Abstract
Background
To assess the postnatal rate of rise (ROR) of total serum bilirubin (TSB) in very low birth weight (VLBW) preterm infants, to determine risk factors associated with a rapid rise (>90th percentile), and to compare ROR and hour-specific TSB at postnatal 12–48 h with data of term infants retrieved from the literature.
Methods
Retrospective analysis of 2430 routine TSB concentrations obtained between birth and initiation of phototherapy in 483 VLBW infants.
Results
TSB increased by a median (interquartile range) ROR of 0.15 (0.11–0.19) mg/dL/h. The 50th percentile of TSB was below the 40th percentile of (near-)term counterparts at 12–48 h. TSB ROR correlated with the age at initiation (RS = −0.687; p < 0.001) and the duration (RS = 0.444; p < 0.001) of phototherapy. ROR >90th percentile (>0.25 mg/dL/h) was associated with lower gestational ages [27.2 (25.4–29.3) vs. 28.4 (26.4–30.4) weeks], lower birth weights [978 (665–1120) vs. 1045 (814–1300) g], and lower 5-min Apgar scores [7 (7–8) vs. 8 (7–9)].
Conclusion
ROR of TSB is an indicator for early and prolonged phototherapy. While hour-specific TSB and ROR at 12–48 h are lower than those reported for (near-)term infants, TSB appears to rise more rapidly in infants with low gestational age, low birth weight, and low 5-min Apgar score.
Similar content being viewed by others
Introduction
All newborn infants display a rise of total serum bilirubin (TSB) concentrations after birth.1 While low concentrations are considered to have neuroprotective effects,2,3 high concentrations can lead to irreversible damage of the central nervous system (bilirubin-induced neurologic dysfunction).4
Preterm and very low birth weight infants (VLBW) are considered at high risk for bilirubin-mediated brain damage and are generally subjected to phototherapy at much lower thresholds than term infants.5,6,7,8 However, unlike term and late preterm infants, whose screening and management of hyperbilirubinemia are based on universally accepted guidelines referring to a percentile-based nomogram of TSB values,9 similar universal accepted guidelines are not available for preterm infants <35 weeks of gestation. A variety of different approaches have been proposed, including potential risk factors for “subsequent hyperbilirubinemia,” such as low gestational age (GA), low birth weight (BW), high postnatal weight loss, history of jaundice, bruising, cephalhematoma, Asian race, glucose-6 phosphate deficiency, and exclusive breastfeeding.5,10,11,12
To identify infants who are at risk for severe hyperbilirubinemia, previous investigators suggested the rate of rise (ROR) of transcutaneous bilirubin (TcB) (TcB ROR) per hour (mg/dL/h) as a predictor of subsequent need of phototherapy by demonstrating that neonates who require phototherapy have consistently higher postnatal TcB RORs than neonates who do not need phototherapy.13 A predictive model based on TcB ROR was proposed with a sensitivity of 83%, a negative predictive value of almost 98%, and a specificity of 47% for the 50% percentile TcB ROR to predict the need for phototherapy and for early discharge policy in term and late preterm neonates within the first 18 to 24 h.13
However, the ROR of TSB (TSB ROR) and associated risk factors for rapidly rising bilirubin concentrations have not yet been studied in preterm neonates, especially not in those with a BW <1500 g.
In this study, we examined the natural course of hour-specific TSB concentrations in preterm neonates <1500 g BW, covering the period of time from birth until phototherapy was set up according to the current German hyperbilirubinemia guidelines.14 Furthermore, we evaluated TSB ROR as an indicator for timing and duration of phototherapy, determined risk factors associated with a rapid rise (>90th percentile), and compared hour-specific TSB and TSB ROR between 12 and 48 h of life, with data of term infants retrieved from the literature. We hereby aim to evolve a better understanding of postnatal TSB increase in VLBW preterm infants.
Methods
Study design
This retrospective study was conducted at the Department of Neonatology, Charité - Universitätsmedizin Berlin, Germany, based on routine capillary TSB measurements obtained during a 3-year study period each time a blood gas analysis was sampled. The study was approved by the local ethics committee (EA2/085/16) and the data protection officer (380/16).
Study population
Basic data of all newborn infants with a BW <1500 g (n = 548), who were born between September 1, 2012 and August 31, 2015 and were cared for in our neonatal intensive care unit (NICU), were retrieved from the electronic hospital information system. Infants with a GA ≥37 weeks, infants who were admitted to the NICU >72 h after birth or who died <24 h after birth, infants without TSB measurements obtained from an automated blood gas analyzer (ABL800 FLEX), or whose clinical charts were not fully available were excluded from this study.
Bilirubin measurements
Simultaneous measurements of blood gases, pH, glucose, Na+, K+, Ca2+, hemoglobin, and bilirubin were done in 0.1 mL blood samples using an ABL800 FLEX blood gas analyzer (Radiometer Medical, Brønshøj, Denmark). As this study intends to cover the period of time from birth until phototherapy was set up, only TSB samples prior to the infant’s individual phototherapy threshold were included in this analysis. Therefore, as soon as an infant hit its individual phototherapy threshold, it was excluded from further analysis. The exact timing of phototherapy was retrieved from clinical charts. Phototherapy was applied according to common clinical practice based on the German national hyperbilirubinemia guideline published in 2010.14 As stated by this guideline, the phototherapy threshold for preterm infants is calculated by GA (weeks) minus 20 (mg/dL), and there is a further reduction of 2 mg/dL for each day before the age of 3 days. The 2010 guideline also recommended to reduce the threshold by additional 2 mg/dL if the direct antiglobulin test (DAT) is positive. The minimum threshold for initiation of phototherapy is 5 mg/dL.14 TSB measurements of infants who were not treated with phototherapy were included up to postnatal peak concentration, whereas subsequent tests were excluded from the analysis. Phototherapy was stopped as soon as the infant’s TSB concentration dropped and consistently stayed below the individual phototherapy threshold within routine measurements.
Potential risk factors
Clinical characteristics of infants and mothers who were assumed to be associated with postnatal TSB increase were retrieved from clinical charts, including GA in weeks, BW in gram (g), sex, mode of delivery, 5-min Apgar score, umbilical artery pH (pH), hemoglobin in g/dL analyzed by an ABL800 FLEX blood gas analyzer at the time of birth, maximum postnatal weight loss in percent (%), DAT, ABO blood group constellation between mother and infant, feto-fetal transfusion syndrome (FFTS), antenatal betamethasone administration, and rates of intraventricular hemorrhage (IVH). ABO blood group compatibility was defined as the absence of one of the following constellations: mother, 0—infant, A or B or AB; mother, A—infant, B or AB; mother, B—infant, A or AB.
Statistical analysis
Statistical analysis was carried out using Statgraphics Centurion® (version 16.0, Statpoint Inc., Herndon, VA, USA), MEDCALC® (version 9.1.0.1, MedCalc Software, Mariakerke, Belgium), and SPSS Statistics® (version 24.0, IMB Corporation, Armonk, NY, USA).
Data are given as median (interquartile range (IQR)) for continuous and ordinal variables and as numbers (percentages) for categorical variables. P values <0.05 were considered significant.
Hour-specific total serum bilirubin concentrations
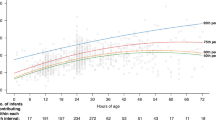
Linear regression analysis was applied to describe the course of hour-specific postnatal TSB concentrations. All TSB samples of all study subjects (n = 483) were included in this analysis. Pearson’s correlation coefficient R was used to find the best-fitted curvilinear model. We calculated percentile curves of hour-specific postnatal TSB concentrations. With permission from Pediatrics®, the official journal of the American Academy of Pediatrics, we plotted our smoothed 50th percentile into the nomogram of hour-specific TSB values in term and near-term appropriate for GA infants (BW ≥2000 g for GA ≥36 weeks or BW ≥2500 g for ≥35 weeks) published by Bhutani et al.9 and thereby compared hour-specific TSB of VLBW preterm infants with term and near-term infants between 12 and 48 h.
Rate of rise of total serum bilirubin
For each study subject who had at least two TSB recordings prior to the initiation of phototherapy or alternatively, if no phototherapy was implemented two TSB recordings up to postnatal peak concentration, the mean “individual TSB ROR” (mg/dL/h) was determined by linear regression analysis (n = 467). To evaluate TSB ROR as an indicator for phototherapy, Spearman’s rank coefficient (RS) was applied between TSB ROR and age (h) at initiation as well as duration (h) of phototherapy. Based on their “individual TSB ROR,” the infants were divided into two groups (group 1: ROR >90th percentile; group 2: ROR ≤90th percentile). Infants whose mean “individual ROR” was above the 90th percentile of all “individual RORs” were considered as “rapid risers.” The infants’ age (h) at the time of initiation of phototherapy as well as the duration of phototherapy (h) was compared between the two groups by using the Mann–Whitney U test. Likewise, we compared “individual TSB RORs” between infants who received phototherapy and those who did not. Moreover, we compared TSB ROR between VLBW preterm and (near-) term infants within postnatal 12 to 48 h by visual comparison of graphs after having plotted our smoothed 50th percentile of TSB into the nomogram published by Bhutani et al.9
Risk factor analysis
Differences of clinical characteristics that were assumed to be potential risk factors of a “rapid TSB rise” (>90th percentile) were compared between the two defined groups. Continuous or ordinal variables were compared by using the Mann–Whitney U test. Differences of categorical variables were compared by Fisher’s exact test.
Results
Patient’s characteristics
A total of 548 VLBW neonates were cared for at the NICU at Charité - Universitätsmedizin Berlin, Germany between September 2012 and August 2015, of whom 65 were excluded for various reasons given below (Fig. 1). The final study population consisted of 483 preterm neonates with a median (IQR) GA of 28.3 (26.3–30.4) weeks and a BW of 1040 (810–1295) g. Of all study subjects, 88.8% were treated with phototherapy. The number of available TSB samples prior to the initiation of phototherapy or alternatively up to postnatal TSB peak concentration ranged from 1 to 17 samples for each infant. A total of 467 infants had at least two TSB samples and were included to determine the ROR of postnatal TSB concentrations. Patient’s characteristics are given in Table 1.
Hour-specific postnatal total serum bilirubin concentrations
An overall of 2430 bilirubin samples were obtained between birth and initiation of phototherapy. The first sample was obtained at 2.58 (1.37–5.37) h of age displaying a TSB level of 1.9 (1.4–2.5) mg/dL. Regression analysis between TSB samples and the neonates’ age resulted in a square root curve model depicting the course of hour-specific postnatal TSB concentrations in preterm infants <1500 g. The curvilinear regression line with 95% prediction limits is plotted in Fig. 2. In Fig. 3, the 50th percentile curve of postnatal TSB is plotted into the nomogram of hour-specific TSB concentrations of term and near-term appropriate for GA infants by Bhuntani et al.,9 which is widely recommended as a reference for screening of postnatal rising bilirubin concentrations in various guidelines.14 As depicted, at postnatal 12 to 48 h, the 50th percentile of hour-specific TSB concentrations of the cohort of VLBW preterm infants is consistently somewhat below the 40th percentile of hour-specific TSB concentrations of (near-) term appropriate for GA infants reported by Bhutani et al.9
Fiftieth percentile of postnatal hour-specific total serum bilirubin (TSB) concentrations in preterm neonates <1500 g birth weight in relation to data of term and near-term appropriate for gestational age neonates by Bhutani et al.9
Rate of rise of total serum bilirubin
Of all study subjects, 467 infants had at least two TSB recordings up to the initiation of phototherapy or alternatively, if no phototherapy was implemented, prior to postnatal TSB peak concentration, and were therefore included to calculate mean “individual TSB ROR.” Postnatal TSB concentrations increased alongside postnatal age, with an overall TSB ROR of 0.15 (0.11–0.19) mg/dL/h prior to the initiation of phototherapy at 30.23 (22.57–40.86) h of age. Between postnatal 12 to 48 h, TSB increases slower in the cohort of VLBW preterm infants compared to the (near-) term infants reported by Bhutani et al.9 (Fig. 3). The infants’ individual postnatal TSB ROR was negatively correlated with the age at initiation of phototherapy (RS = −0.687; p < 0.001) and positively correlated with the duration of phototherapy (RS = 0.444; p < 0.001). A total of 46 infants displayed rapidly rising TSB concentrations >90th percentile (>0.25 mg/dL/h), while 421 infants had a mean “individual ROR” below 0.25 mg/dL/h. Comparing these two groups, phototherapy was initiated at an earlier median (IQR) age [16.80 (14.58–20.0) vs. 31.98 (25.46–42.48) h; p < 0.001] and the overall duration was longer [132.3 (95.3–161.8) vs. 87.0 (27.5–138.5) h; p < 0.001] in “rapid risers” (Fig. 4). Likewise, neonates who were treated with phototherapy had generally higher “individual TSB RORs” than those who did not receive phototherapy during hospitalization [0.15 (0.12–0.20) vs. 0.08 (0.06–0.11) mg/dL/h; p < 0.001].
Risk factor analysis
Low GA (p = 0.014), low BW (p = 0.027), and lower 5-min Apgar scores (p = 0.045) were the only variables that were significantly lower in “rapid risers” (ROR >90th percentile; >0.25 mg/dL/h), as compared to “non-rapid risers” (ROR ≤90th percentile; ≤0.25 mg/dL/h). In contrast, sex, mode of delivery, umbilical artery pH, hemoglobin concentrations, maximum postnatal weight loss, DAT, blood group constellations, FFTS, antenatal betamethasone administration, or IVH did not differ significantly between the two groups (Table 1). The result of IVH did not even become significant when combining all infants with IVH ≥grade III (23 of 421 vs. 6 of 46; odds ratio 0.385 (95% confidence interval: 0.148–1.002; p = 0.054).
Discussion
Hour-specific postnatal total serum bilirubin concentrations
Our study presents a systematical analysis of postnatal TSB concentrations in a large cohort of VLBW preterm infants prior to the initiation of phototherapy. In a similar study by Mayer et al.,15 hour-specific TSB concentrations were generally 1–2 mg/dL higher compared to our results, which is due to a lower mean GA and lower mean BW of our study population.
By plotting our data into the nomogram published by Bhutani et al.,9 which were retrieved from the literature, we observed hour-specific TSB within postnatal 12–48 h to be lower in VLBW preterm infants compared to term and near-term appropriate for GA neonates (BW ≥2000 g for GA ≥36 weeks or BW ≥2500 g for ≥35 weeks). Even though we are well aware that term and preterm infants cannot be compared, setting data in relation to each other will contribute to a better overall understanding of postnatal bilirubin increase. Even though hyperbilirubinemia is generally considered to be “more prevalent, more severe and its course more protracted” in preterm than in term infants,16 our results demonstrate that preterm infants have a lower hour-specific TSB concentration compared to (near-) term counterparts.
Rate of rise of total serum bilirubin
Prior to the initiation of phototherapy, VLBW preterm infants display an average TSB increase of 0.15 (0.11–0.19) mg/dL/h. Similar studies have not yet been performed in preterm infants with a VLBW. However, estimations on TSB ROR from a chart by Mayer et al.15 (GA ≤35 weeks, BW ≤2000 g) result in an average TSB increase of 0.15 mg/dL/h within postnatal 12 to 24 h, affirming the result of our study. By visual comparison of graphs, we observed TSB ROR to be lower in VLBW preterm infants compared to term and near-term infants between postnatal 12 to 48 h. However, visual comparison based on graphs is rather vague and more research has to be done to further validate our observation in future studies.
In this study, the infants’ “individual postnatal TSB ROR” was confirmed an indicator for timing and duration of phototherapy as a higher TSB ROR correlates with younger age at initiation and longer duration of phototherapy. Likewise, neonates who were treated with a phototherapy had generally higher “individual TSB RORs” prior to the initiation of phototherapy than those who did not receive a phototherapy. Similar to our study, Fouzas et al.17 calculated rates of postnatal bilirubin rise and thereby demonstrated that preterm infants requiring phototherapy have higher rates of rise than those who do not require phototherapy. However, in contrast to our study, Fouzas et al.’s17 analysis was based on TcB measurements and their results were not significant for the first postnatal hours of life [0.13 (0.12–0.14) vs. 0.14 (0.11–0.17) mg/dL/h; p = 0.62 for hours 24–48].17
Risk factors associated with a rapid rise of TSB
In this study, rapidly rising TSB concentrations (>90th percentile; >0.25 mg/dL/h) were associated with lower GA, lower BW, and lower 5-min Apgar scores, implying that the combination of low GA, low BW, and low 5-min should draw one’s attention to a possible subsequent rapid TSB rise, which has been shown to correlate with a relatively young age at initiation as well as a long duration of phototherapy. However, GA, BW, and 5-min Apgar score are often co-linear variables and it has to be taken into account that the study only presents statistical associations but not independent cause-and-effect relationships between the variables. Therefore, it could be possible that only GA and not BW or 5-min Apgar score actually affects TSB ROR. Additionally, it has to be taken into consideration that the national phototherapy thresholds that were used in this study are GA-dependent and therefore timing and duration of phototherapy are GA-dependent variables.
All other analyzed variables that been assumed to affect postnatal TSB ROR such as sex, mode of delivery, pH, hemoglobin level, maximum postnatal weight loss, DAT, blood group constellations, FFTS, antenatal betamethasone administration, and IVH were not significant in this study, implying that the so-called “risk factors” might only play a minor role in the process of postnatal bilirubin increase. However, it has to be taken into account that the study was not statistically powered based on the risk factors.
Especially blood group incompatibility and a positive DAT are generally assumed to influence postnatal TSB concentration.18 A study by Mantagou et al.11 previously demonstrated a significant effect of ABO as well as rhesus incompatibility on the ROR of postnatal bilirubin concentrations within the first 36 to 48 postnatal hours of life. However, this was based on TcB measurements in term and late preterm neonates (GA ≥35 weeks, BW ≥2000 g) and therefore cannot be compared due to major methodical differences. At time of birth, ABO blood group-specific characteristics are usually not fully developed, especially not at a median GA of 28.4 weeks of our study population, which might be another explanation for the missing effect of ABO incompatibility on TSB ROR. In contrast to our results, Mantagou et al.11 also noticed a significant effect of postnatal weight loss on TcB ROR after the first 48 h of life. However, our analysis only covers TSB ROR prior to the initiation of phototherapy, which was initiated at a median (IQR) age of 30.23 (22.57–40.86) h.
Strengths and limitations
By providing data on hour-specific TSB, TSB ROR, and associated risk factors, this study contributes to a better understanding of postnatal bilirubin increase in preterm infants with a birth weight <1500 g. Nevertheless, this study has some limitations. Most of the study subjects received phototherapy within the first hours of life and dropped out from further analysis after they had reached their individual treatment threshold. Therefore, the analysis is restricted to the first hours of life and does not cover the entire natural course of postnatal bilirubin increase. If we had allowed the infants to continue on their natural course and had not used phototherapy, we might as well have documented TSB values or TSB RORs that are higher than our current results.19 For ethical reasons this is the closest we will get to the natural course without harming infants by denying them treatment considered helpful. Because all TSB samples of all study subjects were used to analyze the course of hour-specific TSB, it has to be acknowledged that infants with many measurements - up to 17, have a higher effect on the correlation than infants with a small number of measurements. As the study is based on retrospective data, we could not influence TSB sampling. The analysis of potential risk factors is limited to variables that were available for most preterm infants even though many more have been discussed to influence postnatal bilirubin concentrations or might be different following different guidelines, for example, jaundice observed in the first 24 h, type of diet and breastfeeding, ethnic and genetic factors, drugs, blood transfusions, hemolytic disorders, and maternal factors.19 Most of the analyzed variables are unspecific and common in most newborn infants. The individual use of these variables as indicators for rapidly rising bilirubin concentrations may therefore be of limited use,20 especially because nearly all preterm neonates <1500 g (approximately 90%) develop a need of phototherapy while hospitalized at the NICU. Infants who received blood transfusions were not excluded from the analysis, which is a possible confounder as well as the possible effect of intrauterine growth restriction, which was not recorded in this study. Some clinicians might criticize that our analysis based on TSB samples should be replaced by TcB measurements, as this method provides good predictive accuracy, is less invasive, and more convenient for the neonate, with the potential to reduce painful blood sampling.21,22 However, at the NICU all neonates undergo frequent routine blood testing. All TSB samples analyzed in this study were obtained during routine determination of blood glucose, pO2, and pH, which was sufficient to schedule infants for timely phototherapy.
By presenting a systematical analysis of TSB concentrations prior to initiation of phototherapy, the study contributes to a better understanding of postnatal bilirubin increase in VLBW preterm infants. However, our findings do not have an effect on clinical practice at this stage.
Conclusion
In this cohort of 483 preterm infants <1500 g birth weight, TSB ROR was confirmed as indicator for timing and duration of phototherapy. Rapidly rising TSB concentrations >90th percentile are associated with lower GA, lower BW, and lower 5-min Apgar scores. Between postnatal 12 and 48 h, hour-specific TSB concentrations and TSB ROR are lower in VLBW preterm infants compared to term and near-term infants.
References
Newman, T. B. et al. Frequency of neonatal bilirubin testing and hyperbilirubinemia in a large health maintenance organization. Pediatrics 104, 1198–1203 (1999).
McDonagh, A. F. Is bilirubin good for you? Clin. Perinatol. 17, 359–369 (1990).
Dani, C., Poggi, C. & Pratesi, S. Bilirubin and oxidative stress in term and preterm infants. Free Radic. Res. 53, 2–7 (2019).
Watchko, J. F. & Tiribelli, C. Bilirubin-induced neurologic damage—mechanisms and management approaches. N. Engl. J. Med. 369, 2021–2030 (2013).
Maisels, M. J. et al. An approach to the management of hyperbilirubinemia in the preterm infant less than 35 weeks of gestation. J. Perinatol. 32, 660–664 (2012).
Oh, W. et al. Association between peak serum bilirubin and neurodevelopmental outcomes in extremely low birth weight infants. Pediatrics 112, 773–779 (2003).
Moll, M. et al. Are recommended phototherapy thresholds safe enough for extremely low birth weight (ELBW) infants? A report on 2 ELBW infants with kernicterus despite only moderate hyperbilirubinemia. Neonatology 99, 90–94 (2011).
Bhutani, V. K., Wong, R. J. & Stevenson, D. K. Hyperbilirubinemia in preterm neonates. Clin. Perinatol. 43, 215–232 (2016).
Bhutani, V. K., Johnson, L. & Sivieri, E. M. Predictive ability of a predischarge hour-specific serum bilirubin for subsequent significant hyperbilirubinemia in healthy term and near-term newborns. Pediatrics 103, 6–14 (1999).
Bratlid, D., Nakstad, B. & Hansen, T. W. National guidelines for treatment of jaundice in the newborn. Acta Paediatr. 100, 499–505 (2011).
Mantagou, L. et al. Trends of transcutaneous bilirubin in neonates who develop significant hyperbilirubinemia. Pediatrics 130, e898–e904 (2012).
Amos, R. C., Jacob, H. & Leith, W. Jaundice in newborn babies under 28 days: NICE guideline 2016 (CG98). Arch. Dis. Child Educ. Pract. Ed. 102, 207–209 (2017).
Thakkar, P., Chavda, H. & Doshi, V. Transcutaneous bilirubin nomogram for healthy term and late preterm neonates in first 96 h of life. Indian Pediatr. 54, 369–372 (2017).
Bührer C. et al. Hyperbilirubinämie des Neugeborenen—Diagnostik und Therapie. AWMF online. 2015. https://www.awmf.org/leitlinien/detail/ll/024-007.html.
Mayer, I. et al. Value of twelfth hour bilirubin level in predicting significant hyperbilirubinemia in preterm infants. J. Clin. Med Res 6, 190–196 (2014).
Watchko, J. & Maisels, M. Jaundice in low birthweight infants: pathobiology and outcome. Arch. Dis. Child Fetal Neonatal Ed. 88, F455–F458 (2003).
Fouzas, S. et al. Transcutaneous bilirubin levels in late preterm neonates. J. Pediatr. 157, 762–6.e1 (2010).
Kaplan, M. et al. Hemolysis and hyperbilirubinemia in antiglobulin positive, direct ABO blood group heterospecific neonates. J. Pediatr. 157, 772–777 (2010).
Maisels, M. J. & Newman, T. B. in Care of the Jaundiced Neonate (eds Stevenson, D. K., Maisels, M. J., & Watchko, J. F.) 97–107 (McGraw-Hill Companies, United States, 2012).
Bhutani, V. K. & Wong, R. J. Risk profiles for haemolytic and nonhaemolytic neonatal jaundice. Acta Paediatr. 105, 1387–1388 (2016).
Maisels, M. J., Coffey, M. P. & Kring, E. Transcutaneous bilirubin levels in newborns <35 weeks’ gestation. J. Perinatol. 35, 739–744 (2015).
Schmidt, E. T. et al. Evaluation of transcutaneous bilirubinometry in preterm neonates. J. Perinatol. 29, 564–569 (2009).
Acknowledgements
We thank Petra Blank and Regina Nagel (secretaries), Jessica Blank (study assistant), Frank Ording (quality system records), and Anita Pierschke (functional diagnostics) for their helpful assistance in this study.
Author contributions
S.H. designed the data collection instruments, collected data, carried out the initial analyses, drafted the initial manuscript, and reviewed and revised the manuscript. C.B. conceptualized and designed the study, interpreted the data, and reviewed and revised the manuscript critically. G.S. and B.M. coordinated and supervised data collection, and carried out the final analyses and interpretation. M.B. conceptualized and designed the study, coordinated and supervised data collection and interpretation, and reviewed and revised the manuscript. Final approval of the version is to be published. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hahn, S., Bührer, C., Schmalisch, G. et al. Rate of rise of total serum bilirubin in very low birth weight preterm infants. Pediatr Res 87, 1039–1044 (2020). https://doi.org/10.1038/s41390-019-0415-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-019-0415-7
This article is cited by
-
Maternal and infant probiotic administration for morbidity of very low birth weight infants: a three-arm randomized placebo-controlled trial
European Journal of Nutrition (2022)
-
Repetitive bilirubin measurements in preterm infants prior to phototherapy: is it wise to use the rate of rise?
Pediatric Research (2020)
-
Insights Image for “Rate of rise of total serum bilirubin in very low birth weight preterm infants”
Pediatric Research (2020)