Abstract
Objective
The objective of this study was to describe a novel amplitude-integrated electroencephalography (aEEG) pattern in infants with hypoxic–ischemic encephalopathy (HIE) and to assess the clinical significance.
Methods
The aEEG traces of infants with HIE who were treated with therapeutic hypothermia (TH) from 2012 to 2017 were analyzed. A pseudo-sawtooth (PST) pattern was defined as a periodic increase of the upper and/or lower margin of the trace on aEEG without showing seizure activities on conventional EEG (CEEG).
Results
Of the 46 infants, 6 (13%) had the PST pattern. The PST pattern appeared following a flat trace or a continuous low-voltage pattern and was followed by a burst-suppression pattern. On CEEG, the PST pattern consists of alternating cycles of low-voltage irregular activities and almost flat tracing. The PST pattern was associated with neuroimaging abnormalities and with various degrees of neurodevelopmental outcomes. Positive predictive values of the PST or worse pattern for adverse outcomes were high at 12 h after birth.
Conclusion
A novel aEEG background pattern in infants with HIE was reported. The PST pattern likely indicates a suppressed background pattern and may be linked to unfavorable outcomes. Further multicenter validation study is needed to clarify its clinical significance.
Similar content being viewed by others
Introduction
Amplitude-integrated electroencephalography (aEEG) provides a simplified cerebral function monitoring tool and is rapidly gaining popularity among neonatologists worldwide. Today, it is widely used in bedside decision-making and seizure screening for hypoxic–ischemic encephalopathy (HIE) in neonates.1,2,3,4,5,6 One of the most-cited classifications of the aEEG background is pattern recognition, reported by Hellström-Westas.7,8 It is categorized into five patterns as follows: flat trace (FT), continuous low voltage (CLV), burst suppression (BS), discontinuous normal voltage (DNV), and continuous normal voltage (CNV). A recent systematic review and meta-analysis concluded that a significant aEEG background abnormality (i.e., FT/CLV/BS patterns), as recorded during the first 72 h after birth, has strong predictive value in infants with HIE.9
A sawtooth pattern is another characteristic aEEG pattern. It represents repetitive rises of the upper and/or lower margins of the aEEG trace, and it is known to correspond to status epilepticus.7 Previous studies have suggested that neonatal seizures due to HIE are associated with long-term neurodevelopmental outcomes,10 and the presence of a sawtooth pattern is a main predictor of neurodevelopmental outcomes.11 However, we have found a sawtooth-mimicking aEEG pattern that never meets the definition of electrographic seizures on conventional EEG (CEEG). Therefore, we called it a “pseudo-sawtooth (PST)” pattern and have regarded it as a profoundly suppressed background pattern.
The aim of this study was to investigate the clinical significance of the PST pattern in a series of HIE infants treated with therapeutic hypothermia (TH).
Methods
Patients
This was a single-center, cohort study of infants with HIE treated with TH from January 2012 to December 2017. During this period, 46 infants were treated with TH for perinatal asphyxia. Inclusion criteria for TH were gestational age ≥36 weeks and birth weight ≥1800 g showing clinical signs of moderate-to-severe neonatal encephalopathy, as well as evidence of hypoxia–ischemia (HI) defined with one or more of the followings: (a) Apgar score ≤5 at 10 min; (b) need for respiratory support 10 min after birth; (c) blood pH < 7.00; or (d) base excess ≥16 mmol/L.
This study was approved by the Institutional Ethics Committee of Anjo Kosei Hospital. Written, informed consent was obtained from each parent.
TH protocol
All infants were considered eligible for TH according to the current recommendation.12 The target core temperature of 34.0 °C was maintained for 72 h, using a whole-body, servo-controlled, water-circulated blanket. After completion of the TH maintenance period, controlled rewarming was performed at the rate of 0.25 °C per hour until the infant’s temperature reached 36.0 °C. All infants received continuous infusion of fentanyl (1–3 µg/kg/h) and midazolam (0.1–0.3 mg/kg/h) for analgesia and sedation during cooling. Analgesia and sedation were reduced during the rewarming period and discontinued when the core temperature reached 36.0 °C. All infants underwent mechanical ventilation with the same synchronized intermittent mandatory ventilation mode and the target range for PaCO2 was set at 40–50 mm Hg during cooling. Inotropic agents and/or steroids were used to avoid hypotension.
EEG recording and assessment
Continuous CEEG recording with aEEG display was begun immediately after admission as a routine clinical practice and was continued for several days, basically until the aEEG recovered to a normal CNV pattern. CEEGs were recorded at the bedside using a bipolar montage with at least eight surface electrodes (Fp1–C3, C3–O1, Fp2–C4, C4–O2, Fp1–T3, T3–O1, Fp2–T4, and T4–O2) according to the international 10–20 system, as previously reported.13 This was combined with electrocardiogram, electrooculogram, and respiration recording, using a Nihon Kohden EEG Neurofax (Nihon Kohden, Tokyo, Japan). The low cutoff filter was fixed at 0.5 Hz, and the raw CEEG was displayed at 3 cm/s and 100 μV/10 mm. Impedance was <20 kΩ. Simultaneous aEEG traces were converted from CEEG data using the EEG Trend program QP-160A (Nihon Kohden).
All aEEG data were reanalyzed by three experienced readers (T.K., M.T., and H.K.) blinded to the CEEG data and the patients’ history. The aEEG background was analyzed using pattern recognition reported by Hellström-Westas et al. as follows: FT, very low voltage, mainly inactive (isoelectric) tracing with activity <5 μV; CLV, continuous background pattern of very low voltage (around or <5 μV); BS, discontinuous background pattern, periods of very low voltage (inactivity) intermixed with bursts of higher amplitude; DNV, discontinuous trace, where the voltage is predominantly >5 μV; and CNV, continuous activity with voltage of 10–25 (−50) μV.8
Whether a seizure pattern was present on aEEG was also evaluated. Seizure patterns on aEEG were divided into two categories (isolated seizure pattern and “sawtooth pattern”) according to a previously reported definition.11 A sawtooth pattern on aEEG was defined as a periodic increase of the upper and/or lower margin of the trace. In this study, CEEG-confirmed seizures were regarded as true seizures. The seizure definition by CEEG was based on a widely recognized definition in which a seizure must show an evolving, repetitive, stereotyped waveform, lasting for ≥10 s.14 If a sawtooth pattern on aEEG was not defined as epileptic seizures on CEEG, it was designated a “PST pattern” (Fig. 1). Possible artifacts, including biological and non-biological artifacts, were also evaluated on aEEG and were confirmed with CEEG. Because the duration of the artifacts was <1 h, we ignored the artifacts. When the three observers’ judgments differed, consensus was reached after a discussion.
A representative sample of the pseudo-sawtooth (PST) pattern. a A PST pattern demonstrating cyclical elevations of both lower and upper borders of the aEEG trace. b The simultaneous eight-channel raw EEG corresponding to the top of the increase of the aEEG trace (red line in a) does not show any ictal changes, but it rather shows low-voltage irregular activity mixed with medium-voltage slow waves
Clinical data
Clinical data were collected from medical records. The clinical severity of HIE was defined based on the Sarnat classification.15 Brain magnetic resonance imaging (MRI) was performed once soon after TH, using a 1.5-T MRI scanner with conventional T1- and T2-weighted images, FLAIR images, and diffusion-weighted images. The MR images were assessed by two experienced investigators (M.T., H.K.) according to the National Institute of Child Health and Human Development (NICHD) classification.16 Patients were followed up in our outpatient clinic and assessed using a standardized neurological examination. Gross motor function was assessed based on the Gross Motor Function Classification System (GMFCS): levels 3–5 were considered severe, grade 1–2 mild, and score 0 was given to infants without cerebral palsy.17,18 Global development was assessed at any age by physicians and also by psychologists at 18 months based on a Kyoto Scale of Psychological Development test, which has been widely used in Japan.19 Developmental delay was defined as having a developmental quotient <70 or physicians presuming its equivalent, and infants who were bed-ridden and could not understand any language were regarded as having severe developmental delay. In addition, whether epilepsy has been diagnosed or not at the last follow-up was assessed.
Statistical analysis
Sensitivity, specificity, and positive and negative predictive values for neonatal MRI abnormalities or clinical outcomes at 12–18 months of age were calculated by contingency table analysis with χ2 test or Fisher’s exact test. In the analysis, an abnormal outcome was defined as the presence of severe cerebral palsy and/or developmental delay and/or epilepsy at 12–18 months of age. The SPSS software package (SPSS Version 24; Chicago, IL) was used, and p < 0.05 was considered significant.
Results
Characteristics of the study population
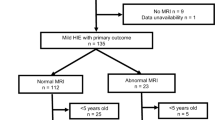
The characteristics of the 46 infants are shown in Table 1. Mean (standard deviation: SD) gestational age was 39.6 (1.5) weeks, and mean (SD) body weight at birth was 2996 (399) g. Severity of HIE before cooling was mild in 7 infants (15%), moderate in 30 (65%), and severe in 9 (20%). In all infants, TH was started within 6 h of life. Brain MRI was performed after cooling at a median (range) age of 10 (6–28) days of life. MRI identified abnormalities in 21 infants (46%; NICHD classification 1A in 8 infants, 1B in 2 infants, 2A in 2 infants, 2B in 5 infants, and 3 in 4 infants). No infants died before Neonatal Intensive Care Unit discharge.
Evolutional changes of the aEEG background pattern
Figure 2 shows the evolution of the aEEG background pattern over time in each infant. EEG was started at a median (range) age of 2.8 (0.5–7.7) h after birth and was continued during the median (range) duration of 82.5 (15.3–211.0) h. The worst aEEG pattern was FT in 4 infants (9%), CLV in 12 (26%), BS in 20 (44%), and DNV in 10 (22%). CEEG-confirmed neonatal seizures were observed in 12 infants (26%) between 11.5 and 89 h after birth. The sawtooth pattern with aEEG was suspected in nine parts of aEEG traces of six infants, but three of them were confirmed as epileptic seizures by CEEG. The other six were not confirmed as epileptic seizures by CEEG and were thus PST patterns (Fig. 1).
Evolutional changes of aEEG background and outcome The x axis in aEEG evolution indicates the time point after birth (h). Abnormal findings on neonatal MRI are graded based on the National Institute of Child Health and Human Development classification. Dagger (†) indicates death. Parallel line (II) represents lost to follow-up. FT flat trace, CLV continuous low voltage, PST pseudo-sawtooth pattern, BS burst suppression, ST sawtooth pattern, DNV discontinuous normal voltage, CNV continuous normal voltage, CP cerebral palsy, DD developmental disability, f/u follow-up
The detailed characteristics of the PST pattern
Table 2 shows the characteristics of the six infants with the PST pattern. In all infants, the PST pattern appeared following the FT or CLV pattern and was followed by the BS pattern (Fig. 2). The mean cyclicity of transient increases of the aEEG trace was 1 per 3.3–4.6 min. On CEEG, the PST pattern consists of alternating cycles of low-voltage irregular activities that nest oscillations at many delta/theta frequencies for approximately 60 s and an almost flat tracing for 120–180 s (Supplementary Fig. S1). The alternating rhythm was synchronous across derivations in which the PST pattern was observed (Supplementary Fig. S2). Two infants, in whom the PST pattern was initially suspected as status epilepticus, received phenobarbital (20 mg/kg/dose), but the PST pattern never changed (Supplementary Fig. S3). All six infants had CEEG-confirmed isolated seizures during the BS or DNV pattern following the PST pattern, and two of the six infants showed CEEG-confirmed repetitive seizures (sawtooth pattern on aEEG) (Fig. 2 and Table 2). One of them also had isolated seizures during the PST pattern (Fig. 3).
Association between aEEG evolution and neurodevelopmental outcome
The median (range) follow-up period was 18 (3–60) months. Four infants were lost to follow-up due to drop-out (n = 2) or moving abroad (n = 2). Out of the remaining 42 infants, 5 infants were still under aged <12 months at the last follow-up. Two infants died by the last follow-up. One infant died at 15 months of age, and another at 23 months of age, both due to respiratory infections. Seven infants had cerebral palsy (GMFCS grade 1 in 1 infant, grade 5 in 6 infants). Seven infants had developmental delay, and six of them were diagnosed as having severe developmental delay. Two infants developed epilepsy, one focal epilepsy and the other West syndrome.
Based on evolutional changes of the aEEG pattern within 48 h of life (Fig. 2), three different groups were identified: Group A (n = 3), continuously showing FT pattern; Group B (n = 6), evolving PST pattern within 48 h; and Group C (n = 37), recovering to BS or better pattern without transiently showing the PST pattern. All three infants in Group A had severe, extensive brain damage including the brainstem on neonatal MRI. They all had severe motor and developmental disabilities. Six infants in Group B had varied outcomes, but none of them had normal MRI. In contrast, 12 of the 37 infants in Group C had mild-to-moderate MRI abnormalities, but none of the infants had abnormal outcomes, at least until the last follow-up.
Table 3 shows the predictive values of the aEEG pattern for moderate–severe neuroimaging abnormalities or short-term neurodevelopmental outcomes. Nine of the 46 infants were excluded in the neurodevelopmental analysis of 12–18 months of age due to drop-out (n = 2), moving abroad (n = 2), or still aged <12 months (n = 5). The positive predictive value of a BS or worse pattern at 12 or 24 h was lower than that at 48 h. In contrast, positive predictive values of the PST or worse pattern were high already at 12 h after birth.
Discussion
So far, a sawtooth pattern on aEEG has been widely recognized as a status epilepticus pattern.7,11 However, the novel PST pattern presented in this paper is not a seizure pattern, but a profoundly suppressed background pattern on aEEG seen transiently during the recovery phase of HI insult. With aEEG alone, the PST pattern looks quite similar to a sawtooth pattern. Therefore, the distinction between PST and real-sawtooth aEEG patterns with CEEG is critical for avoiding unnecessary treatment with antiepileptic drugs. In addition, the PST pattern may be a useful early marker for unfavorable outcomes in the TH era.
In infants with HIE, around 6–24 h after birth is a critical period, with seizures often occurring.20,21 In a fetal sheep model of HI insult, delayed onset seizures also occur during a secondary phase of deterioration (6–15 h after the insult) when cytotoxic edema and accumulation of excitotoxins, with near-complete failure of cerebral mitochondrial activity, occur.22 In the present study, the PST pattern also first appeared between 6.5 and 28 h after birth. Therefore, the similarity of the PST and real-sawtooth patterns in the timing of their appearance, as well as the morphology on aEEG trace, may lead to a further misinterpretation of PST as status epilepticus. Looking at simultaneous raw EEGs, however, a real-sawtooth pattern must consist of repetitive epileptic activities showing evolutional, stereotypical, repeating activities. In contrast, the PST pattern does not fulfill the criteria of such epileptic activities but rather shows periodic alternation between low-voltage irregular activities with nested oscillations at many delta/theta frequencies and an almost isoelectric pattern on scalp EEG. Existence of such periodic activities on CEEG has never been recognized, not only in human subjects but also in animal models.
The neurophysiological basis generating the PST pattern remains unanswered, yet the present results that the PST pattern always preceded the BS pattern give rise to some hypotheses. Regarding the BS pattern, previous studies have shown the involvement of different cortical and subcortical cell types within bursts and suppression.23,24 The generation of individual bursts is thought to be caused by basal cortical dynamics often provoked by input from thalamic relay cells, while suppression is caused by a functional deafferentation between subcortical and cortical structures.25 Recently, using computational methods, Ching et al. suggested that BS may arise through the interaction between neuronal dynamics and brain metabolism. In a state of reduced neuronal activity and cerebral metabolism in BS, insufficient production of ATP in local cortical networks can gate neuronal potassium channels, leading to suppression of action potentials.26 Because the PST pattern appears between the FT/CLV and BS patterns, it seems to reflect more rudimentary neural dynamics and may reflect reduced, disorganized cortical activities under cessation of neural projections from subcortical structures. The more constant periodic EEG activity in the PST pattern than in the BS pattern may be caused by ATP depletion in local cortical activities, with disruption of the excitatory–inhibitory balance. On the other hand, recent studies have also indicated that BS is associated with enhanced excitability in cortical networks.27,28 Furthermore, the activities of the hippocampus are enriched as suppression of the EEG deepens and reaches a high level of organization that will entrain the cortex, thus preserving a rudiment of network activity.28 Like this scenario, some rhythmic oscillator intrinsic to the subcortical structure(s) may generate the alternating pattern in the impaired cortical activities.
Typically, artifacts from the surrounding environment (e.g., mechanical ventilation, water-circulated blanket) on aEEG show repetitive, monotonous activity with almost the same frequency on CEEG. However, the PST pattern consists of the periodic alternation of low-frequency-dominant and various frequency irregular activities and almost flat activities, as shown in Supplementary Fig. 1. In addition, cyclicity of the PST pattern was not exactly the same, ranging from 3.3 to 4.6 min, as shown in Table 2. Furthermore, the PST pattern on aEEG was never seen in the infants who had a flat aEEG during the first 48 h after birth. Therefore, we consider that the PST pattern is not an artifact.
Sedative drugs such as fentanyl and midazolam could affect the background traces on EEG, typically suppressing the background.29,30 However, we have rarely seen a similar PST pattern in infants who received fentanyl and midazolam with different etiologies, such as respiratory illnesses or surgical conditions. In addition, all 46 infants received fentanyl and midazolam in this study, but only six of them had the PST pattern. Therefore, we do not consider that the PST pattern is derived from the sedative drugs.
Careful observation of the aEEG trace enables us to differentiate the PST pattern from the BS pattern. The PST pattern can be characterized by regularity of periodic alterations between low-voltage irregular activities and an almost flat tracing, while burst activity of the BS pattern emerges erratically. In addition, the low-voltage activities in the PST pattern persist longer than bursts in the BS pattern. Therefore, the PST pattern typically shows periodic rises of the lower margin of the aEEG trace. In contrast, bursts in the BS pattern persist only for several seconds. Such short bursts cannot cause a rise of the lower margin of the aEEG trace when displayed at 6 cm/h (Supplementary Fig. S4).
The PST pattern likely reflects ongoing brain damage and is associated with diverse neurodevelopmental outcomes. In general, the aEEG background evolves during the first days depending on the duration and severity of the HI insult. The duration of aEEG abnormalities is associated with neurodevelopmental outcomes. In mild patients, as in Group C, rapid recovery occurs, and the prognosis is excellent; in contrast, infants with a persistent severe aEEG abnormality, as in Group A, have the worst outcomes.10,11,26 Infants with the PST pattern seem to be intermediate between the groups. In addition, recent evidence suggests that the predictive value of aEEG is delayed from 24–36 to 48–72 h of life.31,32,33,34 The delay in the predictive reliability of the aEEG for adverse outcomes can be explained by the beneficial effect of TH on the ongoing brain damage. It is further reported that TH prolongs the time to the presence of cyclicity on aEEG and pseudo-normalization on diffusion-weighted MRI.33,35 In the present study, the PST pattern, always associated with ongoing brain damage, occurred within about 24 h after birth, before the BS pattern. Since the BS pattern does not necessarily indicate ongoing brain damage, the PST pattern may provide valuable information for early prediction of the outcome in the TH era.
The strength of the present study was the standardized treatment protocol for TH. All infants in the present study were treated with TH according to the same protocol and sedated with the same medications with similar dosages. Another strength was the accurate detection of neonatal seizures and localization of the PST pattern, with gold-standard multichannel CEEG monitoring. The PST pattern was not observed on all of the channels simultaneously, suggesting that limited channel recording can lead to overlooking the presence of the PST pattern. Nevertheless, there are several limitations in this study. First, the sample size was relatively small from a single hospital, and the follow-up period was short. Second, EEG recordings were discontinued before recovery to the CNV pattern in some infants. Third, it remains unproven whether the generation of the PST pattern does not depend on the particular circumstance of mild hypothermia and the use of fentanyl and midazolam in the present cohort. Future multicenter studies on aEEG/EEG evolution in human subjects and corroboration by animal studies and computational models are needed.
Conclusions
A novel aEEG background pattern seen transiently during the recovery phase in TH-treated infants with HIE was reported. The PST pattern does not indicate status epilepticus, but it likely indicates a profoundly suppressed background pattern. Recognition of the PST pattern is clinically important for avoiding unnecessary treatment and probably for early prediction of unfavorable outcomes in the current cooling era. Further multicenter validation study is needed to more clarify the clinical significance of the pattern.
References
Hellstrom-Westas, L., Rosen, I. & Svenningsen, N. W. Predictive value of early continuous amplitude integrated EEG recordings on outcome after severe birth asphyxia in full term infants. Arch. Dis. Child. Fetal Neonatal Ed. 72, F34–F38 (1995).
Toet, M. C. et al. Amplitude integrated EEG 3 and 6 h after birth in full term neonates with hypoxic-ischaemic encephalopathy. Arch. Dis. Child. Fetal Neonatal Ed. 81, F19–F23 (1999).
Toet, M. C. et al. Comparison between simultaneously recorded amplitude integrated electroencephalogram (cerebral function monitor) and standard electroencephalogram in neonates. Pediatrics 109, 772–779 (2002).
al Naqeeb, N. et al. Assessment of neonatal encephalopathy by amplitude-integrated electroencephalography. Pediatrics 103, 1263–1271 (1999).
Shah, N. A., Van Meurs, K. P. & Davis, A. S. Amplitude-integrated electroencephalography: a survey of practices in the United States. Am. J. Perinatol. 32, 755–760 (2015).
ter Horst, H. J. et al. Prognostic significance of amplitude-integrated EEG during the first 72 h after birth in severely asphyxiated neonates. Pediatr. Res. 55, 1026–1033 (2004).
Hellström-Westas, L., de Vries L. S. & Rosen, I. An Atlas of Amplitude-Integrated EEGs in the Newborn, 2nd edn (CRC Press, 2002).
Hellström-Westas, L. et al. Amplitude-integrated EEG classification and interpretation in preterm and term infants. Neoreviews 7, e76–e87 (2006).
Del Rio, R. et al. Amplitude integrated electroencephalogram as a prognostic tool in neonates with hypoxic-ischemic encephalopathy: a systematic review. PLoS ONE 11, e0165744 (2016).
McBride, M. C., Laroia, N. & Guillet, R. Electrographic seizures in neonates correlate with poor neurodevelopmental outcome. Neurology 55, 506–513 (2000).
van Rooij, L. G. et al. Neurodevelopmental outcome in term infants with status epilepticus detected with amplitude-integrated electroencephalography. Pediatrics 120, e354–e363 (2007).
Perlman, J. M. et al. Part 11: Neonatal resuscitation: 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Circulation 122(16Suppl 2), S516–S538 (2010).
Hayashi-Kurahashi, N. et al. EEG for predicting early neurodevelopment in preterm infants: an observational cohort study. Pediatrics 130, e891–e897 (2012).
Clancy, R. R. & Legido, A. The exact ictal and interictal duration of electroencephalographic neonatal seizures. Epilepsia 28, 537–541 (1987).
Sarnat, H. B. & Sarnat, M. S. Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Arch. Neurol. 33, 696–705 (1976).
Shankaran, S. et al. Brain injury following trial of hypothermia for neonatal hypoxic-ischaemic encephalopathy. Arch. Dis. Child. Fetal Neonatal Ed. 97, F398–F404 (2012).
Palisano, R. et al. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev. Med. Child Neurol. 39, 214–223 (1997).
Palisano, R. J. et al. Content validity of the expanded and revised Gross Motor Function Classification System. Dev. Med. Child Neurol. 50, 744–750 (2008).
Society for the Kyoto Scale of Psycological Developmental test. Shinpan K Shiki Hattatsu Kensahou 2001 Nenban [The Kyoto Scale of Psychological Development Test 2001] (Nakan-ishiya Shuppan, Kyoto, 2008).
Guidotti, I. et al. Hypothermia reduces seizure burden and improves neurological outcome in severe hypoxic-ischemic encephalopathy: an observational study. Dev. Med. Child Neurol. 58, 1235–1241 (2016).
Lynch, N. E. et al. The temporal evolution of electrographic seizure burden in neonatal hypoxic ischemic encephalopathy. Epilepsia 53, 549–557 (2012).
Williams, C. E. et al. Delayed seizures occuring wih hypoxic-ischemic encephalopathy in the fetal sheep. Pediatr. Res. 27, 561–565 (1990).
Hirose, M. et al. Functional cortical deafferentation from the subcortical structures in a patient with early myoclonic encephalopathy: a functional neuroimaging study. Epilepsia 51, 699–702 (2010).
Steriade, M., Amzica, F. & Contreras, D. Cortical and thalamic cellular correlates of electroencephalographic burst-suppression. Electroencephalogr. Clin. Neurophysiol. 90, 1–16 (1994).
Japaridze, N. et al. Neuronal networks during burst suppression as revealed by source analysis. PLoS ONE 10, e0123807 (2015).
Ching, S. et al. A neurophysiological-metabolic model for burst suppression. Proc. Natl Acad. Sci. USA 109, 3095–3100 (2012).
Amzica, F. What does burst suppression really mean? Epilepsy Behav. 49, 234–237 (2015).
Kroeger, D., Florea, B. & Amzica, F. Human brain activity patterns beyond the isoelectric line of extreme deep coma. PLoS ONE 8, e75257 (2013).
Bernet, V. et al. Effect of sedation and analgesia on postoperative amplitude-integrated EEG in newborn cardiac patients. Pediatr. Res. 67, 650–655 (2010).
van Leuven, K. et al. Midazolam and amplitude-integrated EEG in asphyxiated full-term neonates. Acta Paediatr. 93, 1221–1227 (2004).
Nash, K. B. et al. Video-EEG monitoring in newborns with hypoxic-ischemic encephalopathy treated with hypothermia. Neurology 76, 556–562 (2011).
Hamelin, S. et al. Influence of hypothermia on the prognostic value of early EEG in full-term neonates with hypoxic ischemic encephalopathy. Neurophysiol. Clin. 41, 19–27 (2011).
Thoresen, M. et al. Effect of hypothermia on amplitude-integrated electroencephalogram in infants with asphyxia. Pediatrics 126, e131–e139 (2010).
Hallberg, B. et al. The prognostic value of early aEEG in asphyxiated infants undergoing systemic hypothermia treatment. Acta Paediatr. 99, 531–536 (2010).
Bednarek, N. et al. Impact of therapeutic hypothermia on MRI diffusion changes in neonatal encephalopathy. Neurology 78, 1420–1427 (2012).
Acknowledgements
The authors thank Dr. Yuichi Kato and Dr. Tetsuo Hattori of Anjo Kosei Hospital for data acquisition and helpful comments on our manuscript. This study was supported by a Grant-in-Aid for Scientific Research (C) (no. 26461543) and Grants for Scientific Research on Innovative Areas (15H01578) from the Ministry of Education, Culture, Sports, Science and Technology in Japan.
Author contributions
M.T., H.K., and T. Kubota conceptualized and designed the study, drafted the initial manuscript, and reviewed and revised the manuscript. T.F. collected data, and reviewed and revised the manuscript. Y.O., Y.S., Y.I., H.Y., A. Ohno, T. Nakata, T. Negoro, T. Kato, K.W., and A. Okumura and Y.T. assisted in data interpretation and manuscript preparation and revision. J.N. conceptualized and designed the study, coordinated and supervised data collection, and critically reviewed the manuscript for important intellectual content. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Tanaka, M., Kidokoro, H., Kubota, T. et al. Pseudo-sawtooth pattern on amplitude-integrated electroencephalography in neonatal hypoxic–ischemic encephalopathy. Pediatr Res 87, 529–535 (2020). https://doi.org/10.1038/s41390-019-0567-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-019-0567-5
This article is cited by
-
Review of Noninvasive Neuromonitoring Modalities in Children II: EEG, qEEG
Neurocritical Care (2023)