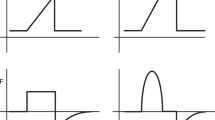Abstract
Most preterm infants breathe at birth, but need additional respiratory support due to immaturity of the lung and respiratory control mechanisms. To avoid lung injury, the focus of respiratory support has shifted from invasive towards non-invasive ventilation. However, applying effective non-invasive ventilation is difficult due to mask leak and airway obstruction. The larynx has been overlooked as one of the causes for obstruction, preventing face mask ventilation from inflating the lung. The larynx remains mostly closed at birth, only opening briefly during a spontaneous breath. Stimulating and supporting spontaneous breathing could enhance the success of non-invasive ventilation by ensuring that the larynx remains open. Maintaining adequate spontaneous breathing and thereby reducing the need for invasive ventilation is not only important directly after birth, but also in the first hours after admission to the NICU. Respiratory distress syndrome is an important cause of respiratory failure. Traditionally, treatment of RDS required intubation and mechanical ventilation to administer exogenous surfactant. However, new ways have been implemented to administer surfactant and preserve spontaneous breathing while maintaining non-invasive support. In this narrative review we aim to describe interventions focused on stimulation and maintenance of spontaneous breathing of preterm infants in the first hours after birth.
Similar content being viewed by others
Introduction
Compared to term infants, the respiratory system of preterm infants is structurally and biochemically immature, with a highly compliant chest wall, a large gas diffusion barrier, and stiff lungs due to structural immaturity and surfactant deficiency.1 Although most preterm infants breathe at birth, respiratory support is often needed to ensure adequate gas exchange.2,3 While traditionally the infant was intubated and mechanically ventilated, there is considerable evidence that this approach increases the risk of lung and brain injury with subsequent long-term impairment of lung function and neurodevelopment.4,5 To avoid injury, the focus of respiratory support has therefore shifted toward more non-invasive approaches, such as applying positive pressure support or ventilation via face mask.6,7,8,9,10 However, little is known on how non-invasive respiratory support interacts with the infant’s changing physiology. It is unknown whether applied strategies are effective, counterproductive, or even injurious.
The effectiveness of non-invasive ventilation might be hampered by a number of reasons. For instance, mask leak is often not recognized by the caregiver and represents one of the major causes of ineffective ventilation, as it reduces the administered tidal volumes.11,12,13 Ventilating non-invasively by face mask without leakage requires training and experience.12,13,14,15 In addition, commercially available face masks are commonly not of an appropriate size for the infant’s face, particularly in preterm infants, making it difficult to avoid placing the rim over the chin or eyes.16,17,18 As a result, efforts have been made to implementing extra training,19 improving ventilation devices,20 and designing different masks.21 Another complication is that, in an effort to minimize mask leak during ventilation, caregivers may also inadvertently further reduce the effectiveness of ventilation by pressing too hard and obstructing the upper airways.13,22,23
The adducted larynx at birth has so far been overlooked as a possible cause for obstruction. Lung aeration can only take place in case of an open airway—including the larynx.2,24,25 However, during fetal life the larynx is chronically adducted to promote lung expansion and thereby lung growth and it is unknown when and how the larynx adapts to the new function after birth.25,26 There is now evidence that immediately after birth the larynx continues to function as it does in fetal life and remains mostly closed, making ventilation strategies inadequate when applied non-invasively.24 This was recently demonstrated in a preterm rabbit model showing that, at birth, the larynx is predominantly closed during apnea and opens only briefly when a breath is taken. This pattern changes and the larynx remains mainly open once a stable breathing pattern has been established.24 This explains the distention of the upper airway that can occur during mask ventilation, as has been demonstrated in preterm lambs and infants by van Vonderen et al.27 This should be taken into account when targeting ventilation, because tidal volumes could be interpreted as “appropriate” during mask ventilation, while the closed larynx prevents lung aeration and gas exchange, which results in inadequate ventilation. Stimulating spontaneous breathing of preterm infants at birth could therefore enhance the success of non-invasive ventilation at birth.
Maintaining spontaneous breathing is also an important goal in the first hours after admission to the Neonatal Intensive Care Unit (NICU). While the majority of preterm infants leave the delivery room supported by non-invasive ventilation, a proportion of them will suffer from severe respiratory distress syndrome, for which administration of exogenous surfactant might be needed.28 Traditionally, intubation and subsequently mechanical ventilation was required to administer surfactant, increasing the risk for ventilation-induced lung injury.29 However, recent trials have demonstrated the feasibility and efficacy of surfactant administration in a minimally invasive way, thereby omitting intubation.30,31 A stable respiratory drive is a prerequisite to make this procedure successful in avoiding intubation and mechanical ventilation.
In this narrative review, we aimed to describe interventions to stimulate and maintain spontaneous breathing of preterm infants at birth and in the first hours after admission in the NICU. An overview of these interventions with outcomes is shown in Table 1.
Stimulating spontaneous breathing at birth
Tactile stimulation
While fetuses utilize breathing movements during pregnancy to promote lung expansion and growth, the purpose of breathing movements after birth changes to establishing lung aeration and gas exchange.32 The triggers for changing from discontinuous fetal breathing movement to a more continuous postnatal breathing pattern are not clear but could include activation of chemoreceptors, increased PCO2 levels, loss of factors inhibiting respiratory center activity (prostaglandins, progesterone metabolites, adenosine), cold stimulus to the skin, and physical stimuli (light, temperature, handling).33
Although there is little data on effectivity of tactile stimulation at birth, it seems logical that this would increase respiratory effort and it has therefore been incorporated in the international resuscitation guidelines. So far, only a few experimental studies have focused on tactile stimulation. Faridy showed that newborn rat pups die from respiratory distress if the mother is prevented to perform her stimulation process (licking, rolling, and biting the pup).34 In addition, the breathing rate is higher in preterm rats receiving tactile stimulation that simulates licking from the mother, compared to rat pups not receiving this stimulation.35 It is possible that physical stimulation increases breathing effort, by causing a change in arousal state of the infant.36,37,38
International resuscitation guidelines recommend tactile maneuvers such as warming, drying, and rubbing the back or soles of the feet to stimulate respiratory activity in (preterm) infants at birth.39,40 However, the guidelines do not specifically indicate timing and methods of stimulation, which is probably the reason why in recent studies a wide variety of practices have been used.41,42,43,44
Dekker et al. and Baik-Schneditz et al. observed that the primary method of stimulation varied from rubbing the soles of the feet to rubbing the chest and/or back.41,42,43 Activation of different sensory pathways could be involved in these methods of application (Fig. 1). Stimulation of proprioceptors activated by changing the position of the feet and legs by rubbing the soles of the feet has been shown to reduce breathing pauses and increase respiratory rate.45,46 Rubbing the chest or back, thereby activating the somatic or visceral mechanoreceptors in the thoracic region, might affect the respiratory center as well.47,48 Activating a larger number of receptors by applying stimulation to a larger cutaneous surface area might increase the effect. However, the most effective method of stimulation remains unclear.
The reported incidence of stimulation varies between 35% in the study of Baik-Schneditz et al. and 90% in the study of van Henten et al.41,42,43,44 Lower percentages of stimulation were reported in infants <30 weeks of gestation, who are wrapped in a polyethylene bag.42,43 The polyethylene bag might form a physical barrier and thereby contributes to the omission of stimulation.49,50 However, these infants usually need more (respiratory) support and could benefit from receiving tactile stimulation.
The true effect of tactile stimulation on respiratory effort is difficult to determine in human preterm infants. Clinical equipoise for omitting stimulation is not possible as tactile stimulation has been common practice for many years and has become a fundamental intervention of resuscitation, even though recent studies described that one third to even two thirds of infants do not receive any stimulation.41,42 Therefore, the effect of standard stimulation (stimulation at the discretion of the caregiver) was recently compared to a strict protocol of repetitive stimulation in a randomized controlled trial (RCT) in preterm infants at birth. It was hypothesized that repetitive stimulation, consisting of stimulation episodes of 10 s alternated with pauses of 10 s, would improve breathing effort. Pauses in stimulation were included in an attempt to avoid habituation of the reflex.51 Respiratory effort was shown to be higher in the repetitive stimulation group, but these differences did not reach statistical significance. However, while infants in the repetitive stimulation group had significantly better oxygenation, despite requiring a significantly lower FiO2, the findings of an increased respiratory effort were clinically relevant and indicate that applying repetitive stimulation may facilitate the respiratory transition of preterm infants at birth.52 It is important to note that, despite the fact that infants in the standard stimulation group were supposedly stimulated based on clinical indication, these infants received much higher levels of stimulation than previously observed in cohort studies (96% vs. 67%).41,42,43,52 It is likely that performing studies on a maneuver like tactile stimulation produced a Hawthorne effect, leading to an increase in application of stimulation in the control group.52 This explains the smaller than expected differences between the intervention and control groups in respiratory effort, resulting in an underestimation of the effect. Although further larger trials are needed to test the effect of repetitive stimulation on clinical outcomes, the demonstrated positive effect on respiratory effort may reduce further our ability to attain clinical equipoise.52
Oxygenation
It is well established that, in utero, when oxygenation levels are reduced below normal (PaO2 <25–30 mm Hg), fetal breathing movements are greatly reduced or even abolished.38,53,54,55,56 On the other hand, hyperoxia can stimulate fetal breathing movements, but the stimulatory effect is not sustained.57,58,59
After birth, it is now well established that the inhibitory effect of hypoxia (PaO2 <20–25 mm Hg) on breathing persists for days–weeks; it diminishes with time and eventually switches to a stimulation of respiratory drive, which persists for the remainder of our lives.60 In addition, intermittent hypoxia during uterine contractions might elevate fetal plasma adenosine concentrations, which also could inhibit peripheral and central chemoreceptors and cause respiratory depression.61
Up until 2005, guidelines recommended that resuscitation of preterm infants commenced with a fraction of inspired oxygen (FiO2) of 1.0 in order to improve oxygenation at birth. However, oxygen saturations (SpO2) were not monitored consistently, which resulted in an increased risk for hyperoxia.62 Excessive oxygen exposure should be avoided in infants during stabilization at birth, as hyperoxia increases free radical production thereby overwhelming the immature antioxidant capacity of the preterm infant, which might lead to damage to cells, enzymes, lipids, DNA, and proteins.63,64,65 Meta analyses have found that resuscitation of term infants at birth with air significantly reduced mortality compared with infants resuscitated with 100% oxygen.66,67,68 Less data are available in preterm infants, although hyperoxia at birth likely increases the risk of bronchopulmonary dysplasia (BPD).69,70 For this reason, international resuscitation guidelines now recommend to initiate resuscitation with low FiO2 levels, which should thereafter be titrated based on SpO2 target ranges.40 However, the SpO2 target ranges are based on data from healthy term and preterm infants who did not need extensive resuscitation.71 As such, the optimal SpO2 target ranges for compromised preterm infants are not clear, although it is possible that better oxygenation is needed for optimal stimulation of spontaneous breathing. Currently, a trial is being performed evaluating clinical outcomes after targeting higher SpO2 values at 5 and 10 min after birth.(Registered in the Australian New Zealand Clinical Trial Registry; ACTRN12615000115538).
Oxygenation is largely defined by the surface area available for gas exchange and the diffusion distance as well as the partial pressure gradient for oxygen between the alveoli and adjacent capillaries. It has become clear that in most very preterm infants clinicians fail to create adequate lung aeration and thus have to use a higher FiO2 to compensate for the suboptimal surface area created for gas exchange. Using the latest resuscitation guidelines, most preterm infants fail to reach the 25th percentile of the SpO2 reference values in the first minutes after birth, despite the use of SpO2-based titration.72,73,74,75,76,77,78 Caregivers thereby appear to accept hypoxia and disregard the effect on respiratory effort. As increasing the FiO2 can reduce the level of hypoxia, this would be expected to increase the respiratory effort in preterm infants. However, this has so far only been demonstrated in an observational study where an increase in respiratory drive was observed after switching fraction of inspired oxygen from 0.21 to 1.0.79 Nevertheless, it is important to recognize that when hypoxia persists for longer than the first 5 min after birth, it is associated with a higher risk of mortality before hospital discharge and development of intraventricular hemorrhage.80 Caregivers should aim for an optimal level of oxygenation in preterm infants directly after birth, while avoiding both hypoxia and hyperoxia.
The effect of oxygenation on respiratory effort at birth was recently demonstrated in a spontaneously breathing preterm rabbit model, while supported non-invasively kittens showed a more stable breathing pattern at birth when FiO2 1.0 was given compared to FiO2 0.21 (unpublished data). Kittens receiving room air suffered from apnea, and the breathing pattern was restored more stable after rescue ventilation was given with a FiO2 of 1.0 compared to room air (unpublished data). In a preterm lamb model, resuscitation with FiO2 of 1.0 led to an increase in pulmonary blood flow equal to the increase occurring in term lambs, permitting optimal gas exchange, while this increase was not observed in lambs resuscitated with FiO2 0.21 with or without subsequent SpO2-based titration of FiO2. However, they spent less time in the SpO2 target range.81
High vs. low FiO2 was compared in recent multicenter trials in human preterm infants.74,75,77,78,82,83,84,85 Infants receiving an initial FiO2 of 1.0 reached their SpO2 target ranges earlier and remained within the target ranges for a longer period in some trials,74,75,76,77,78 while in other trials no difference between high and low FiO2 levels on these outcomes were observed.74,75,77,78,83,84,85,86 The difference in effect might be explained by the differences in titration protocols between studies using different time intervals and magnitude of steps taken.
So far, the studies comparing different initial FiO2 levels did not evaluate the effect on respiratory effort. A trial is currently being performed to test the effect of initial high FiO2 vs. low FiO2 with subsequent titration based on SpO2 on respiratory effort in the first minutes after birth (registered in the Dutch Trial Registry under registry number NTR6878, www.trialregister.nl).
Caffeine
Adenosine acts as a neuromodulator, influencing the tonic modulation of breathing by inhibiting respiratory effort. The level of adenosine is influenced by different variables, including inflammation and hypoxia.61 Caffeine is a methylxanthine that has a molecular structure similar to adenosine and works as an adenosine receptor antagonist to reduce adenosine-induced respiratory depression.87 Although the safety and effectivity of caffeine to prevent apnea of prematurity has been demonstrated in a large randomized trial, the optimal timing and dosage is still unclear.88,89 A systematic review comparing the effects of high vs. low doses of caffeine in the first days after birth demonstrated that a high dose of caffeine led to a decrease in BPD, the combined outcome BPD or death, and extubation failure, although the level of evidence was reported to be low.90 However, these findings endorse the possible advantages of a higher dose of caffeine, which should be confirmed in a large RCT.
Caffeine administered within the first 2 days of life decreases the risk of developing BPD and improves both short- and long-term neurodevelopmental outcomes.88,91,92,93,94 When administered within 2 h after birth, it decreases the incidence of continuous positive airway pressure (CPAP) failure, which in turn could lead to further improvement in outcome.95 One possible explanation is increased diaphragm activity, which occurs after administration of a loading dose of caffeine, leading to higher tidal volumes that are indicative of an increase in respiratory effort.96 At birth, increased adenosine levels might lead to depression of the respiratory center,61 and therefore administering caffeine directly at birth could counteract this effect by antagonizing adenosine. A recent trial evaluated the effect of administration of caffeine base (10 mg/kg, administered by the use of a butterfly needle (21 G) inserted in the umbilical vein) in the delivery room on respiratory effort of preterm infants.97 Infants who received caffeine in the delivery room had a greater respiratory effort, with higher minute volumes, inspired tidal volumes, and recruitment breaths (with a tidal volume >8 ml/kg), as compared with infants receiving caffeine after admission to the NICU.97 Although this trial consisted of a small number of infants with a gestational age of 24–30 weeks, the trial was able to demonstrate a significant positive correlation between minute volume and gestational age. The minute volume increased by 2.4 ml/min/kg with each day of gestational age. This association was even more pronounced when caffeine was administered in the first minutes after birth with an increase in minute volume of 4.1 ml/min/kg with each day of gestational age.97 These results indicate that the stimulatory effect of caffeine is gestational age dependent, and different caffeine dosages per gestational age would be needed to gain the optimal effect on breathing effort.97 More studies on caffeine at birth are needed with respect to relevant clinical outcomes, as well as dose finding, since it has been shown that the required dose might be dependent of gestational age and level of adenosine present at birth. Inflammation leads to an increase in adenosine levels, and also the presence of hypoxia leads to an imbalance between adenosine synthesis and its breakdown.98,99
Because caffeine can freely pass the placenta by passive diffusion, administration to the mother before or during delivery could potentially lead to a direct stimulating effect on respiratory drive of the preterm infant at the time of birth.100 This was demonstrated in the lamb model of Binder-Heschl et al., which showed that a loading dose of caffeine base administered to the ewe resulted in similar plasma caffeine concentration in the mother and the lamb obtained immediately following infusion.101 In addition, the study of Binder-Heschl et al. showed a significant decrease in SpO2 after cord clamping in the lambs not receiving caffeine, while this was absent in the caffeine-treated lambs.101 It is possible that, when antenatal administration of caffeine leads to better aeration of the lung, this could decrease the occurrence of hypoxia after early clamping of the cord.
Delayed cord clamping
Before birth, the placental circulation contains approximately 30–50% of the blood volume of the combined fetal/placental unit. While the lungs remain unaerated and the pulmonary circulation remains vasoconstricted, cardiac output is largely dependent on venous return from the placenta. Clamping the umbilical cord before lung aeration, therefore, causes umbilical venous return to cease, which can lead to a sudden decrease in cardiac output. However, when the lungs aerate before cord clamping, the associated increase in pulmonary blood flow can replace umbilical venous return as the primary source of ventricular preload and as such cardiac output remains unchanged. As such, clamping the cord after ventilation onset has less impact on cardiac output and avoids the cardiovascular instability at the time of clamping.102,103 This has been demonstrated in a preterm lamb model showing a more stable heart rate and arterial pressure when the cord is clamped after lung aeration compared to clamping before lung aeration.104 In addition, ventilation before clamping of the umbilical cord has been shown to increase arterial and cerebral oxygenation.105 Increasing the pulmonary blood flow also leads to a better ventilation/perfusion ratio, thereby optimizing the uptake of oxygen leading to better oxygenation. This will enhance respiratory effort even more.
It is currently unclear how delaying cord clamping affects the respiratory transition after birth. Keeping the cord intact should provide the newborn with a baseline PaO2 that is no lower than that which occurred before birth, assuming that placental gas exchange is still functional. Delaying cord clamping could thereby help in the establishment of a continuous breathing pattern after birth, as severe breathing-inhibitory hypoxia due to cord clamping would be avoided. On the other hand, the placenta releases prostaglandins (of the E series) and adenosine into the fetal circulation that are known to inhibit breathing.106 As such, cutting the cord might be beneficial for breathing activity as it would reduce circulating prostaglandin (and adenosine) levels and thereby reduce any inhibitory effect on breathing.107 However, as circulating prostaglandins are completely metabolized by circulation through the lung, the inhibitory effect of the prostaglandins may only be an issue in apneic infants.108,109 This makes it only more important that lung aeration occurs while delaying cord clamping. Also, it is possible that, for those apneic infants, prostaglandin synthesis inhibitors might result in an increase in respiratory activity.110
So far, there are no studies assessing respiratory effort of infants receiving delayed cord clamping. However, Katheria et al. showed that infants who did not receive respiratory support during delayed cord clamping needed significantly more stimulation to initiate breathing, and the duration of stimulation was longer to maintain spontaneous breathing.111 During delayed cord clamping, respiratory support could enhance spontaneous breathing by improving lung aeration, leading to better oxygenation and reducing hypoxia.105,112 Although timing of the first breath is a measure of respiratory effort, effectivity of spontaneous breathing was not objectively evaluated by using respiratory function parameters. By objectively evaluating respiratory effort during delayed cord clamping, one could determine to what extent circulating prostaglandin affects spontaneous breathing, and thus whether there appears to be an indication for the use of prostaglandin synthesis inhibitors.
Maintaining spontaneous breathing in the first hours after birth
Continuous positive airway pressure
Applying CPAP can be used to facilitate respiratory transition at birth by increasing the pressure gradient, which promotes alveolar fluid reabsorption and prevents end-expiratory alveolar collapse. This in turn increases the surface area available for gas exchange,113 leading to improved oxygen exchange and a decreased risk of hypoxia.114 Also, functional residual capacity (FRC) will increase by maintaining alveolar aeration during both inspiration and expiration, leading to a reduction in work of breathing.115 In infants who breathe spontaneously at birth, CPAP is therefore recommended for use as the initial mode of respiratory support.30,40 Studies showed that the use of CPAP after the initial stabilization at birth led to decreased BPD rates when compared to elective intubation and positive pressure ventilation.4,116
While the beneficial effects of CPAP use in the delivery room have been shown, the optimal CPAP level and strategy remain unclear. A recent review has shown that there is a wide variety of CPAP practices across different units, varying in pressure levels and titration strategies.117 Although the international guidelines recommend the use of CPAP levels between 5 and 8 cm H2O, experimental studies have shown that higher CPAP levels lead to better lung aeration.24,118 Instead of using a fixed CPAP level, we might need to adjust the level according to the phase of respiratory transition. It is likely that higher CPAP levels are initially needed to assist airway liquid clearance during lung aeration, whereas during the subsequent phase, the primary role of CPAP is to minimize airway liquid re-entry when the lung is at FRC.117 On the other hand, sustained high CPAP levels might delay stiffening of the chest wall by opposing lung recoil. Therefore, CPAP levels should be weaned down after respiratory transition at birth and clearance of lung liquid from the interstitial space. However, more data are needed to define the optimal CPAP strategy for facilitating and maintaining lung aeration at birth in order to improve oxygenation without causing overdistension of the lung.119
Surfactant
After transition has been successfully established, respiratory distress syndrome (RDS) can cause difficulties in obtaining an appropriate level of oxygenation, which might lead to CPAP failure.8,10,120 In this stage, oxygenation can be improved by treating RDS with exogenous surfactant, as this results in improved lung compliance and less work of breathing.121 Traditionally, surfactant treatment requires intubation and mechanical ventilation.122,123 However, surfactant treatment via an endotracheal tube is usually associated with a loss of spontaneous breathing and the requirement for mechanical ventilation.
Recently, minimal or less invasive surfactant administration techniques have gained increasing favor.31,124 This involves administering surfactant via nasogastric tubes, angiocatheters, or specially designed catheters positioned in the trachea while the infant is spontaneously breathing on CPAP. These techniques have shown to be effective, resulting in increased breathing effort.125,126,127,128,129,130 It is apparent that administering surfactant using these techniques results in successful surfactant application without mechanical ventilation in 60% of infants with a birth weight <1500 g124 and in 92% of infants with a gestational age of 29–32 weeks.131
Surfactant administration via these approaches have been termed “less or minimally invasive (minimally invasive surfactant therapy (MIST))”, but this terminology is potentially misleading as the procedure still involves placement of a catheter in the trachea using a laryngoscope to visualize the vocal cords.31,124,132 It is known that laryngoscopy is highly uncomfortable, and while this is performed in an awake infant, his/her attempts to resist this procedure might lead to negative cardiovascular responses.133,134,135 As experiencing pain during procedures might affect neurodevelopment of preterm infants, efforts should be taken to reduce pain or discomfort during a procedure.136 In addition, the use of sedation to enhance the comfort of the infant during MIST could increase the chance of an uneventful procedure. On the other hand, caution is needed as the use of sedation during the procedure might impair the infant’s respiratory drive.
The choice of sedative during MIST is dependent of the level of sedation/analgesia that can be achieved, counterbalanced by the negative effect it may have on respiratory drive. Remifentanil was thought to be promising sedative for procedures such as intubation–surfactant–extubation (INSURE) due to its rapid distribution and redistribution.137 However, remifentanil is a potent respiratory depressant and the level of sedation during INSURE was not shown to be effective.138 Recent trials have shown an adequate level of sedation when Propofol is used and it is now widely used for intubation and other procedures.139,140,141 However, side effects such as hypotension have been described.142 There is much controversy whether Propofol provides analgesia next to sedation.143 Nevertheless, the sedative effect of Propofol might result in better comfort and thus less stress, thereby avoiding possible negative effects of MIST on neurodevelopment.144 An additional benefit of Propofol is its short-acting anesthetic property, which, in animals, minimizes the swallowing reflex allowing the larynx to relax and provides easier access to the upper trachea.
Recent trials have assessed the effect of low-dose Propofol as pre-medication for MIST.144,145 The number of infants who were assessed to be comfortable (COMFORTneo score <14) was significantly higher in the group who received low-dose Propofol compared to no premedication. However, Propofol led to significantly more desaturations and the need for nasal intermittent mandatory ventilation (NIMV) also increased, although temporarily.144 This indicates a decrease in respiratory drive or respiratory effort, which might be counterproductive as the maintenance of spontaneous breathing is essential for successful administration of surfactant in a minimal invasive way. However, this effect was transient and did not lead to an increased need for intubation. In addition, the maturity of the respiratory center evolves during gestation, which might influence the response to pre-medication as well. Indeed, most infants with a gestational age <32 weeks receiving Propofol needed NIMV.144 It might be because of a higher risk on side effects that those infants with a low gestational age receive the lowest amount of analgesic interventions, while those infants undergo the largest amount of (painful) procedures.146 Administration of low-dose Propofol could therefore be considered in obtaining a better level of comfort during MIST.
Taken altogether, available data indicate that sedatives can be used to decrease discomfort of infant receiving surfactant non-invasively, but dose finding and alternative drugs need to be investigated to decrease the side effect on respiratory effort.
Future Perspectives
While in this review we have described different ways to stimulate breathing at birth, it is also important to recognize that using so many interventions in the delivery room should not interfere with applying adequate respiratory support (Fig. 2). A possible solution could be to automate some of the described interventions so that the health-care professional can focus on respiratory support. We described that tactile stimulation is often omitted. The development and use of a device for automated stimulation based on respiratory effort of the infant might assist the caregiver during resuscitation. Hereby, the caregiver can fully focus on applying optimal non-invasive ventilation by face mask, which might lead to less leakage or obstruction. Normoxia is also an important determinant of respiratory drive. Ideally, both hypoxia and hyperoxia should be avoided. Automated oxygen titration used during resuscitation of preterm lambs led to similar time spent below and within SpO2 target range compared to manual FiO2 control, but the time spent above target range was significantly shorter when using automated control.147 Optimizing automated oxygen titration in the delivery room by using narrower target ranges or devices with better algorithms could potentially lead to more time spent within the SpO2 target range, resulting in improved respiratory effort.
Interventions focused on stimulation of spontaneous breathing during stabilization at birth. a Application of continuous positive airway pressure, supplemented with inflations if indicated. b Tactile stimulation. c Administration of supplemental oxygen. d Administration of a loading dose of caffeine via the umbilical vein
It has been shown that caffeine can be administered antenatally, ensuring that the required dose is reached immediately after birth. Using this approach, it is possible that fewer interventions will be required in the first minutes after birth that again enables the caregiver to focus on applying stimulation and non-invasive respiratory support. In addition, if caffeine is administered antenatally, the stimulatory effect on respiratory effort might be present as soon as the infant is born, possibly leading to a smoother respiratory transition. However, a large trial is needed to test whether administering caffeine in the delivery room or even antenatally, in combination with other interventions focused on stimulation of breathing, will lead to better clinical outcomes.
In case of signs of RDS after admittance to the NICU, surfactant should be administered in a way that preserves respiratory drive and prevents discomfort. When we are able to determine the optimal dose of Propofol in different gestational age ranges during this procedure, we might avoid adverse effects on neurodevelopmental outcome due to stress during the procedure.
Conclusion
The success of non-invasive ventilation depends on the effectiveness of spontaneous breathing both during transition and at the NICU. At birth, the importance of larynx function has been overlooked in the story of a successful transition of preterm infants. Thus, when non-invasive ventilation is desired, interventions that aide laryngeal patency could be a turning point in current practice. Therefore, the focus of the caregiver needs to shift toward stimulation instead of trying to take over the spontaneous breathing efforts of the infant with positive pressure ventilation. While different ways for supporting and stimulating breathing effort have been investigated separately, it is likely that combining these interventions in a bundle of care will increase the success in maintaining effective breathing of the preterm infant.
References
Wiswell, T. E. Resuscitation in the delivery room: lung protection from the first breath. Respir. Care 56, 1360–1367 (2011). Discussion 1367–1368.
van Vonderen, J. J., Hooper, S. B., Hummler, H. D., Lopriore, E. & te Pas, A. B. Effects of a sustained inflation in preterm infants at birth. J. Pediatr. 165, 903–908 e1 (2014).
O’Donnell, C. P., Kamlin, C. O., Davis, P. G. & Morley, C. J. Crying and breathing by extremely preterm infants immediately after birth. J. Pediatr. 156, 846–847 (2010).
Schmolzer, G. M. et al. Non-invasive versus invasive respiratory support in preterm infants at birth: systematic review and meta-analysis. BMJ 347, f5980 (2013).
Roehr, C. C. et al. Positive effects of early continuous positive airway pressure on pulmonary function in extremely premature infants: results of a subgroup analysis of the COIN trial. Arch. Dis. Child. Fetal Neonatal Ed. 96, F371–F373 (2011).
Mehler, K. et al. Outcome of extremely low gestational age newborns after introduction of a revised protocol to assist preterm infants in their transition to extrauterine life. Acta Paediatr. 101, 1232–1239 (2012).
Thomas, C. W., Meinzen-Derr, J., Hoath, S. B. & Narendran, V. Neurodevelopmental outcomes of extremely low birth weight infants ventilated with continuous positive airway pressure vs. mechanical ventilation. Indian J. Pediatr. 79, 218–223 (2012).
Morley, C. J. et al. Nasal CPAP or intubation at birth for very preterm infants. N. Engl. J. Med. 358, 700–708 (2008).
Wintermark, P., Tolsa, J. F., Van Melle, G., Forcada-Guex, M. & Moessinger, A. C. Long-term outcome of preterm infants treated with nasal continuous positive airway pressure. Eur. J. Pediatr. 166, 473–483 (2007).
SUPPORT Study Group of the Eunice Kennedy Shriver NICHD Neonatal Research Network et al. Early CPAP versus surfactant in extremely preterm infants. N. Engl. J. Med. 362, 1970–1979 (2010).
Schilleman, K. et al. Evaluating manual inflations and breathing during mask ventilation in preterm infants at birth. J. Pediatr. 162, 457–463 (2013).
Schmolzer, G. M. et al. Assessment of tidal volume and gas leak during mask ventilation of preterm infants in the delivery room. Arch. Dis. Child. Fetal Neonatal Ed. 95, F393–F397 (2010).
Schilleman, K. et al. Leak and obstruction with mask ventilation during simulated neonatal resuscitation. Arch. Dis. Child. Fetal Neonatal Ed. 95, F398–F402 (2010).
Palme, C., Nystrom, B. & Tunell, R. An evaluation of the efficiency of face masks in the resuscitation of newborn infants. Lancet 1, 207–210 (1985).
Wood, F. E., Morley, C. J., Dawson, J. A. & Davis, P. G. A respiratory function monitor improves mask ventilation. Arch. Dis. Child. Fetal Neonatal Ed. 93, F380–F381 (2008).
UK RC. Newborn Life Support, 3rd edn (UK Resuscitation Council, London, 2011).
Kattwinkel, J. Textbook of Neonatal Resuscitation, 6th edn (AAP, Elk Grove, Chicago, IL, 2011).
Wood, F. E. et al. Assessing the effectiveness of two round neonatal resuscitation masks: study 1. Arch. Dis. Child. Fetal Neonatal Ed. 93, F235–F237 (2008).
van Vonderen, J. J., Witlox, R. S., Kraaij, S. & te Pas, A. B. Two-minute training for improving neonatal bag and mask ventilation. PLoS ONE 9, e109049 (2014).
Narayanan, I., Mendhi, M., Bansil, P. & Coffey, P. S. Evaluation of simulated ventilation techniques with the upright and conventional self-inflating neonatal resuscitators. Respir. Care 62, 1428–1436 (2017).
O’Donnell, C. P. et al. Neonatal resuscitation 2: an evaluation of manual ventilation devices and face masks. Arch. Dis. Child. Fetal Neonatal Ed. 90, F392–F396 (2005).
Schmolzer, G. M. et al. Airway obstruction and gas leak during mask ventilation of preterm infants in the delivery room. Arch. Dis. Child. Fetal Neonatal Ed. 96, F254–F257 (2011).
Finer, N. N., Rich, W., Wang, C. & Leone, T. Airway obstruction during mask ventilation of very low birth weight infants during neonatal resuscitation. Pediatrics 123, 865–869 (2009).
Crawshaw, J. R. et al. Laryngeal closure impedes non-invasive ventilation at birth. Arch. Dis. Child. Fetal Neonatal Ed. 103, F112–F119 (2018).
Harding, R., Bocking, A. D. & Sigger, J. N. Influence of upper respiratory tract on liquid flow to and from fetal lungs. J. Appl Physiol. (1985) 61, 68–74 (1986).
Harding, R., Bocking, A. D. & Sigger, J. N. Upper airway resistances in fetal sheep: the influence of breathing activity. J. Appl Physiol. (1985) 60, 160–165 (1986).
van Vonderen, J. J., Hooper, S. B., Krabbe, V. B., Siew, M. L. & Te Pas, A. B. Monitoring tidal volumes in preterm infants at birth: mask versus endotracheal ventilation. Arch. Dis. Child. Fetal Neonatal Ed. 100, F43–F46 (2015).
Sweet, D. G. et al. European Consensus Guidelines on the Management of Respiratory Distress Syndrome - 2016 update. Neonatology 111, 107–125 (2017).
Ammari, A. et al. Variables associated with the early failure of nasal CPAP in very low birth weight infants. J. Pediatr. 147, 341–347 (2005).
Kribs, A. Minimally invasive surfactant therapy and noninvasive respiratory support. Clin. Perinatol. 43, 755–771 (2016).
Dargaville, P. A., Aiyappan, A., Cornelius, A., Williams, C. & De Paoli, A. G. Preliminary evaluation of a new technique of minimally invasive surfactant therapy. Arch. Dis. Child. Fetal Neonatal Ed. 96, F243–F248 (2011).
Hooper, S. B., Te Pas, A. B. & Kitchen, M. J. Respiratory transition in the newborn: a three-phase process. Arch. Dis. Child. Fetal Neonatal Ed. 101, F266–F271 (2016).
te Pas, A. B. & Hooper, S. B. in Fetal & Neonatal Lung Development (eds Jobe, A. H., Whitsett, J. A. & Abman, S. H.) Ch. 9 (Cambridge University Press, New York, 2016).
Faridy, E. E. Instinctive resuscitation of the newborn rat. Respir. Physiol. 51, 1–19 (1983).
Ronca, A. E. & Alberts, J. R. Cutaneous induction of breathing in perinatal rats. Psychobiology 23, 261–269 (1995).
Tattersall, G. J. & Milsom, W. K. Hypothermia-induced respiratory arrest and recovery in neonatal rats. Respir. Physiol. Neurobiol. 137, 29–40 (2003).
Ioffe, S., Jansen, A. H., Russell, B. J. & Chernick, V. Respiratory response to somatic stimulation in fetal lambs during sleep and wakefulness. Pflug. Arch. 388, 143–148 (1980).
Boddy, K., Dawes, G. S., Fisher, R., Pinter, S. & Robinson, J. S. Foetal respiratory movements, electrocortical and cardiovascular responses to hypoxaemia and hypercapnia in sheep. J. Physiol. 243, 599–618 (1974).
Lee, A. C. et al. Neonatal resuscitation and immediate newborn assessment and stimulation for the prevention of neonatal deaths: a systematic review, meta-analysis and Delphi estimation of mortality effect. BMC Public Health 11(Suppl 3), S12 (2011).
Wyllie, J. et al. European Resuscitation Council Guidelines for Resuscitation 2015: Section 7. Resuscitation and support of transition of babies at birth. Resuscitation 95, 249–263 (2015).
Dekker, J. et al. Tactile stimulation to stimulate spontaneous breathing during stabilization of preterm infants at birth: a retrospective analysis. Front. Pediatr. 5, 61 (2017).
Gaertner, V. D., Flemmer, S. A., Lorenz, L., Davis, P. G. & Kamlin, C. O. F. Physical stimulation of newborn infants in the delivery room. Arch. Dis. Child. Fetal Neonatal Ed. 103, F132–F136 (2018).
Baik-Schneditz, N. et al. Tactile stimulation during neonatal transition and its effect on vital parameters in neonates during neonatal transition. Acta Paediatr. 107, 952–957 (2018).
van Henten, T. M. A. et al. Tactile stimulation in the delivery room: do we practice what we preach? Arch. Dis. Child. Fetal Neonatal Ed. https://doi.org/10.1136/archdischild-2018-316344 (2019).
Ishida, K., Yasuda, Y. & Miyamura, M. Cardiorespiratory response at the onset of passive leg movements during sleep in humans. Eur. J. Appl. Physiol. Occup. Physiol. 66, 507–513 (1993).
Kesavan, K., Frank, P., Cordero, D. M., Benharash, P. & Harper, R. M. Neuromodulation of limb proprioceptive afferents decreases apnea of prematurity and accompanying intermittent hypoxia and bradycardia. PLoS ONE 11, e0157349 (2016).
Remmers, J. E. & Marttila, I. Action of intercostal muscle afferents on the respiratory rhythm of anesthetized cats. Respir. Physiol. 24, 31–41 (1975).
Trippenbach, T., Kelly, G. & Marlot, D. Respiratory effects of stimulation of intercostal muscles and saphenous nerve in kittens. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 54, 1736–1744 (1983).
Morley, C. New Australian Neonatal Resuscitation Guidelines. J. Paediatr. Child Health 43, 6–8 (2007).
Rohana, J., Khairina, W., Boo, N. Y. & Shareena, I. Reducing hypothermia in preterm infants with polyethylene wrap. Pediatr. Int. 53, 468–474 (2011).
Castellucci, V. F. & Kandel, E. R. A quantal analysis of the synaptic depression underlying habituation of the gill-withdrawal reflex in Aplysia. Proc. Natl Acad. Sci. USA 71, 5004–5008 (1974).
Dekker, J. et al. Repetitive versus standard tactile stimulation of preterm infants at birth - a randomized controlled trial. Resuscitation 127, 37–43 (2018).
Murphy, P. J. The fetal circulation. Continuing Educ. Anesth. Crit. Care Pain 5, 107–112 (2005).
Patrick, J., Fetherston, W., Vick, H. & Voegelin, R. Human fetal breathing movements and gross fetal body movements at weeks 34 to 35 of gestation. Am. J. Obstet. Gynecol. 130, 693–699 (1978).
Hooper, S. B. & Harding, R. Changes in lung liquid dynamics induced by prolonged fetal hypoxemia. J. Appl Physiol. (1985) 69, 127–135 (1990).
Baier, R. J. et al. Effects of various concentrations of O-2 and umbilical-cord occlusion on fetal breathing and behavior. J. Appl Physiol. 68, 1597–1604 (1990).
Dawes, G. in Foetal and Neonatal Physiology 125–159 (Year Book, Chicago, IL, 1968).
Baier, R. J. et al. Effects of various concentrations of O2 and umbilical cord occlusion on fetal breathing and behavior. J. Appl Physiol. (1985) 68, 1597–1604 (1990).
Gluckman, P. D., Gunn, T. R. & Johnston, B. M. The effect of cooling on breathing and shivering in unanaesthetized fetal lambs in utero. J. Physiol. 343, 495–506 (1983).
Davey, M. G., Moss, T. J., McCrabb, G. J. & Harding, R. Prematurity alters hypoxic and hypercapnic ventilatory responses in developing lambs. Respir. Physiol. 105, 57–67 (1996).
Irestedt, L., Dahlin, I., Hertzberg, T., Sollevi, A. & Lagercrantz, H. Adenosine concentration in umbilical cord blood of newborn infants after vaginal delivery and cesarean section. Pediatr. Res. 26, 106–108 (1989).
Whyte, S. D., Sinha, A. K. & Wyllie, J. P. Neonatal resuscitation–a practical assessment. Resuscitation 40, 21–25 (1999).
Clyman, R. I., Saugstad, O. D. & Mauray, F. Reactive oxygen metabolites relax the lamb ductus arteriosus by stimulating prostaglandin production. Circ. Res. 64, 1–8 (1989).
Saugstad, O. D. Resuscitation with room-air or oxygen supplementation. Clin. Perinatol. 25, 741–756 (1998). xi.
Chen, Y., Whitney, P. L. & Frank, L. Comparative responses of premature versus full-term newborn rats to prolonged hyperoxia. Pediatr. Res. 35, 233–237 (1994).
Tan, A., Schulze, A., O’Donnell, C. P. & Davis, P. G. Air versus oxygen for resuscitation of infants at birth. Cochrane Database Syst. Rev. CD002273 (2005).
Saugstad, O. D., Ramji, S. & Vento, M. Resuscitation of depressed newborn infants with ambient air or pure oxygen: a meta-analysis. Biol. Neonate 87, 27–34 (2005).
Rabi, Y., Rabi, D. & Yee, W. Room air resuscitation of the depressed newborn: a systematic review and meta-analysis. Resuscitation 72, 353–363 (2007).
Saugstad, O. D. Bronchopulmonary dysplasia and oxidative stress: are we closer to an understanding of the pathogenesis of BPD? Acta Paediatr. 86, 1277–1282 (1997).
Davis, J. M. Role of oxidant injury in the pathogenesis of neonatal lung disease. Acta Paediatr. Suppl. 91, 23–25 (2002).
Dawson, J. A. et al. Defining the reference range for oxygen saturation for infants after birth. Pediatrics 125, e1340–e1347 (2010).
Goos, T. G. et al. Observing the resuscitation of very preterm infants: are we able to follow the oxygen saturation targets? Resuscitation 84, 1108–1113 (2013).
White, L. N. et al. Achievement of saturation targets in preterm infants < 32 weeks’ gestational age in the delivery room. Arch. Dis. Child. Fetal Neonatal Ed. 102, F423–F427 (2017).
Armanian, A. M. & Badiee, Z. Resuscitation of preterm newborns with low concentration oxygen versus high concentration oxygen. J. Res. Pharm. Pract. 1, 25–29 (2012).
Kapadia, V. S. et al. Resuscitation of preterm neonates with limited versus high oxygen strategy. Pediatrics 132, e1488–e1496 (2013).
Oei, J. L. et al. Targeted oxygen in the resuscitation of preterm infants, a randomized clinical trial. Pediatrics 139, e20161452 (2017).
Rook, D. et al. Resuscitation of preterm infants with different inspired oxygen fractions. J. Pediatr. 164, 1322–6 e3 (2014).
Wang, C. L. et al. Resuscitation of preterm neonates by using room air or 100% oxygen. Pediatrics 121, 1083–1089 (2008).
van Vonderen, J. J. et al. The administration of 100% oxygen and respiratory drive in very preterm infants at birth. PLoS ONE 8, e76898 (2013).
Oei, J. L. et al. Outcomes of oxygen saturation targeting during delivery room stabilisation of preterm infants. Arch. Dis. Child. Fetal Neonatal Ed. 103, F446–F454 (2018).
Chandrasekharan, P. et al. Effect of various inspired oxygen concentrations on pulmonary and systemic hemodynamics and oxygenation during resuscitation in a transitioning preterm model. Pediatr. Res. 84, 743–750 (2018).
Boronat, N. et al. Survival and neurodevelopmental outcomes of preterms resuscitated with different oxygen fractions. Pediatrics 138, e20161405 (2016).
Escrig, R. et al. Achievement of targeted saturation values in extremely low gestational age neonates resuscitated with low or high oxygen concentrations: a prospective, randomized trial. Pediatrics 121, 875–881 (2008).
Kumar, V. H. et al. Oxygen resuscitation and oxidative-stress biomarkers in premature infants. Res. Rep. Neonatol. 4, 91–99 (2014).
Vento, M. et al. Preterm resuscitation with low oxygen causes less oxidative stress, inflammation, and chronic lung disease. Pediatrics 124, e439–e449 (2009).
Rabi, Y., Singhal, N. & Nettel-Aguirre, A. Room-air versus oxygen administration for resuscitation of preterm infants: the ROAR study. Pediatrics 128, e374–e381 (2011).
Julien, C. A., Joseph, V. & Bairam, A. Caffeine reduces apnea frequency and enhances ventilatory long-term facilitation in rat pups raised in chronic intermittent hypoxia. Pediatr. Res. 68, 105–111 (2010).
Schmidt, B. et al. Caffeine therapy for apnea of prematurity. N. Engl. J. Med. 354, 2112–2121 (2006).
Kreutzer, K. & Bassler, D. Caffeine for apnea of prematurity: a neonatal success story. Neonatology 105, 332–336 (2014).
Vliegenthart, R., Miedema, M., Hutten, G. J., van Kaam, A. H. & Onland, W. High versus standard dose caffeine for apnoea: a systematic review. Arch. Dis. Child. Fetal Neonatal Ed. 103, F523–F529 (2018).
Henderson-Smart, D. J. & Steer, P. A. Caffeine versus theophylline for apnea in preterm infants. Cochrane Database Syst. Rev. CD000273 (2010).
Dobson, N. R. et al. Trends in caffeine use and association between clinical outcomes and timing of therapy in very low birth weight infants. J. Pediatr. 164, 992–8 e3 (2014).
Lodha, A. et al. Association of early caffeine administration and neonatal outcomes in very preterm neonates. JAMA Pediatr. 169, 33–38 (2015).
Patel, R. M., Leong, T., Carlton, D. P. & Vyas-Read, S. Early caffeine therapy and clinical outcomes in extremely preterm infants. J. Perinatol. 33, 134–140 (2013).
Katheria, A. C. et al. A pilot randomized controlled trial of early versus routine caffeine in extremely premature infants. Am. J. Perinatol. 32, 879–886 (2015).
Kraaijenga, J. V., Hutten, G. J., de Jongh, F. H. & van Kaam, A. H. The effect of caffeine on diaphragmatic activity and tidal volume in preterm infants. J. Pediatr. 167, 70–75 (2015).
Dekker, J. et al. Caffeine to improve breathing effort of preterm infants at birth: a randomized controlled trial. Pediatr. Res. 82, 290–296 (2017).
Dunwiddie, T. V. & Masino, S. A. The role and regulation of adenosine in the central nervous system. Annu. Rev. Neurosci. 24, 31–55 (2001).
Rivkees, S. A. & Wendler, C. C. Adverse and protective influences of adenosine on the newborn and embryo: implications for preterm white matter injury and embryo protection. Pediatr. Res. 69, 271–278 (2011).
Mose, T. et al. Placental passage of benzoic acid, caffeine, and glyphosate in an ex vivo human perfusion system. J. Toxicol. Environ. Health A 71, 984–991 (2008).
Binder-Heschl, C. et al. Haemodynamic effects of prenatal caffeine on the cardiovascular transition in ventilated preterm lambs. PLoS ONE 13, e0200572 (2018).
Sommers, R. et al. Hemodynamic effects of delayed cord clamping in premature infants. Pediatrics 129, e667–e672 (2012).
Meyer, M. P. & Mildenhall, L. Delayed cord clamping and blood flow in the superior vena cava in preterm infants: an observational study. Arch. Dis. Child. Fetal Neonatal Ed. 97, F484–F486 (2012).
Bhatt, S. et al. Delaying cord clamping until ventilation onset improves cardiovascular function at birth in preterm lambs. J. Physiol. 591, 2113–2126 (2013).
Polglase, G. R. et al. Ventilation onset prior to umbilical cord clamping (physiological-based cord clamping) improves systemic and cerebral oxygenation in preterm lambs. PLoS ONE 10, e0117504 (2015).
Sippell, W. G., Becker, H., Versmold, H. T., Bidlingmaier, F. & Knorr, D. Longitudinal studies of plasma aldosterone, corticosterone, deoxycorticosterone, progesterone, 17-hydroxyprogesterone, cortisol, and cortisone determined simultaneously in mother and child at birth and during the early neonatal period. I. Spontaneous delivery. J. Clin. Endocrinol. Metab. 46, 971–985 (1978).
Alvaro, R. E. et al. Prostaglandins are responsible for the inhibition of breathing observed with a placental extract in fetal sheep. Respir. Physiol. Neurobiol. 144, 35–44 (2004).
Piper, P. J., Vane, J. R. & Wyllie, J. H. Inactivation of prostaglandins by the lungs. Nature 225, 600–604 (1970).
Adamson, S. L., Kuipers, I. M. & Olson, D. M. Umbilical cord occlusion stimulates breathing independent of blood gases and pH. J. Appl Physiol. (1985) 70, 1796–1809 (1991).
Kitterman, J. A., Liggins, G. C., Clements, J. A. & Tooley, W. H. Stimulation of breathing movements in fetal sheep by inhibitors of prostaglandin synthesis. J. Dev. Physiol. 1, 453–466 (1979).
Katheria, A. et al. Neonatal resuscitation with an intact cord: a randomized clinical trial. J. Pediatr. 178, 75–80 e3 (2016).
Brouwer, E. et al. Physiological-based cord clamping in preterm infants using a new purpose-built resuscitation table: a feasibility study. Arch. Dis. Child. Fetal Neonatal Ed. https://doi.org/10.1136/archdischild-2018-315483 (2018).
Polglase, G. R. et al. Positive end-expiratory pressure differentially alters pulmonary hemodynamics and oxygenation in ventilated, very premature lambs. J. Appl Physiol. (1985) 99, 1453–1461 (2005).
Probyn, M. E. et al. Positive end expiratory pressure during resuscitation of premature lambs rapidly improves blood gases without adversely affecting arterial pressure. Pediatr. Res. 56, 198–204 (2004).
Dysart, K. C. Physiologic basis for nasal continuous positive airway pressure, heated and humidified high-flow nasal cannula, and nasal ventilation. Clin. Perinatol. 43, 621–631 (2016).
Fischer, H. S. & Buhrer, C. Avoiding endotracheal ventilation to prevent bronchopulmonary dysplasia: a meta-analysis. Pediatrics 132, e1351–e1360 (2013).
Martherus, T. et al. Supporting breathing of preterm infants at birth: a narrative review. Arch. Dis. Child. Fetal Neonatal Ed. 104, F102–F107 (2018).
Kitchen, M. J. et al. Changes in positive end-expiratory pressure alter the distribution of ventilation within the lung immediately after birth in newborn rabbits. PLoS ONE 9, e93391 (2014).
Ho, J. J., Subramaniam, P. & Davis, P. G. Continuous distending pressure for respiratory distress in preterm infants. Cochrane Database Syst. Rev. CD002271 (2015).
Sandri, F. et al. Prophylactic or early selective surfactant combined with nCPAP in very preterm infants. Pediatrics 125, e1402–e1409 (2010).
Fujiwara, T. et al. Artificial surfactant therapy in hyaline-membrane disease. Lancet 1, 55–59 (1980).
Bahadue, F. L. & Soll, R. Early versus delayed selective surfactant treatment for neonatal respiratory distress syndrome. Cochrane Database Syst. Rev. 11, CD001456 (2012).
Verder, H. et al. Nasal continuous positive airway pressure and early surfactant therapy for respiratory distress syndrome in newborns of less than 30 weeks’ gestation. Pediatrics 103, E24 (1999).
Gopel, W. et al. Avoidance of mechanical ventilation by surfactant treatment of spontaneously breathing preterm infants (AMV): an open-label, randomised, controlled trial. Lancet 378, 1627–1634 (2011).
van der Burg, P. S., de Jongh, F. H., Miedema, M., Frerichs, I. & van Kaam, A. H. Effect of minimally invasive surfactant therapy on lung volume and ventilation in preterm infants. J. Pediatr. 170, 67–72 (2016).
de Waal, C. G., Hutten, G. J., de Jongh, F. H. & van Kaam, A. H. The effect of minimally invasive surfactant therapy on diaphragmatic activity. Neonatology 114, 76–81 (2018).
Isayama, T., Iwami, H., McDonald, S. & Beyene, J. Association of noninvasive ventilation strategies with mortality and bronchopulmonary dysplasia among preterm infants: a systematic review and meta-analysis. JAMA 316, 611–624 (2016).
Aldana-Aguirre, J. C., Pinto, M., Featherstone, R. M. & Kumar, M. Less invasive surfactant administration versus intubation for surfactant delivery in preterm infants with respiratory distress syndrome: a systematic review and meta-analysis. Arch. Dis. Child. Fetal Neonatal Ed. 102, F17–F23 (2017).
Lau, C. S. M., Chamberlain, R. S. & Sun, S. Less invasive surfactant administration reduces the need for mechanical ventilation in preterm infants: a meta-analysis. Glob. Pediatr. Health 4, 2333794X17696683 (2017).
Rigo, V., Lefebvre, C. & Broux, I. Surfactant instillation in spontaneously breathing preterm infants: a systematic review and meta-analysis. Eur. J. Pediatr. 175, 1933–1942 (2016).
Dargaville, P. A., Ali, S. K. M., Jackson, H. D., Williams, C. & De Paoli, A. G. Impact of minimally invasive surfactant therapy in preterm infants at 29-32 weeks gestation. Neonatology 113, 7–14 (2018).
Klotz, D., Porcaro, U., Fleck, T. & Fuchs, H. European perspective on less invasive surfactant administration-a survey. Eur. J. Pediatr. 176, 147–154 (2017).
Carbajal, R., Eble, B. & Anand, K. J. Premedication for tracheal intubation in neonates: confusion or controversy? Semin. Perinatol. 31, 309–317 (2007).
Pokela, M. L. & Koivisto, M. Physiological changes, plasma beta-endorphin and cortisol responses to tracheal intubation in neonates. Acta Paediatr. 83, 151–156 (1994).
Stow, P. J., McLeod, M. E., Burrows, F. A. & Creighton, R. E. Anterior fontanelle pressure responses to tracheal intubation in the awake and anaesthetized infant. Br. J. Anaesth. 60, 167–170 (1988).
Grunau, R. E., Holsti, L. & Peters, J. W. Long-term consequences of pain in human neonates. Semin. Fetal Neonatal Med. 11, 268–275 (2006).
Kamata, M. & Tobias, J. D. Remifentanil: applications in neonates. J. Anesth. 30, 449–460 (2016).
de Kort, E. H., Hanff, L. M., Roofthooft, D., Reiss, I. K. & Simons, S. H. Insufficient sedation and severe side effects after fast administration of remifentanil during INSURE in preterm newborns. Neonatology 111, 172–176 (2017).
Shah, P. S. & Shah, V. S. Propofol for procedural sedation/anaesthesia in neonates. Cochrane Database Syst. Rev. CD007248 (2011).
Smits, A., Thewissen, L., Caicedo, A., Naulaers, G. & Allegaert, K. Propofol dose-finding to reach optimal effect for (semi-)elective intubation in neonates. J. Pediatr. 179, 54–60 e9 (2016).
Piersigilli, F. et al. Propofol and fentanyl sedation for laser treatment of retinopathy of prematurity to avoid intubation. J. Matern. Fetal Neonatal Med. 32, 517–521 (2017).
Dekker, J. et al. Sedation during minimal invasive surfactant therapy in preterm infants. Neonatology 109, 308–313 (2016).
Ghanta, S. et al. Propofol compared with the morphine, atropine, and suxamethonium regimen as induction agents for neonatal endotracheal intubation: a randomized, controlled trial. Pediatrics 119, e1248–e1255 (2007).
Dekker, J. et al. Sedation during minimal invasive surfactant therapy: a randomised controlled trial. Arch. Dis. Child. Fetal Neonatal Ed. https://doi.org/10.1136/archdischild-2018-315015 (2018).
Descamps, C. S. et al. Propofol for sedation during less invasive surfactant administration in preterm infants. Arch. Dis. Child. Fetal Neonatal Ed. 102, F465 (2017).
Cruz, M. D., Fernandes, A. M. & Oliveira, C. R. Epidemiology of painful procedures performed in neonates: a systematic review of observational studies. Eur. J. Pain. 20, 489–498 (2016).
Hutten, M. C. et al. Fully automated predictive intelligent control of oxygenation (PRICO) in resuscitation and ventilation of preterm lambs. Pediatr. Res. 78, 657–663 (2015).
Acknowledgements
We would like to thank ir. Sophie Cramer for her assistance in providing us with the figures presented in this paper. A.B.t.P. is recipient of a NWO innovational research incentives scheme (VIDI 91716428).
Author contributions
All authors contributed equally and gave approval for the final version to be published.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dekker, J., van Kaam, A.H., Roehr, C.C. et al. Stimulating and maintaining spontaneous breathing during transition of preterm infants. Pediatr Res 90, 722–730 (2021). https://doi.org/10.1038/s41390-019-0468-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-019-0468-7
This article is cited by
-
The influence of chorioamnionitis on respiratory drive and spontaneous breathing of premature infants at birth: a narrative review
European Journal of Pediatrics (2024)
-
Variation in delivery room management of preterm infants across Europe: a survey of the Union of European Neonatal and Perinatal Societies
European Journal of Pediatrics (2023)
-
Versorgung und Reanimation des Neugeborenen nach der Geburt
Notfall + Rettungsmedizin (2021)