Abstract
Background
The pathogenesis of neonatal group B Streptococcus (GBS) lung infection may be associated with surfactant dysfunction or deficiency. This study aimed to investigate the efficacy of surfactants on early postnatal GBS infection in ventilated newborn rabbit lungs.
Methods
A near-term newborn rabbit model was established by intratracheal GBS instillation immediately at birth, followed by mechanical ventilation. At postnatal 1 h, a porcine surfactant was given intratracheally at 100 or 200 mg/kg. After 6 h, animals were euthanized, and lung and blood samples were collected for bacterial counting. Lung histopathology and messenger RNA (mRNA) expression of inflammatory mediators, surfactant proteins, and growth factors in lung tissue were assessed.
Results
The surfactants significantly suppressed (by >50%) pulmonary bacterial proliferation and systemic translocation, alleviated lung inflammatory injury, and improved alveolar expansion by morphometry, in favor of high-dose surfactants. Though the survival rate and lung mechanics were not improved, the surfactants significantly suppressed mRNA expression of proinflammatory mediators, while that for surfactant proteins and growth factors was differentially expressed, compared to the control and GBS infection groups.
Conclusion
Exogenous surfactants may provide a therapeutic alternative for neonatal lung infection by suppressing pulmonary GBS proliferation and translocation into systemic circulation, alleviating inflammatory injury and regulating growth factor expression.
Similar content being viewed by others
Introduction
Group B Streptococcus (GBS), or Streptococcus agalactiae, is a common microorganism of genital and gastrointestinal flora in healthy humans.1 Vertical transmission of GBS from mother to neonate may occur during pregnancy or at delivery.2 Neonatal GBS infections are typically heralded by respiratory symptoms shortly after birth, and may progress to severe sepsis and meningitis, indicating an initial pulmonary focus of infection.1,2 Although fatal infections have been demonstrated to occur preferentially in preterm and very low birth weight infants, the majority of neonates with GBS infections are born term and late preterm.3,4 Systemic reviews have reported that the case fatality rate of neonatal GBS-associated early-onset sepsis (EOS) may range from 10.5 to 12.1%.5,6 Among survivors of neonatal GBS infections, the risk of adverse neurodevelopmental outcomes has been demonstrated to be significantly higher than non-infected infants.7
The treatment of neonatal GBS infections mainly depends on antibiotics. However, emerging antibiotic resistance of GBS has been reported in recent years.1,4 Surfactants have been widely applied to treat respiratory distress syndrome (RDS) in preterm neonates, especially those born extremely preterm.8 By lowering alveolar surface tension, surfactants facilitate liquid absorption, improve pulmonary compliance, and protect small airway and alveoli from injurious cyclic stretching and hyperoxic stress.8,9 Although surfactants have been explored in the treatment of neonatal lung infection caused by various pathogens, including GBS for nearly three decades, there are controversies over the benefit of surfactants in treating neonates with pulmonary infections,10,11,12,13 and it is still regarded as an off-label use.13 Evidences from in vitro studies have suggested that surfactants may directly exert bactericidal effect on GBS,14,15 and was presumed to inhibit the cytotoxic effect of GBS on lung epithelial barrier during the process of bacterial translocation from neonatal lung to systemic circulation.16,17 In vivo animal experiments also showed that surfactants may inhibit bacterial growth and improve lung function in GBS infections.18,19 Additionally, a potential immunoregulatory role of surfactants in the alveolar defense has long been postulated, which may be of clinical importance to understand the pathogenesis of pulmonary GBS infection.20 Despite multiple rationales in support of the benefits, the surfactant therapy in term and near-term infants with lung infection did not show prominent effects in the past and recent observational studies,10,11,12,13,21,22 presumably due to low surfactant dosage and high costs, as well as complexity in pathogenesis of lung infection. With progression in protective lung ventilation strategy, more knowledge needs to be gained from neonates with severe bacterial infection, such as GBS, and the efficacy of surfactant therapy. To better address this issue, we established a near-term newborn rabbit model with pulmonary GBS infection based on the previous protocol18,19 with modification. Our hypothesis was that surfactants at a higher dose may exert more benefits in terms of increasing animal survival time, improving lung mechanics, suppressing GBS growth and translocation, and mitigating lung infection and inflammation.
Materials and methods
Preparation of bacteria
A clinical GBS strain (201705-WA0216) with hemolytic activity was isolated from the blood culture of a one-day-old term neonate diagnosed with EOS. A single colony of the strain was inoculated into 5 ml of tryptic soy broth (Oxoid, Cambridge, UK) and cultivated at 37 °C with agitation (240 rpm) for 12 h. Bacteria were re-suspended in sterile normal saline and adjusted at an optical density of 600 nm to achieve final concentrations of ~108, ~109, and ~1010 colony-forming units (CFUs)/ml. Since GBS at 109 CFU/ml resulted in both relatively higher survival rate and impaired lung mechanics in our preliminary animal tests, it was considered to be optimal in the main experiment (Table S1 in Supplementary materials). The count of bacteria was confirmed by serial dilution and plate counting on tryptic soy agar.
Animal model
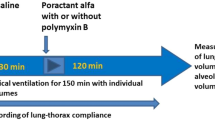
The study protocol was approved by Ethics Committees of Children’s Hospital of Fudan University. Healthy pregnant New Zealand White rabbits at 30 days of gestation (term = 31 days) with an average weight of 3.5–4.5 kg were sedated intramuscularly with 2 ml diazepam (5 mg/ml, Shanghai Xudong Haipu Pharmaceutical Co. Ltd., Shanghai, China) and intravenously with 5 ml/kg 20% urethane (Ethyl carbamate, BBI Life Sciences, Shanghai, China) under continuous face mask oxygen supply. Rabbit pups were delivered by cesarean section, dried, and weighed immediately after birth. After intraperitoneal anesthesia with 0.2 ml mixture of lidocaine (20 mg/ml, Shandong Hualu Pharmaceutical Co. Ltd.) and 10% glucose (volume ratio 1:1), newborn rabbits were tracheotomized and ventilated parallelly in individual boxes in a body plethysmograph kept at 37 °C, as previously described.23
GBS infection and mechanical ventilation
At birth, rabbit pups randomly received GBS suspension (~109 CFU/ml) or saline at 5 ml/kg via a metal tracheal cannula (outer diameter 1.2 mm) followed by mechanical ventilation. The ventilator (Servo 900 C, Siemens-Elema, Solna, Sweden) delivering 100% oxygen was set at 40 breaths/min with a 1:1 inspiratory/expiratory ratio and 2–3 cmH2O positive end expiratory pressure (PEEP). The peak inspiratory pressure (PIP) was recorded with a pressure transducer (Shanghai Yangfan Electronic Co. Ltd., Shanghai, China) and adjusted individually to obtain a tidal volume (Vt) of 4–6 ml/kg body weight, measured by a pneumotachometer (RSS100-HR, Hans Rudulph Inc., Kansas City, KA, USA). Both parameters were monitored by an automated physiologic monitoring system (PowerLab, ADInstruments Pty Ltd, NSW, Australia). Time 0 was set when the pup was connected to the ventilator and placed in the plethysmograph system. Additionally, a mixture of 10% glucose, 5% NaHCO3, and 2% lidocaine (volume ratio 6:3:1) was given intraperitoneally every 1.5–2 h to each pup, 0.1 ml per time.
Administration of surfactants
Surfactant preparation was extracted by methanol/chloroform from porcine lung lavage (40 mg phospholipids/ml), containing >35% disaturated phosphatidylcholine in total phospholipids, and was demonstrated to have high surface activities.9,24 After 1 h of ventilation when lung mechanics of GBS-infected and GBS-non-infected rabbits became stable, GBS-infected animals were randomly allocated to three groups treated with surfactant phospholipids at 100 mg/kg (GS100), 200 mg/kg (GS200) body weight, or sterile saline (GBS). Pups without GBS infection randomly received saline at the same time to serve as a sham control (CON). Some rabbits were euthanized just 1 min after ventilation by intracranial injection of 0.5 ml 2% lidocaine, to serve as a subset control for baseline GBS counts (G1min).18
Survival time and pulmonary function
The color of lips, limbs, and trunk of animals during ventilation was closely observed. Surviving rabbits were visually inspected to ensure that they had regular heart beats by ruling out arrhythmia.18,23 Lung mechanics (PIP, PEEP, Vt) were recorded at 5, 15, 30, 45, 60, 120, 180, 240, 300, and 360 min. Animals surviving after 6 h were euthanized by intracranial injection of 0.5 ml 2% lidocaine. Dynamic compliance of respiratory system (Cdyn, ml/kg/cmH2O) was calculated as Vt/(PIP − PEEP).23
Sampling of blood and lungs
The abdomen of executed animal was opened with sterile instruments, followed by inspection of pneumothorax. Then, the thorax was opened and blood was aspirated from the right ventricle for bacterial counting. The lungs were harvested en bloc aseptically and weighed. The appendix lobe of the right lung was removed for measurement of wet-to-dry weight ratio (W/D)24 or stored at −80 °C for further assessment of cytokine mRNA. Remaining lung lobes were prepared for histopathological examination or homogenized for bacterial counting.
Lung histopathology
Both right and left lungs were fixed with 4% paraformaldehyde, embedded in paraffin, sectioned, and stained with hematoxylin and eosin. Prepared samples were examined under light microscope (Leica Microsystems, Wetzlar, Germany). Leukocyte infiltration, edema, hemorrhage, and bronchiole epithelial desquamation were assessed to obtain lung injury score (LIS). A 4-score scale was used to represent the severity of lung injury: 0 for no or very minor (<1% area), 1 for modest and limited (1–25%), 2 for intermediate (26–50%), 3 for widespread or prominent (51–75%), and 4 for widespread and most prominent (>75%).9,24 A point-counting method was applied to quantify lung expansion, defined as volume density (Vv) of aerated alveolar spaces in total parenchyma.23,24 Coefficient of variation of Vv (CV (Vv)), representing field-to-field variability, was calculated to estimate homogeneity of alveolar expansion.24 The morphological evaluation was performed in blind manner by co-investigators.
Ultrastructural morphology of the lungs
Lung samples freshly excised from the inferior lobe of right lungs with a size of 1 mm3 were fixed with glutaraldehyde at 4 °C for 24 h, dehydrated, embedded, sectioned and stained according to standard procedures, and examined with transmission electron microscope (FEI Tecnai G2 Spirit TWIN, ThermoFisher Scientific, Boston, MA, USA).
mRNA expression of inflammatory mediators, surfactant proteins, and growth factors
Total RNA was extracted from lung tissues using Trizol reagent (Invitrogen, Carlsbad, CA, USA), and the concentration of RNA was measured by colorimetry. PrimeScript RT reagent kit with genomic DNA Eraser (Takara Bio Inc., Otsu, Shiga, Japan) was utilized to synthesize high-capacity complementary DNA. Gene sequence information of detected factors was obtained using nucleotide databases (www.ncbi.nlm.nih.gov/gene/), and primer sequences are listed in Supplemental Table S2. These factors include: (1) inflammatory mediators, such as tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), IL-6, IL-8, IL-10, intracellular adhesion molecule-1 (ICAM-1), inducible nitric oxide synthase (iNOS), as well as toll-like receptor-2 (TLR-2) and nuclear transcription factor-κB (NF-κB) subunit p50; (2) surfactant proteins (SP-A, SP-B, SP-C, SP-D) and cytidine triphosphate: phosphocholine cytidylyltransferase (CCT); (3) growth factors (GFs): vascular endothelial GF, keratinocyte GF, insulin-like GF-1 (IGF-1), IGF-2, IGF-1 receptor (IGF-1R), and IGF-2R. Real-time PCR was performed using Roche LightCycler 480 II (Roche, Basel, Switzerland). The amplification reaction was carried out in 20 μl volume containing 10 μl SYBR®Premix Ex Taq™ (Takara Bio Inc., Otsu, Shiga, Japan). All reactions were run in triplicates. The mRNA expression of each target gene was normalized to β-actin, with mean fold changes calculated using the ΔΔCT method.
Statistical analysis
Statistical analysis was performed using log-rank tests for Kaplan–Meier survival curves. Continuous variables were presented as mean ± standard deviation (SD) and subjected to one-way analysis of variance or Kruskal–Wallis test for differences among the groups, followed by Bonferroni post hoc test or Mann–Whitney U test, respectively, for between-group comparison. Categorical variables were expressed as number (proportion), analyzed by χ2 test. All statistical comparisons were done using the GraphPad Prism software version 6.02 (GraphPad Software, La Jolla, CA, USA) and IBM SPSS 21.0 (IBM, Armonk, NY, USA). A p value <0.05 was considered as statistically significant for the difference.
Results
Survival time of the ventilated animals
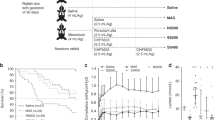
In total, 163 near-term pups from 25 litters were used in experiments. Among the four intervention groups, there were no significant differences in terms of birth weight and lung wet weight (Table 1). At 6 h of ventilation, there were no significant differences in survival rates among group GBS, GS100 and GS200. All groups achieved a survival rate >50%, but more animals in GBS-infected groups died than in CON (p < 0.05) (Fig. 1a).
Survival time and measurement of lung mechanics of the four groups. Definition of the groups: CON, sham control; GBS, group B Streptococcus only; GS100, GBS and surfactants 100 mg/kg body weight; GS200, GBS and surfactants 200 mg/kg. For details of group definition see also Table 1. a Survival time; Kaplan–Meier analysis showing the survival rate in CON (n = 32) is higher than in GBS (n = 45), GS100 (n = 36), and GS200 (n = 38) (p < 0.05); b dynamic compliance of respiratory system (Cdyn); Cdyn is significantly decreased in GBS, GS100, and GS200, compared to CON (p < 0.05)
Lung mechanics
Surfactant treatment exerted no significant effect on Cdyn in GS100 and GS200. However, Cdyn values in all three GBS-infected groups were significantly lower than those in CON (Fig. 1b, Table S3).
Bacterial proliferation
No significant difference existed in birth weight among CON, GBS, GS100, GS200, and G1min subgroup. There was a close correlation (r = 0.88, p < 0.001) between the number of bacteria given per tracheal tube and the CFUs recovered from both left and right lungs after 1 min of ventilation, suggesting that most bacteria were distributed in the ventilated lungs. All animal lungs subjected to bacterial culture were obtained from those surviving after 6 h. The number of CFU (log10) per gram wet lung weight was 8.35 ± 0.34 in G1min. Bacteria recovered in the GBS group were 9.21 ± 0.22 log10 CFU/g lung, showing an order of magnitude (8-fold) increase in bacterial count within 6 h (p < 0.001) (Fig. 2a). In GS100 and GS200 groups, the bacterial counts were 9.03 ± 0.16 and 8.93 ± 0.21 log10 CFU/g lung (p < 0.05, p < 0.01 vs. GBS, respectively). The rate of positive blood culture was 66.7% (16/24) in the GBS group, whereas in GS100 and GS200 the rate was 27.8% (5/18) and 29.4% (5/17) (p < 0.05), respectively. Bloodstream bacterial counts in GS100 and GS200 were reduced by 2 orders of magnitude (median 0), compared to GBS (p < 0.05, p < 0.01) (Fig. 2b).
Bacterial counting in animal lungs and blood. a Number of bacteria (log10 CFU/g lung tissue; mean ± SD) recovered from both lungs in animals ventilated for 6 h in the four groups compared with those from growth control animals executed 1 min after bacterial instillation and ventilation (G1min). b Number of bacteria (log10 CFU/ml blood) recovered from blood in animals after 6 h of ventilation. The horizontal lines represent the median values. The median values of CON, GS100, and GS200 are zero or nearly zero. For group definition, see Table 1 and Fig. 1 legends. §p < 0.05 vs. G1min, *p < 0.05 vs. CON; †p < 0.05, ‡p < 0.01 vs. GBS
Lung histology
No pneumothorax was identified in all ventilated animals. LIS, W/D, Vv, and CV (Vv) in each group are shown in Table 1. Compared to CON, the GBS groups had severe lung inflammatory injuries, represented by significantly higher LIS and W/D, and lower Vv with larger CV (Vv). LIS, especially the score of leukocyte infiltration, were significantly lower in both GS100 and GS200 compared to GBS group (p < 0.05), whereas the alveolar aeration was significantly improved in both groups as shown by higher Vv with smaller CV (Vv) as well (Table 1). The same was true for lower lung W/D. There was a trend towards lower W/D and leukocyte infiltration in GS200. However, GS200 did not improve Vv and other histological changes in lung tissue, as compared to GS100 (Table 1, Fig. 3).
Photomicrographs of representative lung histopathological changes in the four groups after 6 h of ventilation. a Well-aerated alveoli with very mild leukocyte infiltration (black arrow). b Alveolar atelectasis with severe leukocyte infiltration, necrotic cells (black arrow), and hemorrhage (white arrow). c Improved alveolar aeration with mild and focal leukocyte infiltration, and d improved alveolar aeration with mild leukocyte infiltration. For group definition, see Table 1 and Fig. 1 legends. Scale bar = 50 μm. Hematoxylin and eosin, ×200
Lung ultrastructure
Under transmission electron microscopy, surfactant debris, mainly dissociated lamellar bodies were seen in alveolar space in CON, GS100, and GS200 (Fig. 4a, c, d). The structure of type II alveolar epithelial cells (AEC-II) was intact, demonstrating nuclei, lamellar body, and microvilli. Bacteria were found in three GBS-infected groups (Fig. 4b–d).
Expression of mRNA from inflammation-associated factors
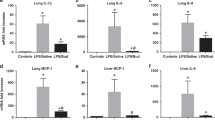
As shown in Fig. 5, the mRNA expression of TLR-2 in the GBS group was significantly enhanced compared to CON (p < 0.01). Reduced TLR-2 expression was found in both GS100 and GS200 compared to GBS (p < 0.05, p < 0.001), and the effect was more prominent in GS200 (p < 0.001 vs. GS100). NF-κB (subunit p50) mRNA expression was significantly increased in all GBS-infected lungs compared to CON (p < 0.001), but was markedly reduced in GS100 and GS200 (p < 0.01, p < 0.001). The same trend was found for TNF-α, IL-1β, IL-6, IL-8, and iNOS, although no statistical significances were shown between GS100 and GS200.
Comparison of relative messenger RNA (mRNA) expression of proinflammatory cytokines in lung tissue after 6 h of ventilation. Abbreviation of variables: TLR, toll-like receptor; NF-κB, nuclear transcription factor-κB (unit p50); IL, interleukin; TNF, tumor necrosis factor; ICAM, intercellular adhesion molecule; iNOS, inducible nitric oxide synthase. The white, black, dark gray, and light gray bar graphs represent CON, GBS, GS100, and GS200 groups, respectively. For group definition, see Table 1 and Fig. 1 legends. Values are presented as means ± SD (n = 12) on a log10 scale on Y-axis. **p < 0.01 vs. CON; †p < 0.05, ‡p < 0.01 vs. GBS; §p < 0.01 vs. other three groups
Expression of surfactant proteins and CCT mRNA
The mRNA expression of SP-A and SP-D was significantly enhanced in the GBS group compared to CON (p < 0.01). Surfactant treatment significantly suppressed mRNA expression of SP-D induced by GBS (p < 0.001) (Fig. 6a). In contrast, surfactants did not exert a significant effect on mRNA expression of SP-A, SP-B, SP-C, and CCT.
Comparison of relative messenger RNA (mRNA) of surfactant proteins and growth factors in lung tissue after 6 h of ventilation. a Surfactant proteins (SP-A, SP-B, SP-C, SP-D) and cytidine triphosphorylate: phosphocholine cytidylyltransferase (CCT). b Various growth factors (GFs). Variable abbreviations: VEGF, vascular endothelial growth factor; KGF, keratinocyte growth factor; IGF, insulin-like growth factor; IGF-R, IGF-receptor. The white, black, dark gray, and light gray bar graphs represent CON, GBS, GS100, and GS200, respectively. For group definition, see Table 1 and Fig. 1 legends. Values are means ± SD (n = 12). *p < 0.05, **p < 0.01 vs. CON; †p < 0.05 vs. GBS, ‡p < 0.01 vs. GBS
Expression of GFs mRNA
The mRNA expression of all GFs, except IGF-2 and IGF-2R, showed no significant differences among all groups. Notably, enhanced expression of IGF-2 and IGF-2R was found in GS200 only, compared to the control (Fig. 6b).
Discussion
This study demonstrated that pulmonary GBS infection was associated with mortality, decreased lung mechanics, and severe lung injury with typical pathologic and molecular biological changes in near-term newborn rabbits. Despite no significant improvement of lung mechanics and survival rate in surfactant-treated animals, surfactants at both concentrations considerably inhibited bacterial proliferation in lung tissues and translocation into systemic circulation, ameliorated lung inflammatory injury, and reduced proinflammatory mediators mRNA expression. We also observed a potentially dose-dependent antimicrobial and anti-inflammatory effect of surfactants.
The rationale of applying exogenous surfactants to treat pulmonary infection partly lies in improving lung function,10,12 as autopsies of neonates who died from GBS pneumonia/sepsis showed surfactant deficiency and dysfunction.25 Several studies have shown that the surfactant therapy improved gas exchange in newborns with GBS pneumonia.10,11 An in vivo animal study showed that the Cdyn in preterm rabbits with GBS infection was significantly enhanced with surfactants at 200 mg/kg19 compared to saline-treated controls with GBS lung injury. However, in our study, both surfactant groups exerted almost no effect on Cdyn improvement of near-term rabbits with GBS infection, whereas the values of W/D, Vv, and CV (Vv) reached levels similar to CON group, significantly different from that of the GBS group, and in general in favor of GS200 (Table 1). These discrepancies may be partly explained by the use of a moderate level (2–3 cmH2O) of PEEP in our ventilator settings, which might have altered lung expansion pattern in the measurement of lung mechanics9,26,27 and by the time of surfactant administration at 1 h post GBS infection, when lung impairment was likely to be at the progressive stage. Our previous surveys, as well as those of retrospective surveys of GBS infection in neonates, demonstrated that the surfactant therapy had mild-to-moderate effects in improvement of survival rate of late preterm, early term, and term neonates with hypoxemic respiratory failure with underlying pathologies (RDS, meconium aspiration, lung infection, and others).10,11,12,13,21,22 Whether surfactants may improve lung mechanics in lung infection, or septic injury, relies on well-designed, clinical investigation to verify.
The efficacy of surfactants may be associated with microbial species, as well as the composition and dosage of surfactants.11,13 Apart from a potentially direct anti-bacterial effect through suppression of proliferation,14,18,19 surfactants may indirectly contribute to bacterial killing by enhancing phagocytic functions of macrophages and neutrophils via expanding alveoli and improving gas exchange.15,18 Moreover, we showed that surfactants may inhibit GBS translocation into systemic circulation, which has been scarcely described before. Herting et al.18,19 demonstrated that almost all animals with GBS infection had positive blood culture of GBS despite surfactant treatment. With surfactant treatment, we found a >95% reduction in systemic bacterial counts, with >50% reduction in the proportion of positive blood samples (Fig. 2b), showing decreased GBS translocation from the lungs to systemic circulation. Invasiveness of GBS is known to be highly dependent on its virulence factor β-hemolysin/cytosin (β-h/c), a pore-forming membrane toxin capable of invading epithelial and endothelial cells and inducing subsequent lung damages, which may contribute to GBS translocation and systemic dissemination.2,16,17,28 As alveolar pool size of surfactant phospholipids is increased following exogenous surfactant therapy, surface-active component, namely dipalmitoyl phosphatidylcholine, may inhibit the cytotoxic effect of β-h/c.16,28 As mentioned above, one of the differences by modified study protocol for the animal model18,19 was addition of 2–3 cmH2O of PEEP in the current ventilator settings.26,27 A combination of high-dose surfactants and PEEP may in part contribute to the alleviation of bacterial translocation. Siew et al.27 demonstrated that, in preterm rabbits (28 days of gestation), ventilation at birth with a fixed PIP of 19–21 cmH2O generated only 1 ml/kg birth weight functional residual capacity (FRC). Surfactants (100 mg/kg) and 3 cm H2O of PEEP generated 2 and 5 ml/kg more FRC, respectively, and the effects were additive. It is probable that surfactants and PEEP improved alveolar aeration after GBS injury, as reflected by significantly improved Vv (and CV (Vv) in both GS100 and GS200 groups (Table 1), suggesting improved FRC. The fact that there was no improvement in the survival in both surfactant-treated groups may be associated with hemodynamic instability, intrapulmonary shunting, acid–base imbalance, and timing and dosage of surfactants.
The anti-inflammatory effect of surfactants as shown by our study may indirectly contribute to the suppression of GBS translocation through protection of alveolar barrier, but the relevant mechanism remains to be clarified. Previous in vivo studies of our research group showed that surfactants may inhibit inflammatory lung injuries induced by bacteria,29 bacterial endotoxin,30 oleic acid,24 meconium aspiration,31 or in primary surfactant deficiency,9,23 as manifested with less leukocyte infiltration and enhanced alveolar expansion in lung histology. Furthermore, GBS significantly stimulated mRNA expressions of proinflammatory mediators, such as IL-1β, TNF-α, IL-6, IL-8, and ICAM-1, and up-regulated TLR-2 and NF-κB expression (Fig. 5). Notably, GBS induced only a modest increase of anti-inflammatory cytokine IL-10 mRNA expression, suggesting an imbalance towards inflammation. With surfactant treatment, median expression of TLR-2, NF-κB, IL-1β, TNF-α, IL-6, and IL-8 mRNA in GBS-infected lungs were reduced by at least 50%, in a surfactant dose-dependent manner (Fig. 5). TLR-2 is considered to be essential for the recognition of Gram-positive bacteria including GBS, and the activation of NF-κB plays a major role in the subsequent release of proinflammatory mediators.31,32,33 This study indicated that the anti-inflammatory effect of surfactants may be mediated via the down-regulation of TLR-2 and NF-κB signaling pathways.
Additionally, iNOS, also regulated by NF-κB, may be involved in the production of nitric oxide (NO), an inflammatory mediator likely to be induced in the context of bacterial infection,34 or hyperoxia,35 or both, potentiating bactericidal or cytotoxic effect. Exogenous NO at therapeutic dosage may modulate this pathophysiological process without jeopardizing surfactant system.9,24,30,35,36 The overall suppressive effect of surfactants on mRNA of proinflammatory cytokines involves iNOS following GBS infection (Fig. 5). Whether it was a causal relationship or just an accompanying phenomenon is unclear. Our previous studies on septic lung injury with systemic and pulmonary infection with Gram-negative bacilli, namely Escherichia coli or Klebsiella pneumonia, suggest that inhaled NO, in combination with surfactants, may suppress inflammation and bacterial proliferation.29,37 Due to scarce relevant literature, interaction between exogenous surfactant and endogenous NO production in GBS infection in neonatal lungs should be further investigated.
The disturbance of surfactant homeostasis is another important factor in the pathogenesis of bacterial infection/pneumonia. The effect of exogenous surfactant on surfactant proteins in GBS-induced lung injury has been seldom explored. SP-A and SP-D are considered to be the major immunomodulatory components of natural surfactants through binding pathogens and modulating inflammatory mediators.20 This study showed that the expression of both SP-A and SP-D mRNA significantly increased in GBS-infected lungs, and surfactant treatment may reduce SP-D mRNA expression. The underlying mechanism and clinical implications of this phenomenon are unknown yet, and requires more experiments to explore.
GFs play a prominent role in lung cell differentiation, growth, and injury reparation.38 In contrast to other GFs, the expression of IGF-2 and IGF-2R mRNA was significantly enhanced in GS200 only, when compared to the GBS group. In a rat model of hyperoxia-induced lung injury, the mRNA expressions of IGF system components including IGF-1, IGF-2, and their receptors were elevated, demonstrating that IGF may function critically in proliferation and differentiation of alveolar epithelium during tissue remodeling.39 However, it is unclear whether the enhancement of IGF-2 and IGF-2R expression is associated with subsequent effective lung reparative process. Our early experimental studies on ventilated preterm piglets with RDS showed 5-fold to 10-fold higher expression of IGF mRNA by inhaled NO (or with surfactants) treatment in the presence of 5–6 cmH2O PEEP.9 Our recent study also revealed a >20-fold expression of IGF-1 mRNA in cultured AEC-II derived from newborn piglet lungs after exposure to corticosteroid priming followed by endotoxin stimulation.40 Taken together, the role of IGF in neonatal septic lung injury and reparation, or remodeling, may be an important target in the future study.
There are several limitations of this study. The serotype and pathogenic factors of GBS strain were not characterized, which may prevent a thorough understanding of GBS-induced lung inflammatory injuries.15,16,28 Moreover, we only assessed the expression of limited inflammatory mediators at the mRNA level. Since this is a preliminary study to explore possible mechanisms involved in anti-bacterial and anti-inflammatory effects of surfactants, further studies are warranted to unravel the underlying mechanisms by conducting translational and transcriptional analysis. Additionally, as this is an in vivo animal model with predetermined time points for analysis, the effect and temporal trend of pro- and anti-inflammatory mediators as well as lung mechanics observed here may vary from that in neonates per se. Also, the pharmacodynamics and pharmacokinetics of surfactants may act differently in animal models as in humans.
In conclusion, by using an established model of near-term newborn rabbits with pulmonary GBS infection, we showed that surfactants may inhibit bacterial proliferation and translocation, ameliorate pulmonary inflammation possibly through reducing the transcription of inflammatory mediators, with a potential dose-dependent effect. Our results may inspire new clinical application of surfactants in the treatment of bacterial infection in the neonatal lungs.
References
Verani, J. R., McGee, L. & Schrag, S. J. Division of bacterial diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention (CDC). Prevention of perinatal group B streptococcal disease-revised guidelines from CDC, 2010. MMWR Recomm. Rep. 59, 1–36 (2010).
Doran, K. S. & Nizet, V. Molecular pathogenesis of neonatal group B streptococcal infection: no longer in its infancy. Mol. Microbiol. 54, 23–31 (2004).
Stoll, B. J. et al. Early onset neonatal sepsis: the burden of group B Streptococcal and E. coli disease continues. Pediatrics 127, 817–826 (2011).
Dong, Y. et al. Group B Streptococcus causes severe sepsis in term neonates: 8 years experience of a major Chinese neonatal unit. World J. Pediatr. 13, 314–320 (2017).
Edmond, K. M. et al. Group B streptococcal disease in infants aged younger than 3 months: systematic review and meta-analysis. Lancet 379, 547–556 (2012).
Madrid, L. et al. Infant group B streptococcal disease incidence and serotypes worldwide: systematic review and meta-analyses. Clin. Infect. Dis. 65, 160–172 (2017).
Yeo, K. T. et al. Long-term outcomes after group B streptococcus infection: a cohort study. Arch. Dis. Child 104, 172–178 (2019).
Seger, N. & Soll, R. Animal derived surfactant extract for treatment of respiratory distress syndrome. Cochrane Database Syst. Rev. 2, CD007836 (2009).
Qian, L. et al. Effects of positive end-expiratory pressure, inhaled nitric oxide and surfactant on expression of proinflammatory cytokines and growth factors in preterm piglet lungs. Pediatr. Res. 64, 17–23 (2008).
Fetter, W. P. et al. Surfactant replacement therapy in neonates with respiratory failure due to bacterial sepsis. Acta Paediatr. 84, 14–16 (1995).
Herting, E. et al. Surfactant treatment of neonates with respiratory failure and group B streptococcal infection. Pediatrics 106, 957–964 (2000).
Tan, K., Lai, N. M. & Sharma, A. Surfactant for bacterial pneumonia in late preterm and term infants. Cochrane Database Syst. Rev. 2, CD008155 (2012).
Taylor G. et al. Surfactant administration in preterm infants: drug development opportunities. J. Pediatr. 208, 163–168 (2019).
Rauprich, P. et al. Influence of modified natural or synthetic surfactant preparations on growth of bacteria causing infections in the neonatal period. Clin. Diagn. Lab. Immunol. 7, 817–822 (2000).
Bouhafs, R. K. et al. Direct and phagocyte-mediated lipid peroxidation of lung surfactant by group B streptococci. Lung 178, 317–329 (2000).
Doran, K. S. et al. Group B streptococcal beta-hemolysin/cytolysin promotes invasion of human lung epithelial cells and the release of interleukin-8. J. Infect. Dis. 185, 196–203 (2002).
Hensler, M. E. et al. Virulence role of group B Streptococcus beta-hemolysin/cytolysin in a neonatal rabbit model of early-onset pulmonary infection. J. Infect. Dis. 191, 1287–1291 (2005).
Herting, E. et al. Experimental neonatal group B streptococcal pneumonia: effect of a modified porcine surfactant on bacterial proliferation in ventilated near-term rabbits. Pediatr. Res. 36, 784–791 (1994).
Herting, E. et al. Surfactant improves lung function and mitigates bacterial growth in immature ventilated rabbits with experimentally induced neonatal group B streptococcal pneumonia. Arch. Dis. Child Fetal Neonatal Ed. 76, 3–8 (1997).
Wright, J. R. Immunoregulatory functions of surfactant proteins. Nat. Rev. Immunol. 5, 58–68 (2005).
Wang, H. et al. Surfactant reduced the mortality of neonates with birth weight >1500g and hypoxemic respiratory failure: a survey from an emerging NICU network. J. Perinatol. 37, 645–651 (2017).
Zhang, L. et al. Mortality of neonatal respiratory failure from Chinese Northwest NICU network. J. Matern. Fetal Neonatal Med. 30, 2105–2111 (2017).
Sun, B. et al. Application of a new ventilator-multi-plethysmograph system for testing efficacy of surfactant replacement in newborn rabbits. Eur. Respir. J. 4, 364–370 (1991).
Zhu, G. F. et al. Combined surfactant therapy and inhaled nitric oxide in rabbits with oleic acid-induced acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 158, 437–443 (1998).
Payne, N. R. et al. Correlation of clinical and pathologic findings in early onset neonatal group B streptococcal infection with disease severity and prediction of outcome. Pediatr. Infect. Dis. J. 7, 836–847 (1988).
Rider, E. D. et al. Different ventilation strategies alter surfactant responses in preterm rabbits. J. Appl. Physiol. 73, 2089–2096 (1992).
Siew, M. L. et al. Surfactant increases the uniformity of lung aeration at birth in ventilated preterm rabbits. Pediatr. Res. 70, 50–55 (2011).
Nizet, V. et al. Group B streptococcal beta-hemolysin expression is associated with injury of lung epithelial cells. Infect. Immun. 64, 3818–3826 (1996).
Zhu, Y. et al. Different effects of surfactant and inhaled nitric oxide in modulation of inflammatory injury in ventilated piglet lungs. Pulm. Pharm. Ther. 18, 303–313 (2005).
Zhao, D. H. et al. Mitigation of endotoxin-induced acute lung injury in ventilated rabbits by surfactant and inhaled nitric oxide. Intens. Care Med. 26, 229–238 (2000).
Hu, X. et al. Mitigation of meconium-induced lung injury by surfactant and inhaled nitric oxide is associated with suppression of nuclear transcription factor kappa B. Biol. Neonate 87, 73–81 (2005).
Puliti, M. et al. Toll-like receptor 2 deficiency is associated with enhanced severity of group B streptococcal disease. Infect. Immun. 77, 1524–1531 (2009).
Glaser, K. & Speer, C. P. Toll-like receptor signaling in neonatal sepsis and inflammation: a matter of orchestration and conditioning. Expert Rev. Clin. Immunol. 9, 1239–1252 (2013).
Goodrum, K. J. & Poulson-Dunlap, J. Cytokine responses to group B streptococci induce nitric oxide production in respiratory epithelial cells. Infect. Immun. 70, 49–54 (2002).
Hu, X., Guo, C. & Sun, B. Inhaled nitric oxide attenuates hyperoxic and inflammatory injury without alteration of phosphatidylcholine synthesis in rat lungs. Pulmon. Pharm. Ther. 20, 75–84 (2007).
Gong, X. et al. Inhaled nitric oxide alleviates hyperoxia suppressed phosphatidylcholine synthesis in endotoxin-induced injury in mature rat lungs. Respir. Res. 7, 5 (2006).
Sun, Z. et al. Anti-inflammatory effects of inhaled nitric oxide are optimized at lower oxygen concentration in experimental Klebsiella pneumoniae pneumonia. Inflam. Res. 55, 430–440 (2006).
Deng, J. C. & Standiford, T. J. Growth factors and cytokines in acute lung injury. Compr. Physiol. 1, 81–104 (2011).
Narasaraju, T. A. et al. Expression profile of IGF system during lung injury and recovery in rats exposed to hyperoxia: a possible role of IGF-1 in alveolar epithelial cell proliferation and differentiation. J. Cell. Biochem. 97, 984–998 (2006).
He, L. et al. Protective role of glucocorticosteroid prior to endotoxin exposure in cultured neonatal type II alveolar epithelial cells. Pulm. Pharm. Ther. 52, 18–26 (2018).
Acknowledgements
We thank Dr. Yi Liu for measurement with transmission electron microscopy, Dr. Dongmei Ding and Prof. Lian Chen for measurement of lung histology and morphometry. This study was supported by a grant from the National Natural Science Foundation (No. 81501288 to Y.D.).
Author information
Authors and Affiliations
Contributions
Y.X. performed experiments, analyzed data, and drafted manuscript; Y.D. conceptualized and supervised the whole study plan and experiment protocol, reviewed, and modified manuscript; X.G. was responsible for measurement of lung mechanics and lung tissue processing. B.S. conceptualized the study plan, designed the experiment, interpreted the data, edited, and finalized the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementaryinformation
Rights and permissions
About this article
Cite this article
Xu, Y., Dong, Y., Guo, X. et al. Suppression of pulmonary group B streptococcal proliferation and translocation by surfactants in ventilated near-term newborn rabbits. Pediatr Res 86, 208–215 (2019). https://doi.org/10.1038/s41390-019-0421-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-019-0421-9