Abstract
In recent years, several studies have shown that premature infants who develop NEC frequently display enteric dysbiosis with increased Gram-negative bacteria for several days to weeks prior to NEC onset. The importance of these findings, for the possibility of a causal role of these bacteria in NEC pathogenesis, and for potential value of gut dysbiosis as a biomarker of NEC, is well-recognized. In this review, we present current evidence supporting the association between NEC in premature infants and enteric dysbiosis, and its evaluation using the Bradford Hill criteria for causality. To provide an objective appraisal, we developed a novel scoring system for causal inference. Despite important methodological and statistical limitations, there is support for the association from several large studies and a meta-analysis. The association draws strength from strong biological plausibility of a role of Gram-negative bacteria in NEC and from evidence for temporality, that dysbiosis may antedate NEC onset. The weakness of the association is in the low level of consistency across studies, and the lack of specificity of effect. There is a need for an improved definition of dysbiosis, either based on a critical threshold of relative abundances or at higher levels of taxonomic resolution.
Similar content being viewed by others
Introduction
Premature infants are at risk of developing enteric dysbiosis with a preponderance of Gram-negative bacteria of families Enterobacteriaceae, Vibrionaceae, and Pseudomonadaceae in the class Gammaproteobacteria and the phylum Proteobacteria.1,2,3,4 In recent years, several case–control studies have shown that premature infants who develop NEC beyond 3 weeks of postnatal age frequently show such dysbiosis for several days to weeks leading up to NEC onset.1,2,3,5,6,7,8,9 These findings have evoked considerable excitement about the role of these bacteria in NEC pathogenesis, and also for the potential value of enteric dysbiosis as a biomarker for risk-stratification of preterm infants for NEC.
In the following sections, we evaluate the evidence supporting the association between NEC in premature infants and enteric dysbiosis. We collated information from an extensive literature search in the databases PubMed, EMBASE, and Scopus, and applied the Bradford Hill criteria for causality (Table 1).10 To minimize bias, keywords from PubMed’s Medical Subject Heading thesaurus were shortlisted prior to the actual search and combined with text words likely to be used in titles and abstracts. Table 2 provides a glossary of terms frequently used in microbiome studies.
Strength of the association
In the Bradford Hill framework for assessment of causality, strong associations are less likely to be explained by bias or confounding. However, strength is not a requirement because weak associations can also be causal.11 The role of bacteria in NEC pathogenesis has now been recognized since the 1960s.12,13,14,15 Bacterial overgrowth is a prominent histopathological finding in NEC lesions, and, considering that pneumatosis, the pathognomonic sign of NEC, signifies gaseous products of bacterial fermentation entrapped within the bowel wall, these bacteria are believed to be metabolically active.16,17 The occurrence of NEC almost always after the 1st postnatal week and never in utero, the difficulty in inducing NEC-like lesions in germ-free animals, the correlation between bacterial invasion within the bowel wall and mortality in surgical NEC, and the protective effect of enteral antibiotics against NEC and NEC-related mortality, underscore the role of bacteria in NEC pathogenesis.18,19,20
NEC cases are known to cluster in time and space, and these mini-outbreaks have long fueled a quest for transmissible infectious triggers of NEC.21 Cultures of blood and other body fluids from infants with NEC have not consistently implicated a single agent and seem to yield a wide array of microorganisms that are present in the NICU microenvironment and colonize critically-ill preterm infants.21,22 Nevertheless, Gram-negative bacteria have remained key suspects in NEC pathogenesis, perhaps because the clinical presentation of NEC resembles Gram-negative sepsis, and although positive blood cultures are uncommon during acute NEC, Gram-negative bacteria such as Klebsiella, Escherichia coli, Enterobacter, and Pseudomonas are frequently identified in the peritoneal fluid from infants with advanced NEC.23 These agents have also been associated with NEC outbreaks.24,25,26,27
In the last decade, several studies of the gut microbiome in preterm infants have associated Gammaproteobacteria and its constituent families Enterobacteriaceae, Vibrionaceae, and Pseudomonadaceae, with increased risk of NEC.1,2,3,5,6,7,8,9 Wang et al. analyzed fecal samples from a small sample of preterm infants (10 infants with a diagnosis of NEC and 10 gestational age-matched controls), and showed that the stool microbiome from NEC patients clustered separately from controls and showed low bacterial diversity with a marked increase in the relative abundance of Gammaproteobacteria.2 Torrazza et al. also noted a distinct pattern of microbial colonization in infants who developed NEC.28 They observed a higher proportion of Proteobacteria (61%) 2 weeks prior to NEC onset. They also showed that the detection of a novel signature sequence reminiscent of Klebsiella pneumoniae during the first postnatal week was associated with later development of NEC.28 In another study, Morrow et al. noted that the stool microbiome in infants who developed NEC between postnatal days 19–39 days showed more Proteobacteria, specifically Enterobacteriaceae.29 More recently, Warner et al. showed that 28 infants who developed NEC showed increased proportions of Gammaproteobacteria and less Negativicutes, compared to 94 controls.8 Their statistical models showed fecal Gammaproteobacteria to be a significant predictor of NEC.
Consistency
There is greater confidence for causality if an exposure is consistently linked with the outcome by different investigators, in different populations, and with different study designs. However, consistency is not a requirement as individual studies can have limitations of methodology, power, and bias.30 As noted above, the association between Gammaproteobacteria and NEC has been noted in several important studies. However, many others have not found a consistent link: Millar, de la Cochetiere, Mshvildadze, Mai, Smith, Stewart, Normann, McMurtry, Sim, Stewart, Heida, Barron, Ravi, Romano-Keeler, Brown, Wandro, Itani and others have identified Gram-positive bacteria as the dominant taxa in at least some of their patients with NEC, or found no clear patterns at all.3,5,6,31,32,33,34,35,36,37,38,39,40,41,42,43,44 A summary of all studies comparing the enteric microbiome of infants with a diagnosis of NEC vs. those who did not develop NEC is provided in Table 3. Clearly, most of these studies included only a few infants in each group, and therefore, Pammi et al. sought to evaluate the evidence by combining 14 eligible reports in meta-analysis.1 The authors drew attention to the potential fallacies of combining a few small, heterogeneous studies, but cautiously concluded that infants with NEC show a modest, but significantly increased proportion of Proteobacteria, particularly Gammaproteobacteria, and lower proportions of Firmicutes and Bacteroidetes compared to control infants. This increase in Gammaproteobacteria abundance was most evident in infants born at <27 weeks’ gestation, and the pattern change was not evident until after the 3rd postnatal week and ~30 weeks’ corrected gestational age. They also found lower alpha-diversity (fewer taxa, lower Shannon Diversity Index) in these infants. Methodologically, there were concerns that studies targeting the hypervariable regions V3–V5 in the 16S rRNA bacterial gene reported higher relative abundances of Proteobacteria and decreased abundances of Firmicutes compared to those targeting the V1–V3 regions. Overall, the meta-analysis confirmed the statistical significance of the association between Gammaproteobacteria and NEC, but the pooled data did not show distinct clustering of NEC and control samples by unweighted or weighted UniFrac metrics. These findings suggested that differences in the gut microbiome in infants who developed NEC vs. controls might be relatively modest. Alternatively, these finding may also have resulted from the considerable methodological, clinical, and study heterogeneity of the included studies, indicating a need for further investigation of this association.
Specificity
The presence of Gammaproteobacteria in the intestinal microbiome is neither specific, because these pathobionts have also been associated with other neonatal diseases; nor necessary, because all cases of NEC do not display enteric dysbiosis with increased Gammaproteobacteria; nor sufficient, because not all infants with dysbiosis develop NEC. Increasing information indicates that intestinal colonization with Gammaproteobacteria may be a normal maturational event during gut microbiome assembly in preterm infants.45,46,47,48,49,50,51,52,53,54 La Rosa et al. evaluated the fecal microbiome of 58 premature infants and showed that the gut microbiota progressed through a choreographed succession of bacterial classes from Bacilli to Gammaproteobacteria to Clostridia.45 They showed that the Gammaproteobacteria abundance peaked between 28 and 34 weeks’ post-menstrual age. To investigate the drivers for a possible Gammaproteobacterial bloom in some infants, they also evaluated some of the better-known selection pressures. Antibiotic use was associated with increased proportions of Gammaproteobacteria, but only for infants with ≥26 weeks’ gestation. Human milk feedings were associated with increasing proportions of Gammaproteobacteria in the most premature infants. However, these exogenous drivers of gut microbial content did not fundamentally alter the trends in population evolution, only its pace.
In another important study, Gregory et al.47 showed that infant gut microbiome is influenced by postnatal age, birth weight, gestational age, and nutrition.47 They also found a relatively ordered succession in bacterial taxa with initial colonization dominated by Bacilli, followed by Gammaproteobacteria, and finally Clostridia and Bifidobacteria. They found an important effect of diet, where infants fed mother’s own milk had greater initial diversity in their microbiome that was most strongly influenced by the presence of a variety of phylotypes that include lower levels of Bacillales and Lactobacillales, in favor of Clostridia, and Enterobacteriales as early as 26 weeks of adjusted gestational age.
We have recently investigated the clinical antecedents of increased fecal abundance of Gammaproteobacteria in premature infants.55 In this study, we enrolled 45 premature infants born with a birth weight ≤1500 g and analyzed their fecal microbiome first at an early time-point within the first 2 weeks and then serially during the 3rd and 4th postnatal weeks. Our goal was to identify the clinical characteristics of preterm infants who developed enteral dysbiosis, which in turn, could inform future efforts to direct microbiome screening in a clinical setting. We hypothesized that most premature infants begin with few Gammaproteobacteria in their stool and acquire these bacteria from the hospital microenvironment or from human interaction50,56,57,58 as a function of postnatal age. Consistent with this hypothesis, we found that the overall proportion of fecal Gammaproteobacteria increased with postnatal age. Interestingly, about half of the infants in our cohort started with a low relative abundance of Gammaproteobacteria (<10%) in early stool samples and gained these bacteria over time. However, a second subgroup within our cohort started with very high relative abundances of Gammaproteobacteria (>90%). This dichotomy in gut microbiome assembly was novel, and in linear mixed models, the high Gammaproteobacteria abundance in our 2nd cluster was associated with vaginal birth, indicating possible vertical, mother-to-infant transmission. During the 3rd and the 4th weeks, these two subgroups began to resemble each other and showed comparable alpha-diversity and Gammaproteobacteria abundance. Overall, a large proportion of infants in our cohort showed a Gammaproteobacteria abundance >50% – 45.5% at ≤2 weeks, 64.3% in the 3rd week, and 79.5% in the 4th week. Our cohort was typical for a regional referral NICU in the United States, without an unusually high exposure to factors typically identified with dysbiosis in premature infants.48,59,60,61,62 For instance, 42 of our 45 infants (93.3%) were receiving either mother’s own or donor human milk even in the 4th postnatal week, and did not receive substantial amounts of infant formula. None received acid-blocking drugs. A majority were exposed to antibiotics during evaluation for early-onset sepsis, but the duration was not exceptionally prolonged in most (2.8 ± 2.3 days). Our findings suggest that enteric colonization with Gammaproteobacteria may not be an unusual event in VLBW infants, and considering that NEC occurs only in a minority of these infants, suggests that the association between Gammproteobacteria are neither necessary nor sufficient for NEC pathogenesis. The possibility remains that Gammaproteobacteria may constitute a contributory factor in a larger, multifactorial schematic. These findings also call for cautious interpretation of data from small cohorts for a relatively rare outcome, NEC. Gammaproteobacteria colonization and the incidence of NEC seem to peak during the same post-menstrual epoch (31 ± 3 weeks). There is a need for careful estimation of sample size to study this disorder, and the need to interrogate an abundance of specimens prior to the event to refute the possibility that this association is not merely an alpha error or an artifact of confounding.
Temporality
The detection of increased fecal Gammaproteobacteria prior to NEC is exciting for its potential value as a biomarker for risk-stratification and its potential clinical/therapeutic implications.11 As outlined in Table 2, a number of studies indicate that premature infants who develop NEC beyond 3 weeks of postnatal age may display such dysbiosis for several days to weeks leading up to NEC onset.1,2,3,5,6,7,8,9 Pammi et al. also showed in their meta-analysis that infants who developed NEC showed a consistent rise in Proteobacteria abundance with decreased Firmicutes and Bacteroidetes, as a function of post-menstrual age.1 Infants who did not develop NEC showed lower abundances of Proteobacteria and higher abundances of Firmicutes.
Biological gradient
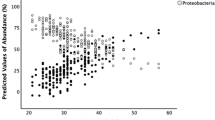
There are no data to suggest that the risk of NEC increases proportionate to the relative abundance of Gammaproteobacteria in the preterm gut microbiome. We recently investigated the relationship between fecal Gammaproteobacteria and fecal calprotectin (FC), which is derived from mucosal leukocytes and is a useful marker of mucosal inflammation.63,64 In our cohort, Gammaproteobacteria abundance did not affect FC expression. Instead, we found FC to be associated specifically with the presence of Klebsiella, and even more strongly, with a single amplicon-sequence variant within this genus. Klebsiella abundance >83% predicted FC > 280 µg/g stool, which have been associated with mucosal inflammation and NEC.65
Our observation that FC correlated with a specific bacterial genus and not the entire class of Gammaproteobacteria suggest that Gammaproteobacteria may be too diverse a group to consistently exert a net inflammatory effect, and perhaps a need for defining dysbiosis at higher levels of taxonomic resolution. There is also a need to confirm whether the dominance of Klebsiella in the preterm gut microbiome in our cohort was specific to our center. The inflammatory effects of Klebsiella in the intestine are plausible, considering the presence of potent virulence factors such as cell wall components and enterotoxins.66,67 Klebsiella are recognized intestinal pathogens of preterm and term neonates, having been identified in diarrhea, ecchymotic colitis, bacteremia during NEC and short-bowel syndrome, and even in NEC outbreaks.24,25,26,68,69 The correlation between fecal Klebsiella and elevated FC has been previously noted in infantile colic.70 Early colonization with Klebsiella has been noted in other preterm cohorts,8,28 but the possibility of finding distinct inflammation-driving pathobiont(s) at other centers cannot be excluded.
Plausibility
Gammaproteobacteria serve an important purpose in the normal newborn. Enterobacteriaceae normally reside in the gut at low levels, localized in close proximity to the mucosa as these bacteria can tolerate relatively high levels of oxygen that diffuses across from the epithelium. In the newborn intestine, Enterobacteriaceae deplete this oxygen and render the microenvironment suitable for colonization of strict anaerobes, such as Bacteroides, Clostridium, and Bifidobacterium.71 During early infancy, breast milk allows oligosaccharide fermenters such as Bifidobacterium to thrive. Subsequent weaning and introduction of solid foods rich in polysaccharides not digestible by host enzymes lead to the expansion of polysaccharide fermenters Bacteroides, Clostridium, Ruminococcus, and simultaneously a decrease in Bifidobacterium and Enterobacteriaceae.72,73
In preterm infants, this normal, seemingly innocuous colonization with Gammaproteobacteria can plausibly turn deleterious. The premature intestine displays heightened sensitivity to Gram-negative bacteria and their products, due to high levels of expression of the Toll-like receptor (TLR)-4, the cognate pathogen recognition receptor for lipopolysaccharides (LPS) and lipid A expressed by coliform bacteria; downstream signaling mediators such as the myeloid differentiation primary response gene 88 (MyD88), the interleukin (IL)-1 receptor-associated kinase 1 (IRAK1), the tumor necrosis factor receptor-associated factor 6 (TRAF6); and the transcriptional regulator nuclear factor kappa B 1 (NF-κB1).74,75,76,77,78 Consistent with these observations, a range of NF-κB-dependent cytokines are increased during NEC, including the tumor necrosis factor (TNF), IL-1, IL-6, IL-8/CXC-motif ligand (CXCL)-8, CXCL1, CXCL2, CC-motif ligand (CCL)-2, CCL3, CCL5, endothelin 1, and the vascular endothelial growth factor.16,32,33,34
The preterm intestine shows a a paucity of normal anti-inflammatory adaptations, which further accentuate its pro-inflammatory bias. In the adult intestine, macrophages display a unique functional dichotomy, where these cells are profoundly anergic to LPS and other bacterial products and yet display avid phagocytic and bacteriocidal properties.79,80 These adaptations of the resident macrophages promote the normal absence of inflammation in the intestine despite close physical proximity to luminal bacteria, and is mediated by transforming growth factor-beta (TGF-β), particularly the isoform TGF-β2, present in the local extracellular matrix.81 The preterm intestine is developmentally deficient in TGF-β2, and this deficiency is further accentuated during NEC due to epigenetic modifications in the TGF-β2 nucleosome.82 During NEC, the newly recruited macrophage precursors also display a high degree of resistance to TGF-β-mediated non-inflammatory differentiation because of increased expression of Smad7.83 Smad7 blocks TGF-β signaling in NEC macrophages by competing with the activating Smads, and sensitizes these cells to LPS through transactivation of IκB kinase-β gene expression and augmentation of NF-κB signaling.83 The midgestation intestine is also deficient in several negative regulators of TLR4-NFκB signaling, including single Ig interleukin-1-related receptor (SIGIRR), IRAK-M, tumor necrosis factor-alpha-induced protein 3 (TNFAIP3), Toll-interacting protein (TOLLIP), and inhibitor of κB (IκB).84
In the preterm intestine, luminal Gammaproteobacteria are more likely to interact with the mucosa because of a developmental paucity of physical and immunological barriers. The mucus layer contains low amounts of the protective mucin 2,85 which is further compromised during NEC.86 There are fewer Paneth cells, and lower expression of antibacterial proteins such as lysozyme and α-defensins.87 The deficiency of secretory IgA (sIgA) is also well known. The appearance of sIgA in mucosal secretions is delayed and increases slowly as a function of post-menstrual age.88,89,90,91 IgA responses are dominated by monomeric sIgA92,93 and the IgA1 sub-class,94 and the antibodies show low antigen affinity, polyreactivity, and autoreactivity.95,96 In addition, the immunoglobulin heavy chains have short complementarity-determining regions,97 which markedly lowers the potential antibody diversity available to premature neonates.97
Coherence
The biological explanations for the association between Gammaproteobacteria and NEC are generally coherent. The two elements that need further scrutiny are the observed lack of any correlation between Gammaproteobacteria and FC, which is a widely accepted marker of mucosal inflammation, and the possibility that high Gammaproteobacteria abundance may not be uncommon in premature infants.55,64
Experiment
In the Bradford Hill framework, a causal inference is supported when interventions (treatments or risk-factor modifications) show a predictable effect on the outcome.11 Several studies show that prolonged exposure to antibiotics, particularly aminoglycosides, can shift the preterm gut microbiome towards decreased alpha diversity and increased Gammaproteobacteria abundance. Fouhy et al. followed the microbiome of 9 infants who received parenteral antibiotic treatment with ampicillin and gentamicin starting within 48 hours of birth.98 Samples collected 4 and 8 weeks later showed significantly higher proportions of Proteobacteria and reduced alpha diversity compared to controls. In another study, Greenwood et al. showed that infants who received 5–7 days of empiric antibiotics during the 1st week showed increased relative abundance of Enterobacter and lower bacterial diversity in the 2nd and 3rd postnatal weeks.59 The effects of antibiotic exposure are not consistent across studies, and a clear effect was not detected in the studies by La Rosa et al. who showed that antibiotics merely influenced the pace, but not the sequence of the patterned colonization in the preterm gut microbiome.45 Torrazza et al. also could not correlate antibiotic usage to specific changes in microbiota.28 Pammi et al. examined the effects of antibiotics in their meta-analysis,1 and showed similar effects of antibiotics in both cases and controls with increased relative abundances of Proteobacteria, and decreased abundances of Firmicutes, Actinobacteria, and Bacteroidetes. At the genus level, antibiotic exposure increased the relative abundances of Klebsiella, unclassified Enterobacteriaceae, Proteus, Paenibacillus, Epulopiscium, and Pseudomonas.
Prolonged empirical antibiotic treatment may increase the risk of NEC in premature infants. Cotten et al. analyzed data from 5693 extremely low birth weight (ELBW) infants admitted to the 19 neonatal research network (NRN) centers.99 The median antibiotic therapy duration was 5 days (range: 1–36 days); 2147 infants (53%) received prolonged (>5 days) empirical therapy (center range: 27–85%) and these infants had increased odds of NEC or death. Similar findings have been reported by Alexander et al., Esmaeilizand et al., Abdel Ghany and Ali, Kuppala et al., and Cantey et al.; some of these studies have used a composite outcome of NEC or late-onset sepsis.100,101,102,103,104 Consistent with these observations, Weintraub et al. noted an association between perinatal exposure to ampicillin and NEC.105 These findings are of interest, but need to be interpreted cautiously. In a recent study, the NRN centers re-examined empiric antibiotic use in 5730 ELBW infants.106 The proportion of infants receiving prolonged early antibiotics varied from 30 to 69% among centers and declined from 49% in 2008 to 35% in 2014. However, prolonged early antibiotic treatment was no longer associated with NEC.
Drugs used to suppress gastric acid production such as histamine (H)-2 receptor blockers have also been examined for potential effects on the preterm gut microbiome and NEC. Gupta et al. compared stool microbiome in 25 preterm infants who received H2 blocker treatment from postnatal days 3–58 vs. 51 controls had not received such treatment.107 The H2 blocker-treated infants showed decreased alpha diversity and a shift towards an increased abundance of Proteobacteria. Guillet et al. examined data from 787 preterm infants from the NRN centers and found antecedent H2-blocker use to be associated with NEC.108 More et al. evaluated this issue in meta-analysis (n = 11,346) and found a significant association between H2 blocker use and NEC (odds ratio 1.78, 95% confidence interval 1.4, 2.27, p < 0.00001).109
Analogous relationships
Gammproteobacterial blooms can be seen during diverse inflammatory conditions of the gastrointestinal tract, including inflammatory bowel disease (IBD), obesity, colorectal cancer, celiac disease, and primary sclerosing cholangitis.110 Intestinal colonization with Proteobacteria has received considerable investigative attention in Crohn’s disease.111 Similar to NEC, a specific organism has not been causally linked with IBD, but abnormalities in the intestinal microbiome are considered part of the underlying pathogenesis. In Crohn’s disease, increased relative abundance of Klebsiella has been linked to an aberrant inflammatory and T-helper cell response.112 Interestingly, a contrarian view is now emerging on Gammaproteobacterial blooms in IBD, where this dysbiosis is believed to be a consequence rather than a cause of inflammation. The selection pressures implicated in these Gammaproteobacterial blooms in the inflamed gut include dietary changes, altered redox potential, mucin utilization, available of metal cofactors, decreased production of antimicrobial peptides, and horizontal gene transfer.113
Reversibility
There are no data yet to show that the correction of the enteric dysbiosis by fecal transplant, specific antibiotics, or other interventions can reduce the risk of NEC.
Conclusions
In above sections, we have presented a detailed appraisal of current evidence supporting an association between Gammaproteobacterial blooms in the intestine and NEC. We looked for, but did not find an objective assessment scale for the Bradford Hill criteria. Therefore, we developed a 5-point scale to systemically evaluate the evidence for each of the 10 Bradford Hill criteria for causal inference (Table 4), and then applied this new assessment metric to the association of enteric dysbiosis and NEC (Table 5). Despite important methodological and statistical limitations, there is support for the association from the larger studies and a meta-analysis.114 The evidence for temporality, that dysbiosis antedated NEC onset, adds strength to a possible causal inference. The role of Gram-negative bacteria in NEC pathogenesis is highly plausible, and is supported by a considerable amount of preclinical evidence. Corroborating observational data on the effects of prolonged exposure to antibiotics and H2-blockers are also supportive.
The weakness in this association is the low level of consistency across studies, and the lack of specificity. Although a large proportion of premature infants may develop an abnormal gut microbiome dominated by Proteobacteria, NEC is still seen only in a minority of these infants. Furthermore, the lack of any correlation between Proteobacteria/Gammaproteobacteria abundance and FC also calls for a better-informed definition of dysbiosis, either by relative abundance or at higher levels of taxonomic resolution. These unresolved concerns indicate that a need for further work before enteric dysbiosis can be causally tied to NEC pathogenesis.
Finally, we need to consider the possibility that the Gammaproteobacterial bloom antedating NEC could be a consequence, not the cause, of mucosal inflammation. This alternative view finds support in evidence that perinatal inflammation arising from chorioamnionitis, prior culture-positive sepsis, or infections such as cytomegalovirus or herpes simplex virus, may predispose to NEC.21,115 In our own cohort,64 the absence of correlation between Gammaproteobacteria abundance and FC may also be consistent with this possibility.
References
Pammi, M. et al. Intestinal dysbiosis in preterm infants preceding necrotizing enterocolitis: a systematic review and meta-analysis. Microbiome 5, 31 (2017).
Wang, Y. et al. 16S rRNA gene-based analysis of fecal microbiota from preterm infants with and without necrotizing enterocolitis. ISME J. 3, 944–954 (2009).
Millar, M. R. et al. Application of 16S rRNA gene PCR to study bowel flora of preterm infants with and without necrotizing enterocolitis. J. Clin. Microbiol. 34, 2506–2510 (1996).
Groer, M. W. et al. Development of the preterm infant gut microbiome: a research priority. Microbiome 2, 38 (2014).
Mshvildadze, M. et al. Intestinal microbial ecology in premature infants assessed with non-culture-based techniques. J. Pediatr. 156, 20–25 (2010).
Mai, V. et al. Fecal microbiota in premature infants prior to necrotizing enterocolitis. PLoS ONE 6, e20647 (2011).
Zhou, Y. et al. Longitudinal analysis of the premature infant intestinal microbiome prior to necrotizing enterocolitis: a case-control study. PLoS ONE 10, e0118632 (2015).
Warner B. B., et al. Gut bacteria dysbiosis and necrotising enterocolitis in very low birthweight infants: a prospective case-control study. Lancet 387, 1928–36 (2016).
Lindberg T. P., et al. Preterm infant gut microbial patterns related to the development of necrotizing enterocolitis. J. Matern. Fetal Neonatal Med. 9, 1–10 (2018).
Hill, A. B. The environment and disease: association or causation? Proc. R Soc. Med. 58, 295–300 (1965).
Fedak, K. M., Bernal, A., Capshaw, Z. A. & Gross, S. Applying the Bradford Hill criteria in the 21st century: how data integration has changed causal inference in molecular epidemiology. Emerg. Themes Epidemiol. 12, 14 (2015).
Polin, R. A. et al. Necrotizing enterocolitis in term infants. J. Pediatr. 89, 460–462 (1976).
Ballance, W. A., Dahms, B. B., Shenker, N. & Kliegman, R. M. Pathology of neonatal necrotizing enterocolitis: a ten-year experience. J. Pediatr. 117, S6–S13 (1990).
Tait, R. A. & Kealy, W. F. Neonatal necrotising enterocolitis. J. Clin. Pathol. 32, 1090–1099 (1979).
Berdon, W. E. et al. Necrotizing enterocolitis in the premature infant. Radiology 83, 879–887 (1964).
Remon, J. I. et al. Depth of bacterial invasion in resected intestinal tissue predicts mortality in surgical necrotizing enterocolitis. J. Perinatol. 35, 755–762 (2015).
Pear, B. L. Pneumatosis intestinalis: a review. Radiology 207, 13–19 (1998).
Bury R. G., Tudehope D. 2001 Enteral antibiotics for preventing necrotizing enterocolitis in low birthweight or preterm infants. Cochrane Database Syst. Rev. CD000405.
Maheshwari, A. Immunologic and hematological abnormalities in necrotizing enterocolitis. Clin. Perinatol. 42, 567–585 (2015).
Gephart, S. M. et al. Changing the paradigm of defining, detecting, and diagnosing NEC: Perspectives on Bell’s stages and biomarkers for NEC. Semin. Pediatr. Surg. 27, 3–10 (2018).
Coggins, S. A., Wynn, J. L. & Weitkamp, J. H. Infectious causes of necrotizing enterocolitis. Clin. Perinatol. 42, 133–154 (2015), ix.
Nanthakumar, N. N., Fusunyan, R. D., Sanderson, I. & Walker, W. A. Inflammation in the developing human intestine: a possible pathophysiologic contribution to necrotizing enterocolitis. Proc. Natl Acad. Sci. USA 97, 6043–6048 (2000).
Mollitt, D. L., Tepas, J. J. 3rd & Talbert, J. L. The microbiology of neonatal peritonitis. Arch. Surg. 123, 176–179 (1988).
Boccia, D., Stolfi, I., Lana, S. & Moro, M. L. Nosocomial necrotising enterocolitis outbreaks: epidemiology and control measures. Eur. J. Pediatr. 160, 385–391 (2001).
Gregersen, N. et al. Klebsiella pneumoniae with extended spectrum beta-lactamase activity associated with a necrotizing enterocolitis outbreak. Pediatr. Infect. Dis. J. 18, 963–967 (1999).
Hill, H. R., Hunt, C. E. & Matsen, J. M. Nosocomial colonization with Klebsiella, type 26, in a neonatal intensive-care unit associated with an outbreak of sepsis, meningitis, and necrotizing enterocolitis. J. Pediatr. 85, 415–419 (1974).
van Acker, J. et al. Outbreak of necrotizing enterocolitis associated with Enterobacter sakazakii in powdered milk formula. J. Clin. Microbiol. 39, 293–297 (2001).
Torrazza, R. M. et al. Intestinal microbial ecology and environmental factors affecting necrotizing enterocolitis. PLoS ONE 8, e83304 (2013).
Morrow, A. L. et al. Early microbial and metabolomic signatures predict later onset of necrotizing enterocolitis in preterm infants. Microbiome 1, 13 (2013).
Hofler, M. The Bradford Hill considerations on causality: a counterfactual perspective. Emerg. Themes Epidemiol. 2, 11 (2005).
McMurtry, V. E. et al. Bacterial diversity and Clostridia abundance decrease with increasing severity of necrotizing enterocolitis. Microbiome 3, 11 (2015).
Smith, B. et al. Investigation of the early intestinal microflora in premature infants with/without necrotizing enterocolitis using two different methods. Pediatr. Res. 71, 115–120 (2012).
Sim, K. et al. Dysbiosis anticipating necrotizing enterocolitis in very premature infants. Clin. Infect. Dis. 60, 389–397 (2015).
de la Cochetiere, M. F. et al. Early intestinal bacterial colonization and necrotizing enterocolitis in premature infants: the putative role of Clostridium. Pediatr Res 56, 366–370 (2004).
Romano-Keeler, J. et al. Distinct mucosal microbial communities in infants with surgical necrotizing enterocolitis correlate with age and antibiotic exposure. PLoS One 13, e0206366 (2018).
Stewart, C. J. et al. The preterm gut microbiota: changes associated with necrotizing enterocolitis and infection. Acta Paediatr 101, 1121–1127 (2012).
Normann, E., Fahlen, A., Engstrand, L. & Lilja, H. E. Intestinal microbial profiles in extremely preterm infants with and without necrotizing enterocolitis. Acta Paediatr 102, 129–136 (2013).
Wandro S., et al. The Microbiome and Metabolome of Preterm Infant Stool Are Personalized and Not Driven by Health Outcomes, Including Necrotizing Enterocolitis and Late-Onset Sepsis. mSphere 3 (2018).
Itani, T. et al. Preterm infants with necrotising enterocolitis demonstrate an unbalanced gut microbiota. Acta Paediatr 107, 40–47 (2018).
Barron, L. K. et al. Independence of gut bacterial content and neonatal necrotizing enterocolitis severity. J Pediatr Surg 52, 993–998 (2017).
Brown, C. T. et al. Hospitalized Premature Infants Are Colonized by Related Bacterial Strains with Distinct Proteomic Profiles. MBio 9, e00441–18 (2018).
Ravi, A. et al. Association of the gut microbiota mobilome with hospital location and birth weight in preterm infants. Pediatr Res 82, 829–838 (2017).
Stewart, C. J. et al. Temporal bacterial and metabolic development of the preterm gut reveals specific signatures in health and disease. Microbiome 4, 67 (2016).
Heida, F. H. et al. A Necrotizing Enterocolitis-Associated Gut Microbiota Is Present in the Meconium: Results of a Prospective Study. Clin Infect Dis 62, 863–870 (2016).
La Rosa, P. S. et al. Patterned progression of bacterial populations in the premature infant gut. Proc Natl Acad Sci U S A 111, 12522–12527 (2014).
Itani, T. et al. Establishment and development of the intestinal microbiota of preterm infants in a Lebanese tertiary hospital. Anaerobe 43, 4–14 (2017).
Gregory, K. E. et al. Influence of maternal breast milk ingestion on acquisition of the intestinal microbiome in preterm infants. Microbiome 4, 68 (2016).
Cong, X. et al. Gut microbiome developmental patterns in early life of preterm infants: impacts of feeding and gender. PLoS ONE 11, e0152751 (2016).
Parm, U., Metsvaht, T., Ilmoja, M. L. & Lutsar, I. Gut colonization by aerobic microorganisms is associated with route and type of nutrition in premature neonates. Nutr. Res. 35, 496–503 (2015).
Raveh-Sadka T., et al. Gut bacteria are rarely shared by co-hospitalized premature infants, regardless of necrotizing enterocolitis development. Elife 4, e05477 (2015).
Arboleya, S. et al. Intestinal microbiota development in preterm neonates and effect of perinatal antibiotics. J. Pediatr. 166, 538–544 (2015).
Arboleya, S. et al. Establishment and development of intestinal microbiota in preterm neonates. FEMS Microbiol. Ecol. 79, 763–772 (2012).
Mai, V. et al. Distortions in development of intestinal microbiota associated with late onset sepsis in preterm infants. PLoS ONE 8, e52876 (2013).
Schwiertz, A. et al. Development of the intestinal bacterial composition in hospitalized preterm infants in comparison with breast-fed, full-term infants. Pediatr. Res. 54, 393–399 (2003).
Ho, T. B. T. et al. Dichotomous development of the gut microbiome in preterm infants. Microbiome 6, 157 (2018).
Gibson, M. K. et al. Developmental dynamics of the preterm infant gut microbiota and antibiotic resistome. Nat. Microbiol. 1, 16024 (2016).
Morowitz, M. J. et al. Strain-resolved community genomic analysis of gut microbial colonization in a premature infant. Proc. Natl Acad. Sci. USA 108, 1128–1133 (2011).
Brooks, B. et al. Microbes in the neonatal intensive care unit resemble those found in the gut of premature infants. Microbiome 2, 1 (2014).
Greenwood, C. et al. Early empiric antibiotic use in preterm infants is associated with lower bacterial diversity and higher relative abundance of Enterobacter. J. Pediatr. 165, 23–29 (2014).
Aagaard, K. et al. The placenta harbors a unique microbiome. Sci. Transl. Med. 6, 237ra265 (2014).
Palmer, C., Bik, E. M., DiGiulio, D. B., Relman, D. A. & Brown, P. O. Development of the human infant intestinal microbiota. PLoS Biol. 5, e177 (2007).
Dominguez-Bello, M. G. et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl Acad. Sci. USA 107, 11971–11975 (2010).
MacQueen, B. C. et al. Elevated fecal calprotectin levels during necrotizing enterocolitis are associated with activated neutrophils extruding neutrophil extracellular traps. J. Perinatol. 36, 862–869 (2016).
Ho T. B. T., et al. Enteric dysbiosis and fecal calprotectin expression in premature infants. Pediatr. Res. 85, 361–368 2018. in press.
Zhang, M., Zhang, X. & Zhang, J. Diagnostic value of fecal calprotectin in preterm infants with necrotizing enterocolitis. Clin. Lab 62, 863–869 (2016).
Lu, M. C. et al. Colibactin contributes to the hypervirulence of pks( + ) K1 CC23 Klebsiella pneumoniae in mouse meningitis infections. Front. Cell. Infect. Microbiol. 7, 103 (2017).
Straus, D. C., Lonon, M. K., Woods, D. E. & Garner, C. W. Production of an extracellular toxic complex by various strains of Pseudomonas cepacia. J. Med. Microbiol. 30, 17–22 (1989).
Stone, H. H., Kolb, L. D. & Geheber, C. E. Bacteriologic considerations in perforated necrotizing enterocolitis. South Med. J. 72, 1540–1544 (1979).
Canioni, D. et al. Histopathology and microbiology of isolated rectal bleeding in neonates: the so-called ‘ecchymotic colitis’. Histopathology 30, 472–477 (1997).
Rhoads, J. M. et al. Altered fecal microflora and increased fecal calprotectin in infants with colic. J. Pediatr. 155, 823–828 e821 (2009).
Arrieta, M. C., Stiemsma, L. T., Amenyogbe, N., Brown, E. M. & Finlay, B. The intestinal microbiome in early life: health and disease. Front. Immunol. 5, 427 (2014).
Koenig, J. E. et al. Succession of microbial consortia in the developing infant gut microbiome. Proc. Natl Acad. Sci. USA 108(Suppl 1), 4578–4585 (2011).
Fallani, M. et al. Determinants of the human infant intestinal microbiota after the introduction of first complementary foods in infant samples from five European centres. Microbiology 157, 1385–1392 (2011).
MohanKumar, K. et al. Trinitrobenzene sulfonic acid-induced intestinal injury in neonatal mice activates transcriptional networks similar to those seen in human necrotizing enterocolitis. Pediatr. Res. 81, 99–112 (2016).
MohanKumar, K. et al. Cytokines and growth factors in the developing intestine and during necrotizing enterocolitis. Semin. Perinatol. 41, 52–60 (2016).
Good, M. et al. Amniotic fluid inhibits Toll-like receptor 4 signaling in the fetal and neonatal intestinal epithelium. Proc. Natl Acad. Sci. USA 109, 11330–11335 (2012).
Hackam, D. J., Upperman, J. S., Grishin, A. & Ford, H. R. Disordered enterocyte signaling and intestinal barrier dysfunction in the pathogenesis of necrotizing enterocolitis. Semin. Pediatr. Surg. 14, 49–57 (2005).
Leaphart, C. L. et al. A critical role for TLR4 in the pathogenesis of necrotizing enterocolitis by modulating intestinal injury and repair. J. Immunol. 179, 4808–4820 (2007).
Smythies, L. E. et al. Human intestinal macrophages display profound inflammatory anergy despite avid phagocytic and bacteriocidal activity. J. Clin. Invest. 115, 66–75 (2005).
Maheshwari, A. et al. Cytomegalovirus blocks intestinal stroma-induced down-regulation of macrophage HIV-1 infection. J. Leukoc. Biol. 80, 1111–1117 (2006).
Maheshwari, A. et al. TGF-β2 suppresses macrophage cytokine production and mucosal inflammatory responses in the developing intestine. Gastroenterology 140, 242–253 (2011).
Namachivayam, K. et al. Smad7 inhibits autocrine expression of TGF-beta2 in intestinal epithelial cells in baboon necrotizing enterocolitis. Am. J. Physiol. Gastrointest. Liver Physiol. 304, G167–G180 (2013).
MohanKumar, K. et al. Smad7 interrupts TGF-β signaling in intestinal macrophages and promotes inflammatory activation of these cells during necrotizing enterocolitis. Pediatr. Res. 79, 951–961 (2016).
Nanthakumar, N. et al. The mechanism of excessive intestinal inflammation in necrotizing enterocolitis: an immature innate immune response. PLoS ONE 6, e17776 (2011).
Montgomery, R. K., Mulberg, A. E. & Grand, R. J. Development of the human gastrointestinal tract: twenty years of progress. Gastroenterology 116, 702–731 (1999).
Martin, N. A. et al. Active transport of bile acids decreases mucin 2 in neonatal ileum: implications for development of necrotizing enterocolitis. PLoS ONE 6, e27191 (2011).
Zhang, C. et al. Paneth cell ablation in the presence of Klebsiella pneumoniae induces necrotizing enterocolitis (NEC)-like injury in the small intestine of immature mice. Dis. Model Mech. 5, 522–532 (2012).
Haworth, J. C. & Dilling, L. Concentration of gamma-A-globulin in serum, saliva, and nasopharyngeal secretions of infants and children. J. Lab Clin. Med. 67, 922–933 (1966).
Brandtzaeg, P., Nilssen, D. E., Rognum, T. O. & Thrane, P. S. Ontogeny of the mucosal immune system and IgA deficiency. Gastroenterol. Clin. North Am. 20, 397–439 (1991).
Gleeson, M. et al. Ontogeny of the secretory immune system in man. Aust. N. Z. J. Med. 12, 255–258 (1982).
Mellander, L., Carlsson, B. & Hanson, L. A. Appearance of secretory IgM and IgA antibodies to Escherichia coli in saliva during early infancy and childhood. J. Pediatr. 104, 564–568 (1984).
Cripps, A. W., Gleeson, M. & Clancy, R. L. Ontogeny of the mucosal immune response in children. Adv. Exp. Med. Biol. 310, 87–92 (1991).
Weemaes, C. et al. Development of immunoglobulin A in infancy and childhood. Scand. J. Immunol. 58, 642–648 (2003).
Fitzsimmons, S. P. et al. Immunoglobulin A subclasses in infants’ saliva and in saliva and milk from their mothers. J. Pediatr. 124, 566–573 (1994).
Bhat, N. M. et al. The ontogeny and functional characteristics of human B-1 (CD5 + B) cells. Int. Immunol. 4, 243–252 (1992).
Chen, Z. J. et al. Polyreactive antigen-binding B cells are the predominant cell type in the newborn B cell repertoire. Eur. J. Immunol. 28, 989–994 (1998).
Bauer, K. et al. Diversification of Ig heavy chain genes in human preterm neonates prematurely exposed to environmental antigens. J. Immunol. 169, 1349–1356 (2002).
Fouhy, F. et al. High-throughput sequencing reveals the incomplete, short-term recovery of infant gut microbiota following parenteral antibiotic treatment with ampicillin and gentamicin. Antimicrob. Agents Chemother. 56, 5811–5820 (2012).
Cotten, C. M. et al. Prolonged duration of initial empirical antibiotic treatment is associated with increased rates of necrotizing enterocolitis and death for extremely low birth weight infants. Pediatrics 123, 58–66 (2009).
Alexander, V. N., Northrup, V. & Bizzarro, M. J. Antibiotic exposure in the newborn intensive care unit and the risk of necrotizing enterocolitis. J. Pediatr. 159, 392–397 (2011).
Esmaeilizand, R. et al. Antibiotic exposure and development of necrotizing enterocolitis in very preterm neonates. Paediatr. Child Health 23, e56–e61 (2018).
Abdel Ghany, E. A. & Ali, A. A. Empirical antibiotic treatment and the risk of necrotizing enterocolitis and death in very low birth weight neonates. Ann. Saudi Med. 32, 521–526 (2012).
Kuppala, V. S., Meinzen-Derr, J., Morrow, A. L. & Schibler, K. R. Prolonged initial empirical antibiotic treatment is associated with adverse outcomes in premature infants. J. Pediatr. 159, 720–725 (2011).
Cantey, J. B., Pyle, A. K., Wozniak, P. S., Hynan, L. S. & Sanchez, P. J. Early antibiotic exposure and adverse outcomes in preterm, very low birth weight infants. J. Pediatr. 203, 62–67 (2018).
Weintraub, A. S. et al. Antenatal antibiotic exposure in preterm infants with necrotizing enterocolitis. J. Perinatol. 32, 705–709 (2012).
Greenberg R. G., et al., Eunice Kennedy Shriver National Institute of Child H, Human Development Neonatal Research N Prolonged duration of early antibiotic therapy in extremely premature infants. Pediatr. Res. 85, 994–1000 (2019).
Gupta, R. W. et al. Histamine-2 receptor blockers alter the fecal microbiota in premature infants. J. Pediatr. Gastroenterol. Nutr. 56, 397–400 (2013).
Guillet, R. et al. Association of H2-blocker therapy and higher incidence of necrotizing enterocolitis in very low birth weight infants. Pediatrics 117, e137–e142 (2006).
More, K., Athalye-Jape, G., Rao, S. & Patole, S. Association of inhibitors of gastric acid secretion and higher incidence of necrotizing enterocolitis in preterm very low-birth-weight infants. Am. J. Perinatol. 30, 849–856 (2013).
Winter, S. E. & Baumler, A. J. Dysbiosis in the inflamed intestine: chance favors the prepared microbe. Gut Microbes 5, 71–73 (2014).
Atarashi, K. et al. Ectopic colonization of oral bacteria in the intestine drives TH1 cell induction and inflammation. Science 358, 359–365 (2017).
Larmonier, C. B., Shehab, K. W., Ghishan, F. K. & Kiela, P. R. T lymphocyte dynamics in inflammatory bowel diseases: role of the microbiome. Biomed. Res. Int. 2015, 504638 (2015).
Zeng, M. Y., Inohara, N. & Nunez, G. Mechanisms of inflammation-driven bacterial dysbiosis in the gut. Mucosal Immunol. 10, 18–26 (2017).
Friede, T., Rover, C., Wandel, S. & Neuenschwander, B. Meta-analysis of few small studies in orphan diseases. Res. Synth. Methods 8, 79–91 (2017).
Been, J. V., Lievense, S., Zimmermann, L. J., Kramer, B. W. & Wolfs, T. G. Chorioamnionitis as a risk factor for necrotizing enterocolitis: a systematic review and meta-analysis. J. Pediatr. 162, 236–242 e232 (2013).
Acknowledgements
NIH awards HL124078 and HL133022 (to A.M.).
Author information
Authors and Affiliations
Contributions
J.B.F., P.G., D.R.S., M.P., and A.M. reviewed the literature and contributed to the manuscript. A.M. developed the Bradford Hill Causality Score. All the authors reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fundora, J.B., Guha, P., Shores, D.R. et al. Intestinal dysbiosis and necrotizing enterocolitis: assessment for causality using Bradford Hill criteria. Pediatr Res 87, 235–248 (2020). https://doi.org/10.1038/s41390-019-0482-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-019-0482-9
This article is cited by
-
Time to first passage of meconium and defecation frequency preceding necrotizing enterocolitis in preterm infants: a case–control study
European Journal of Pediatrics (2023)
-
Neonatal intermittent hypoxia, fish oil, and/or antioxidant supplementation on gut microbiota in neonatal rats
Pediatric Research (2022)
-
Longitudinal gut virome analysis identifies specific viral signatures that precede necrotizing enterocolitis onset in preterm infants
Nature Microbiology (2022)
-
Multi-strain probiotics for extremely preterm infants: a randomized controlled trial
Pediatric Research (2022)
-
Incomplete resection of necrotic bowel may increase mortality in infants with necrotizing enterocolitis
Pediatric Research (2021)