Abstract
Background
In severe aplastic anemia (SAA), predictive markers of response to immunosuppressive therapy (IST) of porcine antilymphocyte globulin (pALG) have not been well defined. We investigated whether clinical and laboratory findings before treatment could predict response in a pediatric cohort.
Methods
In this study, we included 70 newly diagnosed SAA children and treated them with pALG. The response rate was documented during follow-up. The log-rank test compared response rates between the potential predictive factors.
Results
The response rate was 57.1% at 24 months follow-up. In log-rank test, mild disease severity was the most significant predictive marker of better response (P < 0.001); SAA patients with higher absolute reticulocyte count (ARC) and platelet level showed a higher response rate (both P < 0.001). Although insignificantly, elderly children and male sex show better response rate after treatment. The response rate worsened when the time interval before IST was more than 60 days.
Conclusion
Modified IST with pALG was suitable for SAA children, and favorable response correlates with mild disease severity was identified. ARC and platelet status also appeared to be a reproducible prognostic model for response rate. IST should be started as soon as possible, given that the response rate worsens as the interval between diagnosis and treatment increases.
Similar content being viewed by others
Introduction
Severe aplastic anemia (SAA) in children is a rare, life-threatening disorder characterized by pancytopenia and hypocellular bone marrow.1 Over the past years, bone marrow transplantation (BMT) from a matched related donor has been the treatment of choice for children with SAA. For children without an available matched related donor, immunosuppressive therapy (IST) is indicated.2
IST is usually based on antithymocyte globulin/antilymphocyte globulin (ATG/ALG) polyclonal antibodies from animals’ serum immunized by human thymocytes/lymphocytes.3 The standard IST regimen involves horse ATG (hATG) and cyclosporine A (CSA).4 In the 1980s and 1990s, previous studies in SAA conducted in the United States, Europe, and Japan utilized hATG, with hematologic responses observed in about two-thirds of cases.5,6,7 According to previous experience, hATG yielded hematological improvement in 60–75% SAA patients after initial treatment,8,9 and with 85% 5-year survival.10 For salvaging patients who are refractory or relapse after initial therapy with hATG, treatment with rabbit ATG (rATG) results in a beneficial effect in about 60% of patients.11,12 However, the use of rATG may not be quite as effective as first-line treatment involving hATG,13 and hATG is currently unavailable in some countries including China.3 Alternative ISTs have also been suggested to improve the efficacy of SAA treatment, and many different antibodies have emerged. One of these alternatives is porcine ALG (pALG), which is widely used in China, in part because it lowers the cost of therapy by 30–50% per cycle of treatment.14 There have been many researchers reporting that the safety and efficiency of pALG are similar to hATG. The overall response rate (ORR) of IST with pALG plus CSA was 65–80% in adult.15,16,17
To date, only a few studies have considered the combination of pALG and CSA in separate severity of SAA children in terms of the hematopoietic response and failure-free survival (FFS) in 24 months. Moreover, we investigate whether clinical and laboratory findings before treatment could predict response in a pediatric cohort.
Materials and methods
Study population
A total of 70 children with SAA were enrolled from January 2015 to July 2018 at the Institute of Hematology and Blood Diseases Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College. The SAA diagnostic criteria were based on the International AA Study Group Criteria. SAA patients with malignant disease, myelodysplastic syndrome (MDS), myelofibrosis, paroxysmal nocturnal hemoglobinuria (PNH), or congenital AA-like Fanconi’s anemia were excluded.
AA was considered severe if at least two of the following parameters were reached: platelet (PLT) count <20 × 109/L, absolute neutrophil count (ANC) <0.5 × 109/L, and a reticulocyte count <15 × 109/L with hypocellular bone marrow.18 Very severe AA (vSAA) was defined using the same three criteria, and ANC was <0.2 × 109/L. Patients were considered to be in remission if: (1) there is no bleeding or anemia; (2) transfusion of blood products is not required; (3) white blood cell (WBC) counts >3.5 × 109/L, PLT > 100 × 109/L, ANC ≥ 1.0 × 109/L, the hemoglobin (Hb) level reaches remission standards, and (4) patients were in stable condition or had a substantial improvement after 24 months follow-up.
Immunosuppressive regimens
IST consisted of pALG (Wuhan Institute of Biological Products, China) intravenously 20 mg/kg/day followed by 1 mg/kg/day methylprednisolone on days 1–5 and then 1 mg/kg/day prednisolone for 15–21 days and tapered gradually to prevent serum sickness. CSA 3–5 mg/kg/day was used for at least 2 years.3 The CSA dose was adjusted to achieve a whole-blood trough level of 100–200 ng/mL. Complete blood counts and biochemistry parameters were monitored accordingly.
Response criteria
Complete response (CR) was defined as independence from transfusion, associated with normal hemogram parameters. Partial response (PR) was defined as no longer meeting the criteria for SAA without normalization of blood counts and no transfusion dependence for PLT and/or red blood cells (RBCs). Continuous transfusion dependency and hematopoietic stem cell transplantation (HSCT) within 6 months of IST treatment before CR was classified as no response. Relapse was defined as a decrease in blood counts to values either requiring transfusions or needing reinstitution of IST or HSCT.19,20 The responses to IST were evaluated at 40 days, 3, 6, 12, and 24 months after pALG administration. An overall response (OR) was defined as a CR or PR after initiation of IST. FFS was defined as the time from the first day of IST treatment until the relapse, disease- or treatment-related death, or the date of treatment failure, whichever came first.
Detection of PNH clone
We used a highly sensitive two-color flow cytometry method to detect PNH-type CD11b+CD55−CD59− granulocytes and glycophorin A+ CD55−CD59− RBCs. On the basis of data obtained in healthy population, a universal inter-laboratory threshold of ≥0.01% glycophosphatidyl inositol/FLAER-deficient cells with an apparent cluster-like distribution in granulocytes and RBCs was defined as a criterion for the presence of PNH-type cells.21
Statistical analysis
The FFS rate was analyzed using the Kaplan–Meier method. Differences in continuous variables were analyzed using the Mann–Whitney U test, and differences in frequencies were analyzed using Fisher’s exact test. Log-rank test was used for the comparison between the potential predictive factors. All differences were considered significant at P < 0.05. Data were subjected to statistical analysis using SPSS 22.0 (SPSS Software Inc., Chicago, IL, USA) and GraphPad Prism 6.02 software (GraphPad Software Inc., La Jolla, CA, USA).
The study design and methods complied with the Declaration of Helsinki and was approved by the Ethics Committee and Institutional Review Board of Chinese Academy of Medical Sciences and Peking Union Medical College. Informed consent was obtained from all subjects.
Results
We identified 70 patients (48 males, 22 females) who fulfilled the eligibility criteria during the study period. The median age of SAA children were 5 years (ranging from 1 to 14 years). Population characteristics and main baseline biological results are shown in Table 1.
Bone marrow biopsy was performed on all patients and all patients were transfusion dependent at the time of diagnosis. Overall survival (OS) rate during follow-up period was 100%. A total of 36 of the 70 patients (51.4%) achieved at least a CR or PR at 6 months after IST. There was a significantly higher proportion of response in the SAA group when compared with the vSAA group, 24 (60%) and 12 (40%), respectively (P = 0.098). There were two late responses separately, which occurred at the 12-month time point in both groups. Moreover, the ORR did not change in the 24-month time point in comparison to the 12-month time point. The difference in ORR between the SAA and vSAA group was insignificant at the end of the follow-up period (P = 0.125), which suggested that pALG is an effective treatment for all SAA patients (Table 2
).
Treatment failure was observed in 14 patients in the SAA group and 16 patients in the vSAA group; at the end of follow-up, these patients underwent salvage therapies because of a lack of response to IST (n = 16) or relapse (n = 14). A total of 24 patients underwent HSCT as a salvage therapy. Among these patients, six received HSCT at the 3-month time point, 12 patients received HSCT at the 6-month time point, and six received HSCT at the 12-month time point.
To determine predictors of IST response, we compared differences in potential pre-treatment variables between IST responders and non-responders. The following were analyzed both for prevalence in categorical variables and differences in continuous variables: age at diagnosis, sex, time interval before treatment, PNH clone, absolute lymphocyte count (ALC), Hb level, absolute reticulocyte count (ARC), and PLT count. Table 3 indicated that PNH clone, severity of SAA and baseline ARC, and PLT level showed influence with IST response. We also performed multivariate logistic regression analysis to assess the simultaneous contributions of each of the variables in predicting response. In these analyses, higher ANC (P = 0.0009) and PLT count (P = 0.0007), shorter interval between diagnosis and therapy (P = 0.019), and PNH clone negative (P = 0.009) represented significant predictors of better response (Table 4).
The incidence of adverse responses shown in Table 5 was basically not different between the SAA and vSAA group (P > 0.05). All were controllable, and recovered after symptomatic treatment.
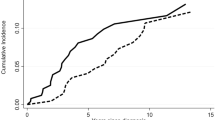
Figure 1 indicated that the FFS rate of the SAA group were significantly higher than the vSAA group (P < 0.001), which indicated a better prognosis of patients with higher ANC. None of the patients died during the 24 months follow-up.
Discussion
IST using ATG/ALG and CSA has been considered as the initial treatment for SAA patients, since most patients lack a minimal residual disease or are not suitable candidates for HSCT due to the lack of access to the certain modality. In China, rATG was the only available agent before 2004, but some of the patients could not afford this expensive drug, which is not covered by any insurance. In recent years, an alternative is pALG, in part because it is less expensive than rATG. An adult trial comparing the pALG with rATG regimen showed that pALG had a significantly better OS rate than rATG and faster median time to achieve first effectiveness in SAA patients.3 A recent study revealed a large difference in efficacy among patients of different age treated with IST: <20 years (n = 31, 100%), 20–40 years (n = 51, 92%), 40–60 years (n = 51, 71%), and >60 years (n = 56, 56%).22 This finding suggests that the IST treatment results for pediatric and adult patients obtained should be analyzed separately, but the researchers work on this issue is rare.
This was the first and largest study to observe the outcomes following combination therapy with pALG and CSA in children with different severity of SAA, and compare differences in other potential pre-treatment variables between responders and non-responders. We identified a better FFS in the SAA group than in the vSAA group in the treatment of pALG during the 24 months follow-up, thus favorable response correlating well with higher ANC. We also performed univariate analysis to assess the contribution of each variable in predicting response. In these analyses, higher ARC and PLT level before IST, patients without PNH clone, and SAA group represented significant predictors of better response.
In this study, although response rate in the separate time point in the SAA group was basically higher than that in the vSAA group, there was no significant difference after 24 months in pALG treatment, which indicated the good effect in all SAA children. The adverse response after pALG treatment were moderate, so it is relatively safe. A previous study conducted by Cui et al.23 reported the overall response to pALG regimen at 3, 6, and 12 months were 56, 64, and 62%, respectively, in SAA children, which were similar to our study.23 However, they did not perform further analysis on the variables between responders and non-responders, as pre-treatment clinical and laboratory findings may exert an obvious influence on the response to IST. Thus, it was relatively incomplete.
Kulagin et al.21 reported that the presence of PNH clones and ARC were identified as independent predictors for hematological response, which was also validated in our study. A number of previous studies in both adults and children with SAA found a high prevalence (up to 70%) of PNH clones at diagnosis and during IST, which has prompted recommendations for PNH clone screening among SAA patients in a number of guidelines.24 Some studies indicated that the presence of small PNH clones in SAA patients is of potential importance, as it has been shown that these patients have a higher response to IST.25 However, PNH positivity was lack of prognostic value according to the NIH group study.26 In view of this, the value of PNH clone presence in the setting of pALG remains less conclusive. The current data confirmed a distinctly lower response rate in PNH-positive children after pALG regimen.
When it comes to the association between sex and response, we found that the response rate was better in the male group than in the female group, although the difference was not statistically significant, which was also observed in a retrospective study in another Asian cohort, where a young males group experienced faster recovery of bone marrow function following ATG.27 Poor response in girls could be influenced by their incapacity to produce hemopoietic growth factor activity. Nissen et al.28 previously reported that a high production of granulocyte colony is associated with a positive outcome after ALG treatment: hemopoietic reconstitution is invariably preceded by a bout of excess granulocyte colony. Accordingly, low granulocyte colony release, as observed in young girls, may herald poor response. Therefore, it appears that young females get a particular form of SAA characterized by low endogenous factor production and poor response to ALG.
Although insignificantly, response rates to IST were considerably low among SAA children with long-standing disease. Only 50% of patients treated more than 60 days after diagnosis responded, indicating that children may receive irreversible damage to hematopoietic progenitor cells, possibly due to immune attack through autoreactivated lymphocytes.27 The present study suggests the importance of immediate treatment for patients and recommends offering IST as quick as possible in all SAA children who lack a matched sibling donor.
Regarding the association between disease severity and response, conflicting results have been reported. A European study reported superior response rates in children with vSAA compared to SAA,29 but some studies including a recent report of a Korean cohort of adult patients have produced the opposite result.30 The present findings were consistent with those published studies, with favorable responses correlating well with higher ANC rather than ALC. In children with AA, treatment ANC may mainly reflect the size of WBC populations, due to the severe neutropenia in this condition. These results suggest that better response to IST might possibly be ascribed to higher WBC count, that is, a relative increase in neutrophil. When ALC was applied to the analysis, no correlation between ALC and response rate was observed in this study.
In SAA children, pre-treatment ARC and PLT count may mainly reflect the status of hematopoiesis function. The present findings suggested that higher response to IST might possibly be ascribed to improved hematopoiesis function, that is, a higher baseline ARC and PLT count may indicate better residual marrow function and the presence of a sufficient number of stem cells to sustain blood cell production after IST.
OS rate during follow-up period was 100%, thus we also adopted FFS rates following IST in this study; it is much more important than survival alone for evaluating the long-term outcome.31
We also have limitations in this study. First, taking into consideration any variable in the study population, we cannot exclude the possibility that another variable may reflect better hematopoietic reserves in the study cohort. Second, although our data were statistically significant, the small sample size in the study suggests that larger SAA trials are needed to further confirm our results.
Conclusion
In conclusion, modified IST with pALG was suitable for all SAA children, and pre-treatment clinical and laboratory findings influence response to pALG and CSA. A better FFS in the SAA group than in the vSAA group in the treatment of pALG was identified during follow-up, thus favorable response correlates with higher neutrophil count or mild disease severity than lymphocyte count, and this blood count parameter might help in clinically assessing bone marrow function. ARC and PLT status also appeared to be a reproducible prognostic model for response rate in the setting of ALG and CSA for SAA children. It appears that young girls get a particular form of SAA characterized by low endogenous factor production and poor response to pALG. IST should be started as soon as possible after diagnosis of SAA, given that the response rate worsens as the interval between diagnosis and treatment increases.
References
Young, N. S. Acquired aplastic anemia. JAMA 282, 271–278 (1999).
Dufour, C. et al. Outcome of aplastic anaemia in children. A study by the severe aplastic anaemia and paediatric disease working parties of the European group blood and bone marrow transplant. Br. J. Haematol. 169, 565–573 (2015).
Ma, X. et al. Comparison of porcine anti-human lymphocyte globulin and rabbit anti-human thymocyte globulin in the treatment of severe aplastic anemia: a retrospective single-center study. Eur. J. Haematol. 96, 260–268 (2016).
Cabannes-Hamy, A. et al. The effect of age in patients with acquired aplastic anaemia treated with immunosuppressive therapy: comparison of Adolescents and Young Adults with children and older adults. Br. J. Haematol. 183, 766–774 (2018).
Bacigalupo, A. et al. Antilymphocyte globulin, cyclosporine, prednisolone, and granulocyte colony-stimulating factor for severe aplastic anemia: an update of the GITMO/EBMT study on 100 patients. European Group for Blood and Marrow Transplantation (EBMT) Working Party on Severe Aplastic Anemia and the Gruppo Italiano Trapianti di Midolio Osseo (GITMO). Blood 95, 1931–1934 (2000).
Kojima, S. et al. Immunosuppressive therapy using antithymocyte globulin, cyclosporine, and danazol with or without human granulocyte colony-stimulating factor in children with acquired aplastic anemia. Blood 96, 2049–2054 (2000).
Rosenfeld, S. J., Kimball, J., Vining, D. & Young, N. S. Intensive immunosuppression with antithymocyte globulin and cyclosporine as treatment for severe acquired aplastic anemia. Blood 85, 3058–3065 (1995).
Frickhofen, N., Heimpel, H., Kaltwasser, J. P. & Schrezenmeier, H. Antithymocyte globulin with or without cyclosporin A: 11-year follow-up of a randomized trial comparing treatments of aplastic anemia. Blood 101, 1236–1242 (2003).
Peslak, S. A., Olson, T. & Babushok, D. V. Diagnosis and treatment of aplastic anemia. Curr. Treat. Options Oncol. 18, 70 (2017).
Luzzatto, L. & Risitano, A. M. Advances in understanding the pathogenesis of acquired aplastic anaemia. Br. J. Haematol. 182, 758–776 (2018).
Di, B. E. et al. Rabbit antithymocyte globulin (r-ATG) plus cyclosporine and granulocyte colony stimulating factor is an effective treatment for aplastic anaemia patients unresponsive to a first course of intensive immunosuppressive therapy. Gruppo Italiano Trapianto di Midollo Osseo (GITMO). Br. J. Haematol. 107, 330–334 (1999).
Scheinberg, P., Nunez, O. & Young, N. S. Retreatment with rabbit anti-thymocyte globulin and ciclosporin for patients with relapsed or refractory severe aplastic anaemia. Br. J. Haematol. 133, 622–627 (2006).
Scheinberg, P. et al. Horse versus rabbit antithymocyte globulin in acquired aplastic anemia. N. Engl. J. Med. 365, 430–438 (2011).
Bing, H. et al. The use of anti-human T lymphocyte porcine immunoglobulin and cyclosporine a to treat patients with acquired severe aplastic anemia. Acta Haematol. 124, 245–250 (2010).
Chen, M. et al. Long-term follow-up study of porcine anti-human thymocyte immunoglobulin therapy combined with cyclosporine for severe aplastic anemia. Eur. J. Haematol. 96, 291–296 (2016).
Wei, J. et al. Porcine antilymphocyte globulin (p-ALG) plus cyclosporine A (CsA) treatment in acquired severe aplastic anemia: a retrospective multicenter analysis. Ann. Hematol. 94, 955–962 (2015).
Liu, L. et al. Efficacy of porcine antihuman lymphocyte immunoglobulin compared to rabbit antithymocyte immunoglobulin as a first-line treatment against acquired severe aplastic anemia. Ann. Hematol. 94, 729–737 (2015).
Camitta, B. M. et al. Severe aplastic anemia: a prospective study of the effect of early marrow transplantation on acute mortality. Blood 48, 63–70 (1976).
Scheinberg, P. et al. Treatment of severe aplastic anemia with a combination of horse antithymocyte globulin and cyclosporine, with or without sirolimus: a prospective randomized study. Haematologica 94, 348–354 (2009).
Nishikawa, E. et al. Long-term outcomes of 95 children with moderate aplastic anemia treated with horse antithymocyte globulin and cyclosporine. Pediatr. Blood Cancer 64, e26305 (2017).
Kulagin, A. et al. Prognostic value of paroxysmal nocturnal haemoglobinuria clone presence in aplastic anaemia patients treated with combined immunosuppression: results of two-centre prospective study. Br. J. Haematol. 164, 546–554 (2014).
Tichelli, A. et al. A randomized controlled study in patients with newly diagnosed severe aplastic anemia receiving antithymocyte globulin (ATG), cyclosporine, with or without G-CSF: a study of the SAA Working Party of the European Group for Blood and Marrow Transplantation. Blood 117, 4434–4441 (2011).
Cui, Q. et al. Modified immunosuppressive therapy with porcine antilymphocyte globulin plus delayed cyclosporine A in children with severe aplastic anemia. Int J. Hematol. 107, 64–68 (2018).
Borowitz, M. J. et al. Guidelines for the diagnosis and monitoring of paroxysmal nocturnal hemoglobinuria and related disorders by flow cytometry. Cytom. B 78, 211–230 (2010).
Sugimori, C. et al. Minor population of CD55−CD59− blood cells predicts response to immunosuppressive therapy and prognosis in patients with aplastic anemia. Blood 107, 1308–1314 (2006).
Bacigalupo, A. How I treat acquired aplastic anemia. Blood 129, 1428–1436 (2017).
Yoshida, N. et al. Predicting response to immunosuppressive therapy in childhood aplastic anemia. Haematologica 96, 771–774 (2011).
Nissen, C. et al. Gender and response to antilymphocyte globulin (ALG) for severe aplastic anaemia. Br. J. Haematol. 83, 319–325 (1993).
Führer, M. et al. Immunosuppressive therapy for aplastic anemia in children: a more severe disease predicts better survival. Blood 106, 2102–2104 (2005).
Chang, M. H. et al. Predictors of response to immunosuppressive therapy with antithymocyte globulin and cyclosporine and prognostic factors for survival in patients with severe aplastic anemia. Eur. J. Haematol. 84, 154–159 (2010).
Socié, G. et al. Malignant tumors occurring after treatment of aplastic anemia. European Bone Marrow Transplantation-Severe Aplastic Anaemia Working Party. N. Engl. J. Med. 329, 1152–1157 (1993).
Acknowledgements
We would like to acknowledge AiYou Foundation and patients for participating in the follow-up as well as those who provided feedback on the written information. This work was supported by the National Key Research and Development Program of China (2016YFC0901503) and the National Natural Science Foundation of China (81500156, 81170470).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, LP., Chen, XJ., Yang, WY. et al. Predicting response to porcine antilymphocyte globulin plus cyclosporine A in children with acquired severe aplastic anemia. Pediatr Res 86, 360–364 (2019). https://doi.org/10.1038/s41390-019-0437-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-019-0437-1