Abstract
Diseases of the preterm newborn such as bronchopulmonary dysplasia, necrotizing enterocolitis, cerebral palsy, and hypoxic-ischemic encephalopathy continue to be major causes of infant mortality and long-term morbidity. Effective therapies for the prevention or treatment for these conditions are still lacking as recent clinical trials have shown modest or no benefit. Stem cell therapy is rapidly emerging as a novel therapeutic tool for several neonatal diseases with encouraging pre-clinical results that hold promise for clinical translation. However, there are a number of unanswered questions and facets to the development of stem cell therapy as a clinical intervention. There is much work to be done to fully elucidate the mechanisms by which stem cell therapy is effective (e.g., anti-inflammatory versus pro-angiogenic), identifying important paracrine mediators, and determining the timing and type of therapy (e.g., cellular versus secretomes), as well as patient characteristics that are ideal. Importantly, the interaction between stem cell therapy and current, standard-of-care interventions is nearly completely unknown. In this review, we will focus predominantly on the use of mesenchymal stromal cells for neonatal diseases, highlighting the promises and challenges in clinical translation towards preventing neonatal diseases in the 21st century.
Similar content being viewed by others
Introduction
Infant mortality in the United States continues to be high (5.9/1000 live births in 2016)1 compared to other developed nations. Diseases affecting extremely premature infants such as bronchopulmonary dysplasia (BPD) and necrotizing enterocolitis (NEC) remain major contributors to infant mortality and morbidity. Continued efforts to prevent these diseases have met with only modest success in the last decade, mandating new therapeutic strategies to improve disease outcomes in the 21st century. Stem cell (SC) and SC-derived therapies have emerged as promising options with over 900 registered trials on clinicaltrials.gov. Given the plasticity and regenerative potential of developing organs, SC use represents an exciting therapeutic strategy. However, fundamental questions regarding mechanisms of action and optimal treatment strategies remain unanswered, restricting their broad clinical applicability.2 Focusing primarily on mesenchymal stromal cells (MSC), we will discuss aspects of SC therapy relevant to neonatal diseases including mechanisms of action, sources, preclinical studies, and clinical trials herein. We highlight the promises and challenges of this novel therapy and provide a blueprint for successful clinical translation to prevent neonatal diseases in the 21st century.
MSC
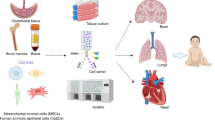
SC-based therapies have received major attention over the past 20 years after the initial discovery that bone marrow-derived cells can regenerate infarcted myocardium.3 This observation ignited a new field of investigation into the capacity of various types of “stem” cells to repair damaged organs including the brain, heart, gut and lung. One cell type, first described in the 1960s as colony forming fibroblasts, has received particular attention.4 These cells had clonogenic capability, and were able to differentiate into chondrocytes, osteocytes and adipocytes, and therefore qualified as “stem” cells. These bone marrow-derived cells, rapidly identified as important niche cells for the hematopoietic SC, were progressively investigated for their apparent repair functions. Since then, similar cells have been isolated from adipose tissue (ADSC), and umbilical cord (UC) and umbilical cord blood (UCB) (Fig. 1).5,6 Since their initial description, these cells have received several names. As of today, the most accepted – yet still evolving – denomination of MSC, is that proposed by the International Society for Cellular Therapy (ISCT).7 MSC characterization is based on expression of cell surface markers, plastic-adherent growth, and differentiation potential into osteocytes, adipocytes, and chondrocytes.7 Although some features unique to the MSC secretome are known,8,9 tissue-specific definitions are lacking. MSC are attractive due to their wide therapeutic potential, low immunogenicity due to lack of MHC class II receptors, ease of isolation, self-renewing capacity, and rapid and extensive ex vivo expansion capacity.10 They exhibit multi-lineage differentiation and their secretome, consisting of a broad array of growth factors, cytokines, chemokines and extracellular vesicles, exhibits pluripotent effects.11
Sources and potential mechanisms of action of stem cells for treating neonatal diseases. Stem cells from various sources have advantages (+) and disadvantages (−). Our understanding of mechanisms of action will inform applicability to neonatal diseases. BDNF brain-derived neurotrophic factor, BPD bronchopulmonary dysplasia, CP cerebral palsy, CTGF connective tissue growth factor, HIE hypoxic-ischemic encephalopathy, IFN interferon, IGF-1 insulin-like growth factor 1, IL interleukin, MMP-9 matrix metalloprotein-9, NEC necrotizing enterocolitis, TNF tumor necrosis factor, PGE2 prostaglandin E2, SDF-1 stromal cell-derived factor 1, TIMP-1 TIMP metallopeptidase inhibitor 1, TGF- β1 transforming growth factor β1, TSG6 tumor necrosis factor-inducible gene 6, VEGF vascular endothelial growth factor. Portion of figure made with resource from freepik.com
Other SC therapies
Some non-MSC stem cells can be similar, but are not identical to MSC; for example, amniotic fluid stem cells (AFSC) have the same cell surface markers and tripotent differentiation potential but also express stem cell embryonic antigen-4 (SSEA-4),12 CD29,13,14 CD49e, OCT-4,13 and may be weakly MHC class II positive.15 Other SCs, such as decidual stromal cells (DSCs), express many of the MSC surface markers, but differentiate poorly into osteocytes, adipocytes, and chondrocytes.16 Similarly, cardiac progenitor cells (CPCs) can be isolated from myocardium during surgical palliation of congenital heart defects, but unlike MSC, express cardiac-specific transcription factors like GATA-417 and critically, can be positive for the classical hematopoietic stem cell marker CD34.18 Finally, umbilical cord blood mononuclear cells (UCB-MNC) refers to the cell fraction obtained by centrifugation which contain UCB-MSC in a small proportion, and are mostly CD133 positive.19 Researchers have also administered minimally processed umbilical cord blood (UCB) without immunophenotyping or ex vivo expansion, to rapidly provide SC therapy.19,20,21,22
Postulated mechanisms of action
It was initially believed that cell replacement at the site of injury by engraftment and differentiation was the key mechanism of MSC action.23 However, extremely low rates of engraftment (typically <1-5%), and recent evidence indicate that MSC exert their therapeutic benefits via cell-to-cell communication and the secretion of bioactive molecules capable of modulating reparative processes24,25 (Fig. 1). These paracrine mechanisms26 include beneficial modifications of the host niche/tissue environment with production of factors important in inflammation/immune signaling (e.g., IL-1Rα,27 tumor necrosis factor-α-induced protein 6 (TSG6),28 prostaglandin E2,29 and IL-1030), angiogenesis (e.g., vascular endothelial growth factor), fibrosis (e.g., stanniocalcin-131 and adrenomedullin32), and cell death/repair (e.g., hepatocyte, insulin, and keratinocyte growth factors25,33,34,35).
The MSC secretome also includes extracellular vesicles such as exosomes, which are nanoparticle-sized, lipid-bilayer-enclosed vesicles that mediate the therapeutic benefit of MSC. Exosomes carry nucleic acids, including microRNAs, and proteins that, upon secretion into the extracellular space fuse with the cell membranes of host cells, effecting transcriptional and post-translational modifications.36,37 This discovery opens new exciting avenues towards cell-free therapy as in vitro38 and in vivo34,39,40,41 experiments demonstrate that the cell-free conditioned media or exosomes obtained from MSC exert the same therapeutic benefit as whole SC therapy. Administering the secretome/exosomes confers advantages related to SC manufacturing, storage, and ability to provide “off-the-shelf” pharmaceuticals,42 while avoiding potential ethical, legal, and scientific challenges, including a concern for tumorigenicity.43,44 Their potential to modulate inflammation/immune signaling, angiogenesis, fibrosis and cell death/repair make them ideal candidates for therapeutic exploration in diseases affecting preterm infants.
Homing & engraftment
MSC45,46 and AFSC12,15 home to sites of injury including the lung in animal models of BPD and the intestinal villi in models of NEC. Administered SC are often undetectable after a few days from the site of implantation,38,40 but may home to remote sites such as the spleen and liver.16,47 Some animal studies suggest that up to 21% of administered UC-MSC differentiate into neurons in the 35 days after transplant48 while others do not.49 Even without engraftment, the effect of SC appears to be long-lasting for up to 14 months.50 This may be related to route of administration, as those given intra-arterially21 and intra-nasally51 engraft, and intravenously49 administered SC do not, or related to SC type, as SC contained in intraperitoneally19 or intravenously20 administered UCB also fail to engraft. Therefore, while engraftment is not necessary for function, and the fate of transfused SC remain unclear, consideration of SC type, and administration near the site of action is important.
Inflammation
MSC were initially noted to be anti-inflammatory, and more recent findings have confirmed their immunomodulatory ability. MSC have several anti-inflammatory effects, inducing a shift from pro-inflammatory cytokines such as IL-1β,52 IL-6,52,53 TNFα,52,53,54 IFNγ,54 IL-1Rα,27 and prostaglandin E229 to anti-inflammatory cytokines like IL-1030,54,55 and TNFα-induced protein 6 (TSG-6).28 Increases in regulatory T-cells54 and a switch from M1 to M2 macrophage polarization51,56 also contribute to the anti-inflammatory signature.
Angiogenesis
MSC have pro-angiogenic effects, exerted primarily through the vascular endothelial growth factor (VEGF) family of pro-angiogenic growth factors essential for normal vascular development.57 MSC from placenta53 and UCB,34 and AFSCs12 secrete VEGF and induce endogenous VEGF secretion, improving lung vascular density in BPD models, and silencing MSC VEGF abolishes this effect.34 In vitro, UCB-MSC conditioned media induces endothelial cell proliferation and tubule formation similar to that induced by direct VEGF application.58
Fibrosis
Fibrosis is a common feature of chronic diseases such as BPD where parenchyma is replaced with scar tissue, putatively via TGF-β1/SMAD2/SMAD3 signaling.59 MSC often reduce fibrosis,53,60 MMP-9/TIMP-1 expression,60 connective tissue growth factor,53 elastin,61 and myofibroblast formation.61 They have also been reported to increase fibrosis62 and TGF-β1,46 and to decrease MMP-963 in BPD. In cardiac disease, BM-MSC64 and CPCs17 reduce fibrosis and collagen I, perhaps via adrenomedullin.32 Overall, the anti-fibrotic impact of MSC is less well-established, but still a likely mechanism of action.
Future mechanistic studies
While inflammation and regeneration are mechanisms relevant to all perinatal diseases, other MSC-functions may not be equally applicable to all diseases. Therefore, it is imperative that we further understand MSC function from a mechanistic perspective. Further, as disease processes are complex with pathogenic mechanisms that vary with stage of disease, consideration of best type of SC therapy and pre-conditioning is important for developing precision SC approaches to prevent or rehabilitate neonatal disease.
Disease outcomes
BPD and pulmonary hypertension
BPD, a chronic lung disease that develops in premature infants, remains a major cause of morbidity and mortality.65 BPD is a phenotype of disrupted lung growth arising from exposure of neonatal lung to chorioamnionitis and nosocomial infection, malnutrition, hyperoxia and positive pressure ventilation.66,67,68,69,70 The multifactorial nature of the disease has challenged development of novel therapies.71 The putative ability of MSC to sense their microenvironment and to modulate the repair response accordingly via pleiotropic secreted factors, makes them appealing for the treatment of BPD.24,72,73
Proof-of-concept experiments suggested a single intravenous39 or intra-tracheal injection38 of BM-MSC was lung-protective in neonatal rodents exposed to hyperoxia, leading to improvements in survival, lung inflammation, pulmonary hypertension and alveolar structure. Similarly, extensive studies have shown that a single intra-tracheal administration of human UC-/UCB-MSC prevents and rescues neonatal rats from hyperoxia-induced lung injury.74 They also have long-term efficacy and safety as exemplified by persistent improvement in lung structure and exercise capacity, with no evidence of tumor formation.40 Similar benefits on lung structure and inflammation in BPD models have been reported using human UCB-MSC.75 Cell-free therapy has considerable promise in BPD, as MSC-derived exosomes administered intravenously, intraperitoneally, or intratracheally prevent oxygen-induced lung injury in neonatal rodents via the modulation of macrophage activity and secretion of miRNA and TSG-6.56,76
The extensive pre-clinical evidence regarding MSC therapy in experimental neonatal lung injury was recently confirmed in a systematic review including 25 studies.26 MSC significantly improved alveolarization irrespective of timing of treatment, source, dose, or route of administration, except for one study using the intra-nasal approach. MSC also significantly ameliorated secondary endpoints including pulmonary hypertension, lung inflammation, fibrosis, angiogenesis, and apoptosis. Notably, numerous risks of bias were identified, highlighting the need for more rigorous experimental design and reporting of pre-clinical studies as set forth by the ARRIVE guidelines for animal studies.77 Furthermore, all 25 studies were performed in the neonatal hyperoxia-induced rodent model of BPD, indicating the need for studies in large animal models that allow the study of more complex disease pathology and in-depth physiologic assessments. Despite these shortcomings, first-in-human trials with MSC have been initiated (Fig. 2).
Current stage of clinical trial development for neonatal diseases. There is accumulating pre-clinical evidence of stem cell efficacy for neonatal diseases, driving initiation of phase I–III clinical trials. No completed phase III or post-marketing phase IV trials have yet been completed for neonatal diseases
The first phase I trial used a single intra-tracheal injection of allogeneic UCB-MSC in nine preterm infants born between 23 and 29 weeks gestation requiring mechanical ventilation between 5 and 14 days of age78 (Table 1). This dose escalation study testing 107 or 2 × 107SC/kg suggested that the procedure was feasible and well tolerated with no serious adverse events reported.78 The follow-up study at 2 years of age indicates no adverse growth, respiratory or neurodevelopmental outcomes.79 Similarly, a phase I/II trial at Rush University Medical Centre (NCT02381366) and phase I trial in Spain (NCT02443961) are pending (Table 2), the latter of which will test the safety and feasibility of up to 3 doses of intravenous UC-MSC in infants born at <28 weeks gestation still requiring mechanical ventilation at 14 days. Finally, a phase II double-blinded, multicenter, randomized controlled trial administering 107 MSC/kg is ongoing (NCT01828957) with a planned long-term follow-up (NCT01897987).
Hypoxic-ischemic encephalopathy
Hypoxic-ischemic encephalopathy (HIE) is caused by an acute reduction in cerebral blood flow and ischemia with necrosis, followed by inflammatory reperfusion injury.80 SC can reduce these phases of injury through anti-inflammatory, pro-angiogenic, anti-oxidant, and anti-apoptotic mechanisms. Pre-clinical studies indicate that when given within hours to days of hypoxia-ischemia, BM-MSC, UC-MSC, and UCB-MSC improve behavioral and motor outcomes,81,82,83 BM-MSC decrease the size of injured brain,84 and adipose-derived MSC and placenta-derived MSC reduce inflammation.52,54 Similar effects are found with administration of minimally processed UCB,85,86 endothelial colony forming cells,85 and neural stem cells,87 potentially making it difficult to investigate mechanisms of action.
Results of one clinical trial suggests that volume- and erythrocyte-reduced umbilical cord blood is safe and benefits neurodevelopmental outcome, although significant differences in gender, severity and attrition at follow-up between the intervention and control groups could have biased results in favor of SC therapy.88 The future of SC therapy for HIE lies in its past and present: identifying the target group of patients (mild, moderate, or severe and term or preterm) and the appropriate timing of intervention (within a certain time-frame), and conducting well-designed clinical trials to answer these fundamental questions.
Cerebral palsy
Cerebral palsy (CP) is a non-progressive motor disorder suffered by both preterm and full-term infants, associated with intellectual disability, impaired mobility, and epilepsy.89 MSC may modulate resident host progenitor cells to enhance plasticity, survival, and differentiation.90,91 Administering MSC in animal models of intraventricular hemorrhage improves behavioral outcomes, fosters growth of oligodendrocytes, and reduces inflammation.92,93 Brain-derived neurotrophic factor (BDNF) and IFNγ appear to be important mediators of this effect as BDNF knockdown eliminates the beneficial effects of MSC94 and the secretome of IFNγ-treated MSC, but not untreated MSC, restores myelination defects.95
In human studies of CP, administration of minimally processed UCB into the cerebrospinal fluid has been generally safe,96 as adverse effects reported relate to lumbar puncture (e.g., headache, vomiting) or mild immunologic reaction (e.g., fever). UCB administration improves motor symptoms,97,98,99 with UCB-MSC also showing benefit to gross and fine motor function for up to two years after administration.100 There may be a genetic basis of response, as twins are more likely to respond or not respond as a pair,101 and not all participants improve, suggesting that unrecognized variables that impact SC therapy efficacy exist.
Necrotizing enterocolitis
Necrotizing enterocolitis (NEC) is a major cause of morbidity and mortality among premature infants with mortality remaining between 20–30% in the last two decades.102 Although the exact pathogenesis of the disease is unknown, infants who develop NEC typically are born prematurely and have low birth weight.103 Additionally, alterations in the intestinal microbiome,104 genetic factors,105,106 and exaggerated inflammatory responses107 are associated with NEC pathogenesis. Survivors of NEC frequently have long term sequelae including short gut syndrome and neurodevelopmental delays.102 SC have been investigated as a possible treatment for NEC due to their ability to reduce inflammation, differentiate, and self-replicate, and they therefore have the potential to improve tissue health, function, and regeneration.108,109,110
Recent studies have investigated the ability of several types of SC and their secreted products to protect the intestines against experimental NEC. Bone marrow-derived MSC (BM-MSC), amniotic fluid-derived MSC (AF-MSC), amniotic fluid-derived neural stem cells (AF-NSC), and enteric neural stem cells (E-NSC) have similar effects on NEC in animal models.111 However, AF-NSC and E-NSC, compared to MSC, are challenging to isolate and culture,15,111 potentially limiting their clinical utility. AFSC administered intraperitoneally significantly reduce the incidence and severity of NEC in animal models,15 significantly decreasing histologic intestinal injury and improving gut barrier function.111,112 Furthermore, exosomes derived from MSC and NSC are just as effective in reducing the incidence and severity of experimental NEC as the SC from which they were derived.13 After intraperitoneal injection, AFSC migrate to bowel, liver, and spleen in healthy animals,113 and within 48–72 h, to tissues with a high level of inflammation and injury in experimental NEC, decreasing ascites114 and improving survival, intestinal function, and inflammation in a COX-2 dependent manner.15 AFSC in NEC-afflicted animals primarily localize in damaged tissue.15,113,115 Preliminary studies also suggest that extracellular vesicles from bovine milk-derived SC may be protective in NEC,116 preventing ileal injury and reduction in goblet cells via enhanced expression of the endoplasmic reticulum chaperone protein glucose-regulated protein 94. Although there have not yet been any clinical trials of SC in NEC, SC or their secreted products could be a promising, novel therapy for NEC and such trials may become a reality in the future (Fig. 3).
Blueprint for developing stem cell therapy for the 21st century. There are a variety of factors, both pre-clinical and clinical, that may impact stem cell efficacy that require further investigation, such as donor, culture methods, stem cell type, quality control, stem cell pre-conditioning, co-treatments, clinical trial design, and long-term follow-up, all of which are centered around studies to elucidate the mechanisms of stem cell action
Heart disease
Congenital heart defects are the most common birth defects and are often repaired in a staged manner, allowing harvesting of autologous material (e.g., cardiac progenitor/stem cells) for ex vivo manipulation prior to direct myocardial administration18,22 during subsequent surgical repair. Pre-clinical studies indicate that BM-MSC64 and CPC17 reduce RV dilation and RV strain and reduce cardiomyocyte apoptosis in mice undergoing left anterior descending artery ligation. A novel prenatal administration of CPC rescued mouse pups from heart failure and increased live births five-fold.117 Cell-free therapy may also be applicable as MSC-derived exosomes significantly alter CPC miRNA which promotes survival, long-term cardiac function, and reduced fibrosis in rats.118
The first human clinical trial for infants with hypoplastic left heart syndrome (HLHS) has been performed and 36 month follow-up data are available. The TICAP (Transcoronary Infusion of CPC in Patients with Single-Ventricle Physiology) pilot trial administered CPC directly into the coronary arteries of fourteen children under 6 years or age before stage 2 or 3 repair. At 36 months post-infusion, right ventricular function and somatic growth improved more in those who received CPC. Interestingly, responses were more favorable in infants with lower ejection fractions and those who were treated earlier.119 The stage I/II ELPIS trial (Allogeneic Human MSC Injection in Patients With HLHS)120 follows up on this study but will administer BM-MSC rather than CPC. ELPIS is enrolling up to thirty patients with HLHS who will receive intramyocardial allogeneic BM-MSC, 2.5 × 105 MSC/kg, at the time of stage 2 repair, with the primary outcome being need for emergent serious adverse event in the first month after infusion in the first ten patients, and the change in right ventricular ejection fraction in the next 20 patients.
Other diseases
Early studies of SC for congenital diaphragmatic hernia (CDH), retinopathy of prematurity (ROP), neonatal stroke, and sepsis show encouraging results. For example, MSC administration in rabbit CDH models improves pulmonary hypoplasia,121 and AFSC administration decreases pulmonary hypertension.14 Similarly, intra-vitreal administration of BM-MSC reduces neovascularization in a mouse model of ROP.122 As these disorders often co-occur with BPD, IVH, and NEC, the first clinical studies may in fact be from coincident findings in studies in which MSC therapy are further developed. Neonatal stroke treatment with MSC reduces infarct size, improves neurodevelopmental outcomes, promotes angiogenesis, and reduces inflammation.123,124 Interestingly, BDNF-overexpressing MSC appear to be more effective than non-transfected SC in reducing injury size and motor deficits in the short-term,125,126 again highlighting the need for understanding mechanism of action in addition to simply observing clinical outcomes. Inflammation from infections and sepsis can be targeted by MSC, improving survival and lung inflammation, though pre-conditioning with IFNγ does not improve efficacy in a model of neonatal sepsis in rats.127
Moving MSC to the bedside—Need for a tiered, evidence-based, pragmatic approach
Due to enthusiasm for novel SC therapeutics, and relative safety, at least for MSC, early phase clinical trials are already underway (Fig. 2). While these will provide some degree of information about the safety and feasibility of this approach, more needs to be learned about the mechanisms of action of MSC in order to harness their full therapeutic potential (Fig. 3). Translation of adult SC studies directly to children and babies without consideration of neonatal physiology and pathogenesis is likely to limit success. Side-by-side comparisons of MSC and their secretome (i.e., exosomes, microvesicles, or conditioned media) are especially important to determine if cell-free therapies are an effective and potentially safer option. Although it is tempting to consider MSC as a universal therapy for any and all patients and diseases, several technical and fundamental aspects must be addressed. Relating MSC therapy to traditional, single-chemical pharmacologic therapies offers a useful framework for considering translation into clinical practice. The core pharmacokinetic principles can be extended, relating absorption to route, distribution to homing, and metabolism to dose and co-treatment interactions, recipient factors and timing. Pharmacodynamically, receptor agonism/antagonism and drug potency relates to MSC pre-conditioning and secretome manipulation. The concepts of additive, antagonistic, or synergistic interactions are important when considering MSC as just one of many therapies an infant may be receiving. Until these factors are better understood, moving forward into large-scale, advanced phase clinical trials requiring years of long-term follow-up may be premature.
Donor
The first step to translation is to identify appropriate donors. Donor age impacts the SC phenotype, with neonatal MSC having greater anti-inflammatory capacity128 and exosomes from preterm UC-MSC being better able to repair ischemic injury compared to exosomes from term UC-MSC.129 Donor sex may impact the MSC secretome as discussed above,55 but has typically been understudied because many studies use male donors and female hosts to identify engraftment. Also to be considered is that early studies show that the health status of donors can impact MSC phenotype and function,130 but studies of this type are in their infancy.
SC type
Numerous tissues sources have been investigated (Fig. 1) with BM-MSC the most well-studied, but collection requires invasive procedures, making them difficult to obtain. Similarly, ADSC are typically obtained from liposuction aspirates.131 MSC from fetal membrane tissues (UC and UCB, placenta and amnion/chorion) and AFSC are especially appealing in the neonatal setting due to accessibility. Efficacy of autologous versus allogeneic SC is unknown, but the former are more likely to be accepted by families,132 and the latter have the advantage of being an “off-the-shelf” product readily available on-demand.
Culture methods
Once the appropriate SC type is identified, the optimal methods of isolation and culture are not yet known.9,133 In addition, traditional SC culture requires fetal bovine serum, which is undesirable for human administration and can change SC phenotype due to batch-to-batch variation.134 Xenobiotic-free culture methods utilizing human plasma or recombinant growth factors exist, but differences between products can alter MSC immunomodulatory capacity135 and cytokine production.136
Quality control and long-term follow up
A lack of in vitro potency assays to predict in vivo efficacy137 is a major challenge to improving the manufacturing of a clinical-grade cellular therapies. Indeed, the “product” is the process” in SC therapy, i.e., the process determines SC phenotype and function. Microarrays and genome sequencing may also be helpful once genetic profiles of various SC types and phenotypes are established. Safety is a critical factor, particularly the concern of carcinogenic transformation, as observed in induced pluripotent stem cells.138 Long-term cultures from higher-passage UC-MSC139 can acquire chromosomal aberrations and proliferative advantage. Therefore, long-term follow-up is required and will rely on phase IV and post-marketing surveillance, but registries of MSC recipients may also foster such monitoring. MSC represent a radical new type of therapy, especially for fragile neonates, so recipients will likely need to be followed into adulthood.
Pre-conditioning
There are a wide variety of chemical agents that could optimize MSC efficacy,140 but the mechanisms of action and improvements in efficacy are incompletely understood. Preliminary studies of pro-inflammatory stimulation with factors like IFNγ95,127 have found this promotes regeneration and anti-inflammatory effects, but pre-conditioning can also decrease efficacy.141 Oxidative stress may also be important, as the usual environment of MSC is relatively hypoxic142, and some neonatal diseases are caused by exposure to hyperoxia (e.g., BPD). For example, hyperoxia pre-conditioning enhances MSC efficacy in preventing pulmonary hypertension and alveolar simplification.41 These experiments also provide insight into how the MSC may respond when placed into the complex in vivo environment. Translating such findings to the bedside will require confirmation of these phenotypic changes with quality control assays as above and consideration of the technical and logistical challenges of pre-conditioning.
Co-treatments
Neonates in the intensive care unit receive many pharmaceuticals,143 as well as many non-pharmacologic treatments such as phototherapy and hypothermia, making it unlikely that MSC will be administered as a single agent. Some treatment-SC therapy interactions will probably be discovered, but studies of such interactions have thus far been limited. For example, inhaled nitric oxide and erythropoietin are synergistic with MSC therapy,60,63 enhancing pro-angiogenic and anti-fibrotic effects in models of BPD. Studies for HIE are more varied, showing therapeutic hypothermia and MSC can be synergistic82 or antagonistic, producing increased brain inflammation.144 These unexpected findings indicate the need for caution as MSC treatment moves from the controlled laboratory setting to the complex and highly variable clinical setting.
Clinical trial design
Clinical trials require definition of appropriate clinical and surrogate endpoints, and should aim to clarify optimal timing, dose, and route of administration; this is especially important for diseases such as HIE which exhibit “critical windows” of susceptibility. Additionally, current therapeutic modalities must be incorporated into study protocols, considered by appropriate target patient population, and statistical analyses to control for patient heterogeneity. Dose is a potentially limiting factor because of the challenges in manufacturing sufficient quantities of MSC from limited donor sources. Generally, higher doses of MSC are more effective, as observed in models of stroke49 and sepsis.145 The therapeutic ceiling of MSC is not yet defined and the dose in pre-clinical experiments can vary by several orders of magnitude. Route of administration affects dosing, as lower doses given directly into the site of injury are as efficacious as higher doses given intravenously by as much as five-fold.146 However, providing higher doses in a less invasive manner may be more acceptable to clinicians and families. Finally, the timing of administration must be investigated. One unique feature of SC-based therapy is its ability to affect initiation, propagation, and repair of disease, whereas conventional single agent therapies typically target one aspect of each phase. It is unclear whether SC should be used in a preventative or therapeutic manner, but efficacy may be diminished with later treatment in models of BPD40,75 and HIE.86,147,148 Timing may also affect the ability to use autologous SC, as UC-MSC take up to three weeks to get to first passage.149
Conclusions
Neonates with acute and chronic illnesses represent a unique clinical challenge, as these complex diseases encompass dynamic physiologic processes in immature developing organs. Current treatment strategies, including agents targeting single pathways, have resulted in small and only incremental improvements, since multi-organ, multi-pathway pathophysiology underpins these complex diseases. MSC and other SC may represent a paradigm shift in the treatment of these diseases as promising pre-clinical studies have led to early clinical trials. However, many challenges remain; including precise characterization of MSC and SC phenotypically, defining mechanisms of action, standardization across preparations and quality control, optimizing treatment protocols with due consideration of disease pathogenesis, and rigorous clinical trials. A systematic and coordinated approach by several teams looking at various aspects of SC therapy ranging from elucidation of mechanisms to clinical trial design, will likely deliver the promise of preventing neonatal disease in the 21st century using SC therapy.
References
Kochanek, K. D., Murphy, S. L., Xu, J., Arias, E. Mortality in the United States, 2016. NCHS Data Brief, no 293. [Internet]. Hyattsville, MD: 2017. Available from: https://www.cdc.gov/nchs/products/databriefs/db293.htm.
Nitkin, C. R., Bonfield, T. L. Concise Review: Mesenchymal Stem Cell Therapy for Pediatric Disease: Perspectives on Success and Potential Improvements. Stem Cells Transl Med 2016 (cited 20 Sep 2016) Available from: http://www.ncbi.nlm.nih.gov/pubmed/27625040.
Orlic, D. et al. Bone marrow cells regenerate infarcted myocardium. Nature 410, 701–705, http://www.nature.com/articles/35070587 (2001).
Friedenstein, A. J., Gorskaja, J. F. & Kulagina, N. N. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp. Hematol. 4, 267–274, http://www.ncbi.nlm.nih.gov/pubmed/976387 (1976).
Kern, S., Eichler, H., Stoeve, J., Klüter, H. & Bieback, K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 24, 1294–1301, http://www.ncbi.nlm.nih.gov/pubmed/16410387 (2006).
Miao, Z. et al. Isolation of mesenchymal stem cells from human placenta: comparison with human bone marrow mesenchymal stem cells. Cell Biol. Int. 30, 681–687, http://www.ncbi.nlm.nih.gov/pubmed/16870478 (2006).
Dominici, M., et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006 8:315–317.
Wegmeyer, H. et al. Mesenchymal stromal cell characteristics vary depending on their origin. Stem Cells Dev. 22, 2606–2618 (2013).
Hoffmann, A., Floerkemeier, T., Melzer, C. & Hass, R. Comparison of in vitro-cultivation of human mesenchymal stroma/stem cells derived from bone marrow and umbilical cord. J. Tissue Eng. Regen. Med. 11, 2565–2581 (2017).
Bernardo, M. E., Pagliara, D. & Locatelli, F. Mesenchymal stromal cell therapy: a revolution in Regenerative Medicine? Bone Marrow Transpl. 47, 164–171 (2012).
Cunningham, C. J., Redondo-Castro, E. & Allan, S. M. The therapeutic potential of the mesenchymal stem cell secretome in ischaemic stroke. J. Cereb. Blood Flow. Metab. 38, 1276–1292 (2018).
Grisafi, D. et al. Human amniotic fluid stem cells protect rat lungs exposed to moderate hyperoxia. Pedia. Pulmonol. 48, 1070–1080 (2013).
McCulloh, C. J. et al. Treatment of experimental necrotizing enterocolitis with stem cell-derived exosomes. J. Pedia. Surg. 53, 1215–1220 (2018).
Dekoninck, P. et al. The use of human amniotic fluid stem cells as an adjunct to promote pulmonary development in a rabbit model for congenital diaphragmatic hernia. Prenat. Diagn. 35, 833–840 (2015).
Zani, A. et al. Amniotic fluid stem cells improve survival and enhance repair of damaged intestine in necrotising enterocolitis via a COX-2 dependent mechanism. Gut 63, 300–309 (2014).
Erkers, T. et al. Treatment of severe chronic graft-versus-host disease with decidual stromal cells and tracing with (111)indium radiolabeling. Stem Cells Dev. 24, 253–263 (2015).
Wehman, B. et al. Cardiac progenitor cells enhance neonatal right ventricular function after pulmonary artery banding. Ann. Thorac. Surg. 104, 2045–2053 (2017).
Avolio, E. et al. Expansion and characterization of neonatal cardiac pericytes provides a novel cellular option for tissue engineering in congenital heart disease. J. Am. Heart Assoc. 4, e002043 (2015).
Nakanishi, K. et al. Rat umbilical cord blood cells attenuate hypoxic–ischemic brain injury in neonatal rats. Sci. Rep. 7, 44111 (2017).
Drobyshevsky, A. et al. Human umbilical cord blood cells ameliorate motor deficits in rabbits in a cerebral palsy model. Dev. Neurosci. 37, 349–362 (2015).
Greggio, S., De Paula, S., Azevedo, P. N., Venturin, G. T. & Dacosta, J. C. Intra-arterial transplantation of human umbilical cord blood mononuclear cells in neonatal hypoxic-ischemic rats. Life Sci. 96, 33–39 (2014).
Brizard, C. P. et al. Safety of intracoronary human cord blood stem cells in a lamb model of infant cardiopulmonary bypass. Ann. Thorac. Surg. 100, 1021–1029 (2015).
Prockop, D. J., Kota, D. J., Bazhanov, N. & Reger, R. L. Evolving paradigms for repair of tissues by adult stem/progenitor cells (MSCs). J. Cell Mol. Med. 14, 2190–2199 (2010).
Fung, M. E. & Thébaud, B. Stem cell-based therapy for neonatal lung disease: it is in the juice. Pediatr. Res. 75, 2–7 (2014).
Lee, J. W., Fang, X., Krasnodembskaya, A., Howard, J. P. & Matthay, M. A. Concise review: Mesenchymal stem cells for acute lung injury: role of paracrine soluble factors. Stem Cells 29, 913–919 (2011).
Augustine, S. et al. Mesenchymal stromal cell therapy in bronchopulmonary dysplasia: systematic review and meta-analysis of preclinical studies. Stem Cells Transl. Med. 6, 2079–2093 (2017).
Ortiz, L. A. et al. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc. Natl Acad. Sci. USA 104, 11002–11007 (2007).
Chaubey, S. et al. Early gestational mesenchymal stem cell secretome attenuates experimental bronchopulmonary dysplasia in part via exosome-associated factor TSG-6. Stem Cell Res Ther. 9, 173 (2018).
Bartosh, T. J. et al. Aggregation of human mesenchymal stromal cells (MSCs) into 3D spheroids enhances their antiinflammatory properties. Proc. Natl Acad. Sci. USA 107, 13724–13729 (2010).
Németh, K. et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat. Med. 15, 42–49 (2009).
Ono, M. et al. Mesenchymal stem cells correct inappropriate epithelial-mesenchyme relation in pulmonary fibrosis using stanniocalcin-1. Mol. Ther. 23, 549–560 (2015).
Li, L. L. et al. Mesenchymal stem cells overexpressing adrenomedullin improve heart function through antifibrotic action in rats experiencing heart failure. Mol. Med Rep. 17, 1437–1444 (2018).
Xu, Y.-X. et al. Mesenchymal stem cell therapy for diabetes through paracrine mechanisms. Med. Hypotheses 71, 390–393 (2008).
Chang, Y. S. et al. Critical role of vascular endothelial growth factor secreted by mesenchymal stem cells in hyperoxic lung injury. Am. J. Respir. Cell Mol. Biol. 51, 391–399 (2014).
Lee, J. W., Fang, X., Matthay, M. A., Serikov, V., Gupta, N. Allogeneic human mesenchymal stem cells for treatment of E. coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. Proc. Natl Acad. Sci. USA 106, 16357–16362 (2009).
van Niel, G., Porto-Carreiro, I., Simoes, S. & Raposo, G. Exosomes: a common pathway for a specialized function. J. Biochem. 140, 13–21 (2006).
Stoorvogel, W. Functional transfer of microRNA by exosomes. Blood 119, 646–648 (2012).
van Haaften, T. et al. Airway delivery of mesenchymal stem cells prevents arrested alveolar growth in neonatal lung injury in rats. Am. J. Respir. Crit. Care Med. 180, 1131–1142 (2009).
Aslam, M. et al. Bone marrow stromal cells attenuate lung injury in a murine model of neonatal chronic lung disease. Am. J. Respir. Crit. Care Med. 180, 1122–1130 (2009).
Pierro, M. et al. Short-term, long-term and paracrine effect of human umbilical cord-derived stem cells in lung injury prevention and repair in experimental bronchopulmonary dysplasia. Thorax 68, 475–484 (2013).
Waszak, P. et al. Preconditioning enhances the paracrine effect of mesenchymal stem cells in preventing oxygen-induced neonatal lung injury in rats. Stem Cells Dev. 21, 2789–2797 (2012).
Vizoso, F. J., Eiro, N., Cid, S., Schneider, J., Perez-Fernandez, R. Mesenchymal stem cell secretome: toward cell-free therapeutic strategies in regenerative medicine. Int. J. Mol. Sci. 18, 1852 (2017). Available from: http://www.ncbi.nlm.nih.gov/pubmed/28841158.
Park, S.-J. et al. Tumorigenicity evaluation of umbilical cord blood-derived mesenchymal stem cells. Toxicol. Res. 32, 251–258 (2016).
Barkholt, L. et al. Risk of tumorigenicity in mesenchymal stromal cell-based therapies - Bridging scientific observations and regulatory viewpoints. Cytotherapy 15, 753–759 (2013).
Hamidian, Jahromi, S., Estrada, C., Li, Y., Cheng, E., Davies, J. E. Human umbilical cord perivascular cells and human bone marrow mesenchymal stromal cells transplanted intramuscularly respond to a distant source of inflammation. Stem Cells Dev. 2018;27:scd.2017.0248. Available from: https://doi.org/10.1089/scd.2017.0248.
Luan, Y. et al. Bone marrow-derived mesenchymal stem cells protect against lung injury in a mouse model of bronchopulmonary dysplasia. Mol. Med. Rep. 11, 1945–1950 (2015).
Gholamrezanezhad, A. et al. In vivo tracking of 111In-oxine labeled mesenchymal stem cells following infusion in patients with advanced cirrhosis. Nucl. Med. Biol. 38, 961–967 (2011).
Zhang, X. et al. Therapeutic effect of human umbilical cord mesenchymal stem cells on neonatal rat hypoxic-ischemic encephalopathy. J. Neurosci. Res 92, 35–45 (2014).
Tanaka, E. et al. Dose-dependent effect of intravenous administration of human umbilical cord-derived mesenchymal stem cells in neonatal stroke mice. Front Neurol. 9, 133 (2018).
Donega, V. et al. Assessment of long-term safety and efficacy of intranasal mesenchymal stem cell treatment for neonatal brain injury in the mouse. Pediatr. Res. 78, 520–526 (2015).
Donega, V. et al. Intranasally administered mesenchymal stem cells promote a regenerative niche for repair of neonatal ischemic brain injury. Exp. Neurol. 261, 53–64 (2014).
Park, D. et al. Transplantation of human adipose tissue-derived mesenchymal stem cells restores the neurobehavioral disorders of rats with neonatal hypoxic-ischemic encephalopathy. Cell Med. 5, 17–28 (2013).
Chou, H. C., Li, Y. T. & Chen, C. M. Human mesenchymal stem cells attenuate experimental bronchopulmonary dysplasia induced by perinatal inflammation and hyperoxia. Am. J. Transl. Res. 8, 342–353 (2016).
Ding, H. et al. Transplantation of placenta-derived mesenchymal stem cells reduces hypoxic-ischemic brain damage in rats by ameliorating the inflammatory response. Cell Mol. Immunol. 14, 693–701 (2017).
Sammour, I. et al. The effect of gender on mesenchymal stem cell (MSC) efficacy in neonatal hyperoxia-induced lung injury. PLoS ONE 11, e0164269 (2016).
Willis, G. R. et al. Mesenchymal stromal cell exosomes ameliorate experimental bronchopulmonary dysplasia and restore lung function through macrophage immunomodulation. Am. J. Respir. Crit. Care Med. 197, 104–116 (2018).
Woik, N. & Kroll, J. Regulation of lung development and regeneration by the vascular system. Cell Mol. Life Sci. 72, 2709–2718 (2015).
Montemurro, T. et al. Angiogenic and anti-inflammatory properties of mesenchymal stem cells from cord blood: soluble factors and extracellular vesicles for cell regeneration. Eur. J. Cell Biol. 95, 228–238 (2016).
Wen, Y.-C. et al. EphA2-positive human umbilical cord-derived mesenchymal stem cells exert anti-fibrosis and immunomodulatory activities via secretion of prostaglandin E2. Taiwan J. Obstet. Gynecol. 57, 722–725 (2018).
Luan, Y. et al. Mesenchymal stem cells in combination with erythropoietin repair hyperoxia-induced alveoli dysplasia injury in neonatal mice via inhibition of TGF-β1 signaling. Oncotarget 7, 47082–47094 (2016).
Hou, C. et al. Human umbilical cord-derived mesenchymal stem cells protect from hyperoxic lung injury by ameliorating aberrant elastin remodeling in the lung of O2-exposed newborn rat. Biochem. Biophys. Res. Commun. 495, 1972–1979 (2018).
Liu, L. et al. Intranasal versus intraperitoneal delivery of human umbilical cord tissue–derived cultured mesenchymal stromal cells in a murine model of neonatal lung injury. Am. J. Pathol. 184, 3344–3358 (2014).
Zhang, Z.-H. et al. Protective effects of BMSCs in combination with erythropoietin in bronchopulmonary dysplasia-induced lung injury. Mol. Med Rep. 14, 1302–1308 (2016).
Wehman, B. et al. Mesenchymal stem cells preserve neonatal right ventricular function in a porcine model of pressure overload. Am. J. Physiol. - Hear Circ. Physiol. 310, H1816–H1826 (2016).
Dumpa, V. & Bhandari, V. Surfactant, steroids and non-invasive ventilation in the prevention of BPD. Semin Perinatol. 42, 444–452 (2018).
Menden, H. L. et al. Nicotinamide adenine dinucleotide phosphate oxidase 2 regulates LPS-induced inflammation and alveolar remodeling in the developing lung. Am. J. Respir. Cell Mol. Biol. 55, 767–778 (2016).
Morty, R. E. Recent advances in the pathogenesis of BPD. Semin Perinatol. 42, 404–412 (2018).
Bhandari, A. & Bhandari, V. Pathogenesis, pathology and pathophysiology of pulmonary sequelae of bronchopulmonary dysplasia in premature infants. Front Biosci. 8, e370–e380 (2003).
Baker, C. D. & Alvira, C. M. Disrupted lung development and bronchopulmonary dysplasia. Curr. Opin. Pediatr. 26, 306–314 (2014).
Álvarez-Fuente, M. et al. Preventing bronchopulmonary dysplasia: new tools for an old challenge. Pediatr. Res 85, 432–441 (2019).
Jobe, A. H. & Bancalari, E. Bronchopulmonary dysplasia. Am. J. Respir. Crit. Care Med 163, 1723–1729 (2001).
Caplan, A. I. Mesenchymal stem cells: time to change the name! Stem Cells Transl. Med. 6, 1445–1451 (2017).
Fischbach, M. A., Bluestone, J. A., Lim, W. A. Cell-based therapeutics: the next pillar of medicine. Sci. Transl. Med. 2013
Pierro, M., Thébaud, B. & Soll, R. Mesenchymal stem cells for the prevention and treatment of bronchopulmonary dysplasia in preterm infants. Cochrane Database Syst Rev. 11, (CD011932 (2017).
Chang, Y. S. et al. Timing of umbilical cord blood derived mesenchymal stem cells transplantation determines therapeutic efficacy in the neonatal hyperoxic lung injury. PLoS ONE 8, e52419 (2013).
Porzionato, A. et al. Intratracheal administration of clinical-grade mesenchymal stem cell-derived extracellular vesicles reduces lung injury in a rat model of bronchopulmonary dysplasia. Am. J. Physiol. Cell Mol. Physiol. 316, L6–L19 (2019).
Kilkenny, C., Browne, W. J., Cuthill, I. C., Emerson, M. & Altman, D. G. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 8, e1000412 (2010).
Chang, Y. S. et al. Mesenchymal stem cells for bronchopulmonary dysplasia: phase 1 dose-escalation clinical trial. J. Pediatr. 164, 966–972.e6 (2014).
Ahn, S. Y., Chang, Y. S., Kim, J. H., Sung, S. I. & Park, W. S. Two-year follow-up outcomes of premature infants enrolled in the phase I trial of mesenchymal stem cells transplantation for bronchopulmonary dysplasia. J. Pediatr. 185, 49–54.e2 (2017).
Douglas-Escobar, M. & Weiss, M. D. Hypoxic-ischemic encephalopathy: a review for the clinician. JAMA Pediatr. 169, 397–403 (2015).
Gu, Y. et al. Mesenchymal stem cells suppress neuronal apoptosis and decrease IL-10 release via the TLR2/NFκB pathway in rats with hypoxic-ischemic brain damage. Mol. Brain 8, 65 (2015).
Park, W. S. et al. Hypothermia augments neuroprotective activity of mesenchymal stem cells for neonatal hypoxic-ischemic encephalopathy. PLoS ONE 10, e0120893 (2015).
Mueller, M. et al. Wharton’s jelly mesenchymal stem cells protect the immature brain in rats and modulate cell fate. Stem Cells Dev. 26, 239–248 (2017).
Donega, V. et al. Intranasal administration of human MSC for ischemic brain injury in the mouse: In vitro and in vivo neuroregenerative functions. PLoS ONE 9, e112339 (2014).
Grandvuillemin, I. et al. Long-term recovery after endothelial colony-forming cells or human umbilical cord blood cells administration in a rat model of neonatal hypoxic-ischemic encephalopathy. Stem Cells Transl. Med. 6, 1987–1996 (2017).
Li, J. et al. Preterm white matter brain injury is prevented by early administration of umbilical cord blood cells. Exp. Neurol. 283, 179–187 (2016).
Braccioli, L., Heijnen, C. J., Coffer, P. J. & Nijboer, C. H. Delayed administration of neural stem cells after hypoxia-ischemia reduces sensorimotor deficits, cerebral lesion size, and neuroinflammation in neonatal mice. Pediatr. Res. 81, 127–135 (2017).
Cotten, C. M. et al. Feasibility of autologous cord blood cells for infants with hypoxic-ischemic encephalopathy. J. Pediatr. 164, 973–979.e1 (2014).
Novak, I. et al. Concise review: stem cell interventions for people with cerebral palsy: systematic review with meta-analysis. Stem Cells Transl. Med. 5, 1014–1025 (2016).
Wang, X. et al. Effects of bone marrow mesenchymal stromal cells on gross motor function measure scores of children with cerebral palsy: a preliminary clinical study. Cytotherapy 15, 1549–1562 (2013).
Finch-Edmondson, M., Morgan, C., Hunt, R. W. & Novak, I. Emergent prophylactic, reparative and restorative brain interventions for infants born preterm with cerebral palsy. Front Physiol. 10, 15 (2019).
Ahn, S. Y. et al. Mesenchymal stem cells prevent hydrocephalus after severe intraventricular hemorrhage. Stroke 44, 497–504 (2013).
Zhu, L. H. et al. Improvement of human umbilical cord mesenchymal stem cell transplantation on glial cell and behavioral function in a neonatal model of periventricular white matter damage. Brain Res 1563, 13–21 (2014).
Ahn, S. Y. et al. Pivotal Role of Brain-Derived Neurotrophic Factor Secreted by Mesenchymal Stem Cells in Severe Intraventricular Hemorrhage in Newborn Rats. Cell Transpl. 26, 145–156 (2017).
Morioka, C. et al. Neuroprotective effects of human umbilical cord-derived mesenchymal stem cells on periventricular leukomalacia-like brain injury in neonatal rats. Inflamm. Regen. 37, 1 (2017).
Feng, M. et al. Safety of Allogeneic Umbilical Cord Blood Stem Cells Therapy in Patients with Severe Cerebral Palsy: A Retrospective Study. Stem Cells Int 2015, 325652 (2015).
Liu, X. et al. Comparative analysis of curative effect of bone marrow mesenchymal stem cell and bone marrow mononuclear cell transplantation for spastic cerebral palsy. J. Transl. Med 15, 48 (2017).
Kang, M. et al. Involvement of Immune Responses in the Efficacy of Cord Blood Cell Therapy for Cerebral Palsy. Stem Cells Dev. 24, 1658–1671 (2015). Available from: http://www.ncbi.nlm.nih.gov/pubmed/25977995.
Romanov, Y. A. et al. Human allogeneic AB0/Rh-identical umbilical cord blood cells in the treatment of juvenile patients with cerebral palsy. Cytotherapy 17, 969–978 (2015).
Huang, L. et al. A Randomized, placebo-controlled trial of human umbilical cord blood mesenchymal stem cell infusion for children with cerebral palsy. Cell Transpl. 27, 325–334 (2018).
Wang, X. et al. Effect of umbilical cord mesenchymal stromal cells on motor functions of identical twins with cerebral palsy: pilot study on the correlation of efficacy and hereditary factors. Cytotherapy 17, 224–231 (2015).
Neu, J. & Walker, W. A. Necrotizing Enterocolitis. N. Engl. J. Med. 364, 255–264 (2011).
Herrmann, K. & Carroll, K. An exclusively human milk diet reduces necrotizing enterocolitis. Breast. Med. 9, 184–190 (2014).
Warner, B. B. et al. Gut bacteria dysbiosis and necrotising enterocolitis in very low birthweight infants: a prospective case-control study. Lancet 387, 1928–1936 (2016).
Cuna, A., George, L. & Sampath, V. Genetic predisposition to necrotizing enterocolitis in premature infants: Current knowledge, challenges, and future directions. Semin Fetal Neonatal Med. 23, 387–393 (2018).
Sampath, V. et al. SIGIRR genetic variants in premature infants with necrotizing enterocolitis. Pediatrics 135, e1530–e1534 (2015).
Hackam, D. J. & Sodhi, C. P. Toll-like receptor-mediated intestinal inflammatory imbalance in the pathogenesis of necrotizing enterocolitis. Cell Mol. Gastroenterol. Hepatol. 6, 229–238.e1 (2018).
Galindo, L. T. et al. Mesenchymal stem cell therapy modulates the inflammatory response in experimental traumatic brain injury. Neurol. Res Int. 2011, 564089 (2011).
Rowart, P. et al. Mesenchymal stromal cell therapy in ischemia/reperfusion injury. J. Immunol. Res. 2015, 1–8 (2015).
Diaco, N., Diamandis, Z. & Borlongan, C. Amniotic fluid-derived stem cells as an effective cell source for transplantation therapy in stroke. Brain Circ. 1, 119 (2015).
McCulloh, C. J., Olson, J. K., Zhou, Y., Wang, Y. & Besner, G. E. Stem cells and necrotizing enterocolitis: a direct comparison of the efficacy of multiple types of stem cells. J. Pediatr. Surg. 52, 999–1005 (2017).
McCulloh, C. J. et al. Evaluating the efficacy of different types of stem cells in preserving gut barrier function in necrotizing enterocolitis. J. Surg. Res. 214, 278–285 (2017).
Ghionzoli, M. et al. Amniotic fluid stem cell migration after intraperitoneal injection in pup rats: implication for therapy. Pediatr. Surg. Int. 26, 79–84 (2010).
Zani, A. et al. Amniotic fluid stem cells prevent development of ascites in a neonatal rat model of necrotizing enterocolitis. Eur. J. Pedia. Surg. 24, 057–060 (2013).
Drucker, N. A. et al. Stem cell therapy in necrotizing enterocolitis: current state and future directions. Semin Pediatr. Surg. 27, 57–64 (2018).
Li, B. et al. Bovine milk-derived exosomes enhance goblet cell activity and prevent the development of experimental necrotizing enterocolitis. PLoS ONE 14, e0211431 (2019).
Liu, X. et al. Rescue of neonatal cardiac dysfunction in mice by administration of cardiac progenitor cells in utero. Nat. Commun. 6, 8825 (2015).
Zhang, Z. et al. Pretreatment of cardiac stem cells with exosomes derived from mesenchymal stem cells enhances myocardial repair. J Am Heart Assoc. 5, e002856 (2016).
Tarui, S. et al. Transcoronary infusion of cardiac progenitor cells in hypoplastic left heart syndrome: Three-year follow-up of the Transcoronary Infusion of Cardiac Progenitor Cells in Patients with Single-Ventricle Physiology (TICAP) trial. J. Thorac. Cardiovasc Surg. 150, 1198–1207 (2015).
Kaushal, S. et al. Study design and rationale for ELPIS: A phase I/IIb randomized pilot study of allogeneic human mesenchymal stem cell injection in patients with hypoplastic left heart syndrome. Am. Heart J. 192, 48–56 (2017).
Yuniartha, R. et al. Therapeutic potential of mesenchymal stem cell transplantation in a nitrofen-induced congenital diaphragmatic hernia rat model. Pedia. Surg. Int. 30, 907–914 (2014).
Wang, J.-D. et al. Human bone marrow mesenchymal stem cells for retinal vascular injury. Acta Ophthalmol. 95, e453–e461 (2017).
Wei, Z. Z. et al. Intranasal delivery of bone marrow mesenchymal stem cells improved neurovascular regeneration and rescued neuropsychiatric deficits after neonatal stroke in rats. Cell Transpl. 24, 391–402 (2015).
van Velthoven, C. T. et al. Mesenchymal stem cells attenuate MRI-identifiable injury, protect white matter, and improve long-term functional outcomes after neonatal focal stroke in rats. J. Neurosci. Res. 95, 1225–1236 (2017).
Van Velthoven, C. T. J. et al. Mesenchymal stem cell transplantation attenuates brain injury after neonatal stroke. Stroke 44, 1426–1432 (2013).
Jeong, C. H. et al. Mesenchymal stem cells expressing brain-derived neurotrophic factor enhance endogenous neurogenesis in an ischemic stroke model. Biomed. Res. Int. 2014, 1–10 (2014).
Zhu, Y. et al. Human umbilical cord mesenchymal stromal cells improve survival and bacterial clearance in neonatal sepsis in rats. Stem Cells Dev. 26, 1054–1064 (2017).
Hamidian, Jahromi, S., Li, Y., Davies, J. E. Effect of tumor necrosis factor alpha dose and exposure time on tumor necrosis factor induced gene-6 activation by neonatal and adult mesenchymal stromal cells. Stem Cells Dev. 27, 44–54 (2018). Available from: http://www.ncbi.nlm.nih.gov/pubmed/29121823.
Panfoli, I. et al. Exosomes from human mesenchymal stem cells conduct aerobic metabolism in term and preterm newborn infants. FASEB J. 30, 1416–1424 (2016).
Montanucci, P. et al. Functional profiles of human umbilical cord-derived adult mesenchymal stem cells in obese/diabetic versus healthy women. Curr. Diabetes Rev. 12, 1–12 (2016).
Turner, L. US stem cell clinics, patient safety, and the FDA. Trends Mol. Med. 21, 271–273 (2015).
Hodges, R. J., Bardien, N. & Wallace, E. Acceptability of stem cell therapy by pregnant women. Birth 39, 91–97 (2012).
Park, W. S., Ahn, S. Y., Sung, S. I., Ahn, J.-Y. & Chang, Y. S. Strategies to enhance paracrine potency of transplanted mesenchymal stem cells in intractable neonatal disorders. Pediatr. Res. 83, 214–222 (2018).
Honn, K. V., Singley, J. A. & Chavin, W. Fetal bovine serum: a multivariate standard. Exp. Biol. Med. 149, 344–347 (1975).
Oikonomopoulos, A. et al. Optimization of human mesenchymal stem cell manufacturing: the effects of animal/xeno-free media. Sci Rep. 2015;5:16570.
Swamynathan, P. et al. Are serum-free and xeno-free culture conditions ideal for large scale clinical grade expansion of Wharton’s jelly derived mesenchymal stem cells? A comparative study. Stem Cell Res. Ther. 5, 88 (2014).
Boregowda, S. V., Phinney, D. G. Quantifiable Metrics for Predicting MSC Therapeutic Efficacy. J Stem Cell Res Ther. 6, pii: 365 (2016). Available from: https://www.omicsonline.org/open-access/quantifiable-metrics-for-predicting-msc-therapeutic-efficacy-2157-7633-1000365.php?aid=82018.
Volarevic, V. et al. Ethical and safety issues of stem cell-based therapy. Int J. Med. Sci. 15, 36–45 (2018).
Borghesi, A. et al. Genomic alterations in human umbilical cord-derived mesenchymal stromal cells call for stringent quality control before any possible therapeutic approach. Cytotherapy 15, 1362–1373 (2013).
Ferreira, J. R. et al. Mesenchymal stromal cell secretome: influencing therapeutic potential by cellular pre-conditioning. Front Immunol. 9, 2837 (2018).
Kilpinen, L. et al. Extracellular membrane vesicles from umbilical cord blood-derived MSC protect against ischemic acute kidney injury, a feature that is lost after inflammatory conditioning. J Extracell vesicles 2013;2. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24349659.
Hoch, A. I. & Leach, J. K. Concise review: optimizing expansion of bone marrow mesenchymal stem/stromal cells for clinical applications. Stem Cells Transl. Med. 3, 643–652 (2014).
Krzyżaniak, N., Pawłowska, I. & Bajorek, B. Review of drug utilization patterns in NICUs worldwide. J. Clin. Pharm. Ther. 41, (612–620 (2016).
Herz, J. et al. Interaction between hypothermia and delayed mesenchymal stem cell therapy in neonatal hypoxic-ischemic brain injury. Brain Behav. Immun. 70, 118–130 (2018).
Perlee D., et al. Intravenous infusion of human adipose mesenchymal stem cells modifies the host response to lipopolysaccharide in humans: a randomized, single-blind, parallel group, placebo controlled trial. Stem Cells 2018. https://doi.org/10.1002/stem.2891.
Ahn, S. Y. et al. Optimal route for mesenchymal stem cells transplantation after severe intraventricular hemorrhage in newborn rats. PLoS ONE 10, 1–14 (2015).
Cameron, S. H. et al. Delayed post-treatment with bone marrow-derived mesenchymal stem cells is neurorestorative of striatal medium-spiny projection neurons and improves motor function after neonatal rat hypoxia-ischemia. Mol. Cell Neurosci. 68, 56–72 (2015).
Park, W. S. et al. Optimal timing of mesenchymal stem cell therapy for neonatal intraventricular hemorrhage. Cell Transpl. 25, 1131–1144 (2016).
Araújo, A. B. et al. Isolation of human mesenchymal stem cells from amnion, chorion, placental decidua and umbilical cord: comparison of four enzymatic protocols. Biotechnol. Lett. 40, 989–998 (2018).
Acknowledgements
This study was supported by the National Institutes of Health (R01 HL128374 [VS]; R01 DK117296-01 [VS]; R01 GM113236 [GB]; R01 HL141345 [JR]), Canadian Institutes of Health Research (BT), Ontario Institute for Regenerative Medicine (BT), Canadian Stem Cell Network (BT), and Children’s Mercy Research Institute (VS, CN).
Author information
Authors and Affiliations
Contributions
All authors made substantial contributions to conception and design, drafting the article, revising it critically for important intellectual content, and gave final approval of the version to be published.
Corresponding author
Ethics declarations
Competing interests
GB is a scientific founder and stock option holder in Scioto Biosciences. CN, RJ, BT, and VS have no financial or other conflicts.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nitkin, C.R., Rajasingh, J., Pisano, C. et al. Stem cell therapy for preventing neonatal diseases in the 21st century: Current understanding and challenges. Pediatr Res 87, 265–276 (2020). https://doi.org/10.1038/s41390-019-0425-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-019-0425-5
This article is cited by
-
Mesenchymal stem cell therapy in perinatal arterial ischemic stroke: systematic review of preclinical studies
Pediatric Research (2024)
-
Comparison of Biological Characteristics of Human Umbilical Cord Wharton’s Jelly-Derived Mesenchymal Stem Cells from Extremely Preterm and Term Infants
Tissue Engineering and Regenerative Medicine (2023)
-
Comparison and Investigation of Exosomes from Human Amniotic Fluid Stem Cells and Human Breast Milk in Alleviating Neonatal Necrotizing Enterocolitis
Stem Cell Reviews and Reports (2023)
-
Stem cell therapy as a promising strategy in necrotizing enterocolitis
Molecular Medicine (2022)
-
Bench to bedside — new insights into the pathogenesis of necrotizing enterocolitis
Nature Reviews Gastroenterology & Hepatology (2022)