ. Microbiol. 79, 5214–5223 (2013).
Bolyen, E. R. J. et al. QIIME 2: reproducible, interactive, scalable, and extensible microbiome data science. PeerJ Prepr. 6, e27295v27292 (2018).
Ladha, F. et al. Sildenafil improves alveolar growth and pulmonary hypertension in hyperoxia-induced lung injury. Am. J. Respir. Crit. Care Med. 172, 750–756 (2005).
Anderson, M. J. A new method for non-parametric multivariate analysis of variance. Austral. Ecol. 26, 32–46 (2001).
Rozance, P. J. et al. Intrauterine growth restriction decreases pulmonary alveolar and vessel growth and causes pulmonary artery endothelial cell dysfunction in vitro in fetal sheep. Am. J. Physiol. Lung Cell. Mol. Physiol. 301, L860–871 (2011).
Zhang, L. et al. Extrauterine growth restriction on pulmonary vascular endothelial dysfunction in adult male rats: the role of epigenetic mechanisms. J. Hypertens. 32, 2188–2198 (2014).
Vyas-Read, S. et al. Early characteristics of infants with pulmonary hypertension in a referral neonatal intensive care unit. BMC Pediatr. 17, 163 (2017).
Griffin, I. J., Tancredi, D. J., Bertino, E., Lee, H. C. & Profit, J. Postnatal growth failure in very low birthweight infants born between 2005 and 2012. Arch. Dis. Child Fetal Neonatal Ed. 101, F50–55 (2016).
Horbar, J. D. et al. Weight growth velocity and postnatal growth failure in infants 501 to 1500 grams: 2000–2013. Pediatrics 136, e84–92 (2015).
La Frano, M. R. et al. Metabolic perturbations of postnatal growth restriction and hyperoxia-induced pulmonary hypertension in a bronchopulmonary dysplasia model. Metabolomics 13, 32 (2017).
Sengupta, P. The laboratory rat: relating its age with human’s. Int. J. Prev. Med. 4, 624–630 (2013).
Genton, L., Cani, P. D. & Schrenzel, J. Alterations of gut barrier and gut microbiota in food restriction, food deprivation and protein-energy wasting. Clin. Nutr. 34, 341–349 (2015).
Gough, E. K. et al. Linear growth faltering in infants is associated with Acidaminococcus sp. and community-level changes in the gut microbiota. Microbiome 3, 24 (2015).
Schwarzer, M. et al. Lactobacillus plantarum strain maintains growth of infant mice during chronic undernutrition. Science 351, 854–857 (2016).
Mai, V. et al. Fecal microbiota in premature infants prior to necrotizing enterocolitis. PLoS ONE 6, e20647 (2011).
Litvak, Y., Byndloss, M. X., Tsolis, R. M. & Baumler, A. J. Dysbiotic Proteobacteria expansion: a microbial signature of epithelial dysfunction. Curr. Opin. Microbiol. 39, 1–6 (2017).
Fiorotto, M. L., Burrin, D. G., Perez, M. & Reeds, P. J. Intake and use of milk nutrients by rat pups suckled in small, medium, or large litters. Am. J. Physiol. 260, R1104–1113 (1991).
Alexeev, E. E., Lonnerdal, B. & Griffin, I. J. Effects of postnatal growth restriction and subsequent catch-up growth on neurodevelopment and glucose homeostasis in rats. BMC Physiol. 15, 3 (2015).
Athalye-Jape, G., Rao, S. & Patole, S. Lactobacillus reuteri DSM 17938 as a probiotic for preterm neonates: a strain-specific systematic review. JJ. Parenter. Enter. Nutr. 40, 783–794 (2016).
Sun, J. et al. Effects of probiotics on necrotizing enterocolitis, sepsis, intraventricular hemorrhage, mortality, length of hospital stay, and weight gain in very preterm infants: a meta-analysis. Adv. Nutr. 8, 749–763 (2017).
Budden, K. F. et al. Emerging pathogenic links between microbiota and the gut–lung axis. Nat. Rev. Microbiol 15, 55–63 (2017).
Vatanen, T. et al. Variation in microbiome LPS immunogenicity contributes to autoimmunity in humans. Cell 165, 842–853 (2016).
Hu, R., Xu, H., Jiang, H., Zhang, Y. & Sun, Y. The role of TLR4 in the pathogenesis of indirect acute lung injury. Front. Biosci. (Landmark Ed.) 18, 1244–1255 (2013).
Young, K. C. et al. Toll-like receptor 4-deficient mice are resistant to chronic hypoxia-induced pulmonary hypertension. Exp. Lung Res. 36, 111–119 (2010).
Liu, Y., Fatheree, N. Y., Mangalat, N. & Rhoads, J. M. Lactobacillus reuteri strains reduce incidence and severity of experimental necrotizing enterocolitis via modulation of TLR4 and NF-kappaB signaling in the intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 302, G608–617 (2012).
Wu, R. Y. et al. Spatiotemporal maps reveal regional differences in the effects on gut motility for Lactobacillus reuteri and rhamnosus strains. Neurogastroenterol. Motil. 25, e205–214 (2013).
Mokoena, M. P. Lactic Acid bacteria and their bacteriocins: classification, biosynthesis and applications against uropathogens: a mini-review. Molecules 22, https://doi.org/10.3390/molecules22081255 (2017).
Acknowledgements
This work was supported by National Institutes of Health: R21 HD096241-01 (to M.A.U.), the Children’s Miracle Network (to S.W.), National Institutes of Health: R01 HL HL085727, R01 HL085844, R01 HL137228, S10 OD010389 shared equipment grant, VA Merit Review Grant I01 BX000576 and I01 CX001490 (to N.C.), and Postdoctoral Fellowship from NIH T32 Training Grant in Basic and Translational Cardiovascular Science (T32 HL86350) (to P.N.T.).
Author information
Authors and Affiliations
Contributions
S.W.: study design, acquisition and analysis of data, drafting, and final approval of the manuscript. C.W.: acquisition and analysis of data, final approval of the manuscript. S.R.A.: acquisition and analysis of data, final approval of the manuscript. P.N.T.: acquisition and analysis of data, final approval of the manuscript. N.C.: study design, final approval of the manuscript. K.M.K.: acquisition and analysis of data, drafting and final approval of the manuscript. S.L.: study design, final approval of the manuscript. R.H.S.: study design, final approval of the manuscript. D.A.M.: study design, data analysis, and final approval of the manuscript. M.A.U.: study design, analysis of data, drafting, and final approval of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wedgwood, S., Warford, C., Agvatisiri, S.R. et al. The developing gut–lung axis: postnatal growth restriction, intestinal dysbiosis, and pulmonary hypertension in a rodent model. Pediatr Res 87, 472–479 (2020). https://doi.org/10.1038/s41390-019-0578-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-019-0578-2
This article is cited by
-
Impact of hyperoxia on the gut during critical illnesses
Critical Care (2024)
-
Intranasal administration of Lactobacillus johnsonii attenuates hyperoxia-induced lung injury by modulating gut microbiota in neonatal mice
Journal of Biomedical Science (2023)
-
Administration of A. muciniphila ameliorates pulmonary arterial hypertension by targeting miR-208a-3p/NOVA1 axis
Acta Pharmacologica Sinica (2023)
-
Malnutrition, poor post-natal growth, intestinal dysbiosis and the developing lung
Journal of Perinatology (2021)
-
Hyperoxia provokes gut dysbiosis in rats
Critical Care (2020)