Abstract
Background
Serum creatinine (SCr)- or urine output-based definitions of acute kidney injury (AKI) have important limitations in neonates. This study evaluates the diagnostic value of urinary biomarkers in very low-birth-weight (VLBW) infants receiving indomethacin for closure of a patent ductus arteriosus (PDA).
Methods
Prospective cohort study in 14 indomethacin-treated VLBW infants and 18 VLBW infants without indomethacin as controls. Urinary biomarkers were measured before, during, and after indomethacin administration.
Results
Indomethacin therapy was associated with significantly higher SCr concentrations at 36, 84, and 120 h compared to controls. At 36 h, three indomethacin-treated patients met the criteria for neonatal modified Kidney Disease: Improving Global Outcomes (KDIGO) AKI. The product of urinary tissue inhibitor of metalloproteinase-2 and insulin-like growth factor-binding protein 7 ([TIMP-2]•[IGFBP7]) was significantly elevated in the AKI subgroup at 12 h (P < 0.05), hence 24 h earlier than the increase in SCr. Urinary neutrophil gelatinase-associated lipocalin (NGAL) and calprotectin were significantly increased in the indomethacin group at 12 h (P < 0.05), irrespective of fulfillment of the AKI criteria. Urinary kidney injury molecule-1 (KIM-1) was not significantly altered.
Conclusion
While urinary [TIMP-2]•[IGFBP7] proves valuable for the early diagnosis of neonatal modified KDIGO-defined AKI, elevated urinary NGAL and calprotectin concentrations in indomethacin-treated VLBW infants not fulfilling the AKI criteria may indicate subclinical kidney injury.
Similar content being viewed by others
Introduction
Very low-birth-weight (VLBW) infants are frequently exposed to nephrotoxic medication during the first days of life.1,2 This includes non-steroidal anti-inflammatory drugs (NSAID) for closure of a patent ductus arteriosus (PDA). Since nephrogenesis is incomplete before the gestational age of 34–36 weeks, the immature kidney in VLBW infants is particularly vulnerable. In addition to acute nephrotoxic effects, long-term adverse sequelae on renal structure and function following drug exposure are increasingly recognized.3,4
Indomethacin treatment for closure of a PDA is an established risk factor for renal impairment.5 However, the diagnosis of acute kidney injury (AKI) remains challenging in the first days of life, particularly in preterm neonates. The difficulty of diagnosing AKI in this period is due to the following factors: (i) serum creatinine (SCr) values partially reflect transplacentally transmitted maternal SCr, (ii) a dynamically developing glomerular filtration rate (GFR), (iii) varying degrees of creatinine reabsorption in the proximal tubule, (iv) varying SCr concentrations depending on the fluid status, and (v) inter-individual maturational differences.2,6 Therefore, in neonates SCr starts from a high maternal level after birth and, following a second peak by day 4 in very preterm neonates, decreases over the first weeks of life until baseline levels are reached.7 Hence, as AKI is known to be associated with poor outcome in very premature patients,2 there is an urgent need for more precise diagnostic tools for early and sensitive detection of kidney injury.
Novel urinary biomarkers are currently being investigated for early detection, differential diagnosis, and prognostic assessment of AKI.8,9 Though predominantly studied in adults, data in the pediatric and neonatal population is increasing.10,11,12,13,14 Urinary neutrophil gelatinase-associated lipocalin (NGAL) and urinary kidney injury molecule-1 (KIM-1) have been investigated in various clinical settings of AKI in preterm and term neonates. This includes AKI due to cardiac surgery, perinatal asphyxia, sepsis, extracorporeal membrane oxygenation, and others.15 Urinary calprotectin has been identified as a valuable marker for the discrimination of prerenal and intrinsic established AKI in neonatal, pediatric, and adult cohorts,16,17 yet there is no data on urinary calprotectin for the prediction of imminent AKI. The product of urinary tissue inhibitor of metalloproteinase-2 (TIMP-2) and insulin-like growth factor-binding protein 7 (IGFBP7) has been identified as a valuable biomarker for the detection of imminent AKI in adults and children.18 However, there is no data on [TIMP-2]•[IGFBP7] for the prediction of AKI in preterm neonates.
Due to the aforementioned limitations in diagnosing AKI in neonates, the primary aim of this study was to investigate urinary [TIMP-2]•[IGFBP7], NGAL, calprotectin, and KIM-1 in VLBW infants undergoing pharmacologic PDA closure with indomethacin. We hypothesized that novel urinary biomarkers allow for an earlier and more sensitive detection of renal damage compared to traditional markers.
Material and methods
Study design and participants
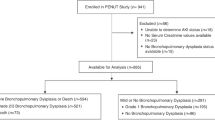
This prospective cross-sectional cohort study was performed at the University Children’s Hospital Heidelberg, Germany, from March 2012 until July 2015. Preterm infants (birth weight ≤1500 g and gestational age ≤31 weeks) admitted to the neonatal intensive care unit were eligible for study inclusion. The enrolled and non-enrolled patients including reasons for exclusion are shown in Fig. 1. Exclusion criteria were congenital kidney diseases and contraindications for indomethacin therapy including active bleeding, thrombocytopenia and/or coagulation defects, suspected or proven necrotizing enterocolitis, and congenital heart disease. Patients with premature discontinuation of indomethacin therapy and patients with relevant skin irritation or technical difficulties in urine collection were withdrawn from the study. Informed consent was obtained from 40 legal guardians. One patient was withdrawn from the study on parental request, and another patient was excluded from the study because of severe clinical deterioration due to sepsis and subsequent discontinuation of indomethacin therapy. Four patients were withdrawn from the study due to technical difficulties in urine collection or relevant skin irritation. Due to non-closure of the PDA, two patients required an extended course of indomethacin therapy.19 These patients were also excluded from the study.
Routine screening for PDA was performed by echocardiography at postnatal age of 2–3 days. Criteria for indomethacin therapy in spontaneously breathing VLBW infants were: PDA >1.5 mm in diameter and/or left-atrium-to-aortic-root ratio >1.4 and/or markedly reduced or retrograde diastolic flow in anterior cerebral artery or celiac trunc with resistance index >0.8 and/or bowing of interatrial septum to right with enlarged left atrium and left ventricle. In ventilated VLBW infants, every PDA >1.5 mm at postnatal age day 2–3 was treated with indomethacin. None of the VLBW infants received prophylactic indomethacin treatment for IVH. Control subjects had either a closed or very thin (<1.5 mm) ductus arteriosus without any signs of hemodynamic significance. Indomethacin was administered intravenously at three doses (0.2 mg/kg body weight per dose) at 12-h intervals. Thereafter, echocardiographic reevaluation was performed and, in case of ductal closure or minimal ductus arteriosus <1.5 mm in diameter, two further indomethacin doses (0.2 mg/kg body weight per dose) were applied at 24-h intervals. All patients with ductus arteriosus’ diameter >1.5 mm after the third dose of indomethacin were excluded from the study.
Total fluid intake was adjusted every 12 h to actual body weight. In the first 7 days after study enrollment, urine output was documented by measuring diaper weight. AKI was defined according to the neonatal Kidney Disease: Improving Global Outcomes (KDIGO) AKI classification.2 Stage 1 corresponded to a SCr increase of ≥0.3 mg/dL or ≥150–200% from the lowest previous value and/or an urinary output >0.5 and ≤1 mL/kg/h over 24 h, stage 2 corresponded to a SCr >200–300% and/or an urinary output >0.3 and ≤0.5 mL/kg/h over 24 h, and stage 3 was defined as SCr >2.5 mg/dL or >300% and/or an urinary output ≤0.3 mL/kg/h for ≥24 h.20
The study protocol was approved by the ethics committee of the Medical Faculty, University of Heidelberg. Written informed consent was obtained by legal guardians of each patient. All clinical investigations were conducted in accordance to the principles expressed in the Declaration of Helsinki.
Sample and data collection
Urine and blood specimens were collected following routine echocardiography and thus immediately before (0 h), during (6, 12, and 36 h), and after (84 and 120 h, days 7, 14, and 28) indomethacin administration in the indomethacin-treated group and at the same time points in control subjects (Table 1). Twelve-hour urine collections for determination of creatinine clearance were obtained at 12–24 and 84–96 h following study enrollment. Creatinine clearance was calculated as CLcr(mL/min/1.73 m2) = (Creatinineurine/Creatinineserum)× (Volumeurine/Time)×1.73. Urine was obtained using adhesive urinary bags (U-Bag® Sterile Cloth Adhesive Premature Size, Briggs Healthcare™, Moines, IA, USA) in accordance with standard procedural recommendations. SCr measurement was performed using an IDMS (isotope dilution mass spectrometry)-traceable enzymatic method. Urine samples for biomarker determinations were centrifuged, the supernatant was stored in aliquots at −80 °C, and thawed prior to analysis.
Urinary biomarkers
The urinary concentrations of TIMP-2 and IGFBP7 were measured using the NephroCheckTM test (Astute Medical, San Diego, CA, USA) as previously described.21 Urinary NGAL concentrations were measured by ARCHITECT Urine NGAL assay (Abbott Diagnostics, Abbott Park, IL, USA) using an ARCHITECT i2000. KIM-1 concentrations were measured by KIM-1 (human) ELISA kit (Enzo Life Sciences GmbH, Lörrach, Germany) and calprotectin concentrations by PhiCal® Calprotectin ELISA kit (Immundiagnostik AG, Bensheim, Germany).16,22
Statistical analysis
Due to non-normal distribution and small sample size, numeric data are presented as median and interquartile range. Non-parametric Mann–Whitney U test was performed for comparison of two groups. For comparison of more than two groups, non-parametric Kruskal–Wallis test with post-hoc Dunn’s test was applied. Comparison of categorical parameters was conducted by Pearson’s χ2 test or, in case of less than five patients per group, by Fisher’s exact test. Receiver operating characteristics (ROC) curves were calculated to evaluate the diagnostic accuracy of the investigated biomarkers. P < 0.05 was regarded as statistically significant. All statistical analyses were performed using IBM® SPSS® Statistics Version 22 and SAS® Version 9.4 WIN (Cary, NC, USA).
Results
Patient characteristics
In all, 32 VLBW neonates participated in the study including 14 patients receiving indomethacin for closure of PDA and 18 control subjects with either closed or very thin (<1.5 mm) ductus arteriosus without any signs of hemodynamic significance. Hemodynamic significance was observed in two patients of the indomethacin group. However, both patients did not develop neonatal KDIGO-defined AKI. Borderline findings of PDA >1.5 mm and moderately enlarged left atrium without any other signs of hemodynamic relevance were observed in three VLBW infants.
As listed in Table 2, there were no significant differences in patient characteristics, medication, and complication rates between both groups beside a higher proportion of patients with umbilical artery catheters and a higher proportion of patients treated with surfactants in the indomethacin group. Of note, antibiotic therapy with gentamicin was initiated immediately following birth. All VLBW infants on gentamicin received only two doses until postnatal age of 48 h, except for one patient of the indomethacin group who was treated with gentamicin for a total of 5 days. This patient, however, did not develop AKI. Maternal characteristics were not significantly different between the indomethacin and the control group (Table 2).
Traditional parameters of kidney function and injury
As expected, due to maternofetal transmission both the control and the indomethacin group initially displayed high SCr concentrations (Fig. 2a). There was a rapid and continuous decline of SCr in the control group, whereas the indomethacin group showed persistently elevated SCr levels until day 5 (48 h after the last indomethacin dose); SCr then declined over the following 48 h to levels comparable to the control group. Hence, there was a statistically significant difference in SCr between the indomethacin-treated and the control group at 36 (P < 0.05), 84 (P < 0.01), and 120 h (P < 0.001).
Serum creatinine (a, b), serum urea (c, d), and serum cystatin C (e, f) in very low-birth-weight (VLBW) infants treated with indomethacin for closure of a patent ductus arteriosus (PDA). Left panels, a, c, e: comparison of indomethacin-treated VLBW infants with PDA (n = 14; gray boxes) to VLBW control infants without PDA (n = 18; white boxes). Right panels, b, d, f: comparison of control VLBW infants (white boxes) to indomethacin-treated VLBW infants not developing AKI (n = 11; light gray boxes) and to indomethacin-treated VLBW infants developing AKI (n = 3; dark gray boxes). Boxplots represent data over a 4-week study period. The lower and upper edges of the box represent the first and third quartile, while the horizontal line within the box indicates the median. The endpoints of the whiskers represent the most extreme sample values (within a distance of 1.5×IQR). Outliers (1.5–3.0×IQR outside the box) are shown as dots, extremes (>3.0 × IQR) as triangles. *P < 0.05, **P < 0.01 by Mann–Whitney U test or Kruskal–Wallis test, respectively. h, hours; d, days
Thirty-six hours after study enrollment, three (21%) indomethacin-exposed VLBW infants developed AKI as defined by the neonatal KDIGO AKI criteria (stage 1, n = 2; stage 2, n = 1), while no patient of the control group developed AKI. Of note, all three patients fulfilled SCr, but only one patient also fulfilled the urine output KDIGO AKI criteria (SCr AKI stage 2; urine output AKI stage 1). Subanalyses of the AKI group yielded significant increases in SCr at 36 h (P < 0.05) and 84 h (P < 0.05) compared to controls (Fig. 2b). Of note, VLBW infants of the indomethacin group without AKI still displayed a delayed decline of SCr compared to control (P < 0.01 at 120 h).
Serum urea was significantly higher in indomethacin-treated VLBW infants compared to control at 12 h (P < 0.01), 36 h (P < 0.05), 84 h (P < 0.01), and 120 h (P < 0.01) (Fig. 2c). It was also significantly increased in non-AKI indomethacin-treated VLBW neonates at 12 and 120 h compared to control (Fig. 2d). Serum cystatin C concentrations were comparable between the indomethacin and the control group at all investigated time points (Fig. 2e). In patients developing KDIGO AKI, slight but not statistically significant increases in serum cystatin C were observed at 36 and 84 h (Fig. 2f). Minimum hourly urine output within the first 7 days of study was significantly reduced (P < 0.01) in the indomethacin group compared to control (Fig. 3a). Creatinine clearance obtained by 12-h urine collections was significantly lower in the indomethacin group at 12–24 h (P < 0.05), but not at 84–96 h (Fig. 3b).
Minimum urine output and creatinine clearance in very low-birth-weight (VLBW) infants treated with indomethacin for closure of a patent ductus arteriosus (PDA). a Minimum urine output per kilogram body weight per hour from the first 7 days after study enrollment in controls (white box) and indomethacin-treated VLBW infants (gray box). b Creatinine clearances as measured by 12-h urine collection in VLBW infants without PDA (white boxes) and in VLBW infants treated with indomethacin for PDA closure (gray boxes) at 12–24 and 84–96 h after initiation of indomethacin therapy. For explanation of boxplots see legend of Fig. 2. *P < 0.05, **P < 0.01 by two-sided t test
Proteinuria, urinary β2-microglobulin, urinary protein-to-creatinine ratio, and urinary β2-microglobulin-to-creatinine ratio were comparable between the indomethacin and the control group (Supplemental Figure S1 A, C, E, G) as well as between the non-AKI indomethacin, the AKI indomethacin, and the control group (Supplemental Figure S1 B, D, F, H).
Novel urinary biomarkers of kidney damage
There was an inverse correlation between initial urinary NGAL concentration and birth weight (r = −0.52, P = 0.028) within the control group. However, no such correlation was observed for urinary KIM-1 (r = −0.38, P = 0.12), [TIMP-2]•[IGFBP7] (r = −0.27, P = 0.27), and calprotectin (r = −0.35, P = 0.16).
Indomethacin-treated VLBW infants displayed 2-fold higher urinary [TIMP-2]•[IGFBP7] values (P < 0.01) compared to control at 36 h (Fig. 4a). Subanalyses revealed significant increases in urinary [TIMP-2]•[IGFBP7] in VLBW infants that developed KDIGO AKI at 36 h already at 12 h (P < 0.05 vs. control, P < 0.05 vs. non-AKI indomethacin), and also at 36 h (P < 0.05 vs. control) and 84 h (P < 0.05 vs. control) (Fig. 4b). Indomethacin-treated VLBW infants without AKI demonstrated significantly higher urinary [TIMP-2]•[IGFBP7] values (P < 0.05) compared to control at 36 h (Fig. 4b). Urinary NGAL was significantly higher in indomethacin-treated VLBW infants compared to control at 12 (3.9-fold) and 36 h (2.6-fold) (P < 0.05 vs. control) (Fig. 4c). In subanalyses, indomethacin-treated patients without AKI displayed higher urinary NGAL concentrations compared to control for most time points investigated, showing statistical significance at 0 h (P < 0.05), 6 h (P < 0.05), 12 h (P < 0.01), and 36 h (P < 0.05) (Fig. 4d). Urinary calprotectin concentrations were significantly higher in indomethacin-treated VLBW infants compared to control at 12 h (2.5-fold, P < 0.05) and 84 h (5.3-fold, P < 0.05) (Fig. 4e). Whereas indomethacin-treated VLBW infants without AKI displayed higher urinary calprotectin concentrations at 12 h (P < 0.05), those with AKI showed significantly higher urinary calprotectin concentrations at 84 h (P < 0.05) compared to control (Fig. 4f).
Urinary [TIMP-2]•[IGFBP7], NGAL, calprotectin, and KIM-1 in very low-birth-weight (VLBW) infants treated with indomethacin for closure of a patent ductus arteriosus (PDA). Left panels, a, c, e, g: comparison of indomethacin-treated VLBW infants with PDA (n = 14; gray boxes) to non-treated VLBW control infants without PDA (n = 18; white boxes). Right panels, b, d, f, h: comparison of control VLBW infants (white boxes) to indomethacin-treated VLBW infants not developing AKI (n = 11; light gray boxes) and to indomethacin-treated VLBW infants developing AKI (n = 3; dark gray boxes). For explanation of boxplots see legend of Fig. 2. *P < 0.05, **P < 0.01 by Mann–Whitney U test or Kruskal–Wallis test, respectively. h, hours; d, days; TIMP-2, tissue inhibitor of metalloproteinase-2; IGFBP7, insulin-like growth factor-binding protein 7; NGAL, neutrophil gelatinase-associated lipocalin; KIM-1, kidney injury molecule-1; AKI, acute kidney injury
Urinary KIM-1 concentrations were slightly but not significantly increased in the indomethacin group at early time points, that is, in those patients developing AKI (Fig. 4g, h). Normalization of urinary biomarker concentrations to urinary creatinine yielded comparable results for all biomarkers, though to a lesser degree of statistical significance (Supplemental Figure S2).
Prognostic accuracy of urinary biomarkers in predicting AKI
ROC AUC values for the prediction of AKI as defined by the neonatal KDIGO classification were calculated for all biomarkers for the following time points: 0, 6, and 12 h. Data are presented in Table 3. Of note, the number of patients developing AKI was small (n = 3). However, at 6 and 12 h after study enrollment, urinary [TIMP-2]•[IGFBP7] showed good performance for the early prediction of AKI (area under the ROC curve, 0.80 (95% confidence interval 0.56–1.00) and 1.00 (1.00–1.00), respectively.
Discussion
This is the first study comparing urinary [TIMP-2]•[IGFBP7], NGAL, calprotectin, and KIM-1 with traditional markers of AKI in VLBW infants undergoing indomethacin therapy for PDA closure. We show that novel urinary biomarkers prove valuable for the detection of renal injury in this clinical setting. Though the percentage of patients developing AKI is small, which parallels previous investigations,23 urinary [TIMP-2]•[IGFBP7] predicts imminent AKI as defined by the neonatal modified KDIGO classification.2 Urinary NGAL and calprotectin, beyond that, likely also detect subclinical structural renal damage in patients not fulfilling the KDIGO AKI criteria. In fact, subclinical kidney damage identified by increased urinary NGAL has been associated with an increased risk of adverse outcomes, that is, the need for renal replacement therapy, in adults.24
The interpretation of the results, due to the absence of a gold standard for AKI definition in neonates, remains challenging. The renal side effects of NSAID in very preterm neonates, alone or in co-administration with nephrotoxic drugs such as aminoglycosides, have been extensively studied.25 Interpreting the results based on the neonatal KDIGO criteria underscores the good performance of urinary [TIMP-2]•[IGFBP7] in predicting AKI 24 h earlier than SCr in VLBW infants. In a screen of >300 biomarker candidates in adults, urinary [TIMP-2]•[IGFBP7] yielded highest accuracy for the prediction of AKI.26 We previously demonstrated that urinary [TIMP-2]•[IGFBP7] is increased in term neonates and older children with established AKI of heterogeneous etiology.21
Several studies have demonstrated an association between increased urinary NGAL and subsequent AKI in VLBW infants.10,27 Lavery et al.12 described a trend towards higher NGAL concentrations in indomethacin-exposed premature infants, but if this was induced by the drug itself or by other prematurity-related factors could not be evaluated due to small patient numbers. Of note, urinary biomarker concentrations per se can depend on gestational age, postnatal age, and birth weight. However, these variations appear to be rather mild and negligible in the clinical context of kidney injury.28 In addition, conflicting data exists on an association between urinary NGAL and a hemodynamically significant PDA itself.29,30 Hence, as seen in our study, higher initial urinary NGAL concentrations in the subgroup of indomethacin-treated VLBW infants that do not develop AKI might be caused by hemodynamic effects of the PDA on the kidney.
Primarily originating from infiltrated neutrophils but also derived by renal collecting duct epithelial cells,31 urinary calprotectin plays a crucial role in controlling macrophage-mediated renal repair following kidney injury.32 Recently, urinary calprotectin has been established as a valuable biomarker for the identification of intrinsic AKI in adults and in a combined neonatal and pediatric cohort.16,17 To our knowledge, however, there is no data on urinary calprotectin in preterm neonates. While NSAID and short-term hemodynamic instability initially induce functional rather than structural AKI,33 elevated urinary calprotectin and NGAL concentrations as observed in our study argue for already established acute tubular damage in the dynamic continuum from functional change to structural renal injury.34 Of note, structural tubular damage as indicated by increased urinary biomarker concentrations can be accompanied by a functional decline but may also be observed independently of relevant changes in GFR. Even in the absence of diagnostic increases in SCr, however, urinary biomarkers can identify patients with likely subclinical AKI who have an increased risk of adverse outcomes.24
Urinary KIM-1 is known to be an early marker of AKI in premature infants.11 Recently, however, it was shown not to be significantly associated with the particular SCr increase in VLBW infants with AKI.10 This might support our observations of only non-significant increases in urinary KIM-1 concentrations in AKI patients.
Interestingly, no significant differences in serum cystatin C, proteinuria, urinary protein-to-creatinine ratio, urinary β2-microglobulin, and urinary β2-microglobulin-to-creatinine ratio were observed among the different groups. Cystatin C is a low-molecular-weight protein that does not pass through the placenta and is therefore indicative of the neonatal GFR. Minor increases in serum cystatin C following indomethacin therapy have been described35 and were also observed in our study, yet were not statistically significant. Urinary β2-microglobulin indicates tubular dysfunction and injury,36 but was recently shown not to be increased in VLBW infants with AKI,10 which is congruent with our findings.
The significant differences in declining SCr patterns between the indomethacin and the control group highlight the difficulties with AKI definitions based on SCr concentrations. During indomethacin therapy, median SCr remained nearly constant, while after cessation SCr rapidly declined to levels of the control group. Of note, significantly different SCr concentrations between indomethacin-treated and control VLBW infants were not detected before 36 h after initiation of indomethacin therapy and thus 24 h later than increases in NGAL and calprotectin.
In addition to nephrotoxic effects mediated by NSAID in our study, significantly more patients of the indomethacin group had an umbilical artery catheter. Koralkar et al.37 reported that VLBW infants developing AKI were more likely to have umbilical artery catheters, assisted ventilation, blood pressure medications, and lower 1- and 5-min Apgar scores. To which extent the presence of an umbilical artery catheter contributes to the development of AKI in this setting, is difficult to assess. Of note, based on the CRIB II score38 there were no significant differences in disease severity between the indomethacin group and the non-treated group in our study (Table 2).
Focusing on the biomarker perspective, transient increases in nearly all biomarkers investigated, even in study participants not fulfilling neonatal KDIGO AKI criteria, might indicate structural kidney injury that partially remains undetected by SCr- or urine output-based diagnostic criteria. The short- and long-term significance of the underlying renal alterations detected by these biomarkers needs to be further clarified in longitudinal studies. From a clinical point of view, however, the early detection of tubular damage by urinary biomarkers can influence physicians’ decision-making in VLBW infants with PDA, for example, when weighing up prolonged or high-dose indomethacin treatment strategies against alternative drugs for PDA closure or surgical PDA ligation.19
Our study has some limitations. First, this is a single-center study in a relatively small patient cohort, which requires validation in larger populations. Due to the low number of study participants and events, multivariable regression analyses for the identification of risk factors for AKI cannot be performed. ROC curve analyses for the prediction of AKI also need to be interpreted with caution due to low patient numbers. Second, because urine collection in male VLBW infants is easier to perform, more males than females were included in this study. Subtle gender differences (higher baseline concentrations and increased variability in females) have been described for urinary NGAL in VLBW infants, which, however, might be due to sample contamination.39
In conclusion, our data strengthen the value of urinary [TIMP-2]•[IGFBP7] for the detection of imminent KDIGO-defined AKI in very preterm neonates undergoing indomethacin treatment for PDA. Increased urinary NGAL and calprotectin concentration in indomethacin-exposed VLBW infants not fulfilling the neonatal KDIGO AKI criteria may indicate subclinical renal damage. Perspectively, a more timely detection of kidney injury may improve medical management in these vulnerable patients with ongoing nephrogenesis. Prevention of severe kidney injury might, in consequence, improve long-term renal outcome. Noteworthy, adding non-invasive diagnostics such as urinary biomarkers to neonatal AKI definitions has the potential to refrain VLBW infants from traumatic blood samplings. In fact, stress and painful events have been linked to negative developmental outcomes.40
References
Hanna, M. H., Askenazi, D. J. & Selewski, D. T. Drug-induced acute kidney injury in neonates. Curr. Opin. Pediatr. 28, 180–187 (2016).
Selewski, D. T. et al. Neonatal acute kidney injury. Pediatrics 136, e463–e473 (2015).
Kent, A. L. et al. Renal glomeruli and tubular injury following indomethacin, ibuprofen, and gentamicin exposure in a neonatal rat model. Pediatr. Res. 62, 307–312 (2007).
Schreuder, M. F. et al. Effect of drugs on renal development. Clin. J. Am. Soc. Nephrol. 6, 212–217 (2011).
Ohlsson, A., Walia, R. & Shah S. S. Ibuprofen for the treatment of patent ductus arteriosus in preterm or low birth weight (or both) infants. Cochrane Database Syst. Rev. Issue 2. Art. No.: CD003481 (2015).
Bateman, D. A. et al. Serum creatinine concentration in very-low-birth-weight infants from birth to 34–36 wk postmenstrual age. Pediatr. Res. 77, 696–702 (2015).
Guignard, J. P. & Drukker, A. Why do newborn infants have a high plasma creatinine? Pediatrics 103, e49 (1999).
McCaffrey, J., Dhakal, A. K., Milford, D. V., Webb, N. J. & Lennon, R. Recent developments in the detection and management of acute kidney injury. Arch. Dis. Child. 102, 91–96 (2017).
Schrezenmeier, E. V., Barasch, J., Budde, K., Westhoff, T. & Schmidt-Ott, K. M. Biomarkers in acute kidney injury—pathophysiological basis and clinical performance. Acta Physiol. (Oxf.). 219, 554–572 (2017).
Askenazi, D. J. et al. Acute kidney injury urine biomarkers in very low-birth-weight infants. Clin. J. Am. Soc. Nephrol. 11, 1527–1535 (2016).
Genc, G., Ozkaya, O., Avci, B., Aygun, C. & Kucukoduk, S. Kidney injury molecule-1 as a promising biomarker for acute kidney injury in premature babies. Am. J. Perinatol. 30, 245–252 (2013).
Lavery, A. P. et al. Urinary NGAL in premature infants. Pediatr. Res. 64, 423–428 (2008).
Sarafidis, K. et al. Urine neutrophil gelatinase-associated lipocalin to predict acute kidney injury in preterm neonates. A pilot study. Pediatr. Nephrol. 29, 305–310 (2014).
Tabel, Y. et al. Urinary neutrophil gelatinase-associated lipocalin as an early biomarker for prediction of acute kidney injury in preterm infants. Am. J. Perinatol. 31, 167–174 (2014).
Sweetman, D. U. Neonatal acute kidney injury—severity and recovery prediction and the role of serum and urinary biomarkers. Early Hum. Dev. 105, 57–61 (2017).
Heller, F., Frischmann, S., Grunbaum, M., Zidek, W. & Westhoff, T. H. Urinary calprotectin and the distinction between prerenal and intrinsic acute kidney injury. Clin. J. Am. Soc. Nephrol. 6, 2347–2355 (2011).
Westhoff, J. H. et al. Urinary biomarkers for the differentiation of prerenal and intrinsic pediatric acute kidney injury. Pediatr. Nephrol. 31, 2353–2363 (2016).
Vijayan, A. et al. Clinical use of the urine biomarker [TIMP-2] x [IGFBP7] for acute kidney injury risk assessment. Am. J. Kidney Dis. 68, 19–28 (2016).
Sperandio, M. et al. Effectiveness and side effects of an escalating, stepwise approach to indomethacin treatment for symptomatic patent ductus arteriosus in premature infants below 33 weeks of gestation. Pediatrics 116, 1361–1366 (2005).
Kellum, J. A., Lameire, N. & Group KAGW. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Crit. Care 17, 204 (2013).
Westhoff, J. H. et al. Urinary tissue inhibitor of metalloproteinase-2 (TIMP-2) * insulin-like growth factor-binding protein 7 (IGFBP7) predicts adverse outcome in pediatric acute kidney injury. PLoS ONE 10, e0143628 (2015).
Seibert, F. S. et al. Calprotectin and neutrophil gelatinase-associated lipocalin in the differentiation of pre-renal and intrinsic acute kidney injury. Acta Physiol. (Oxf.). 207, 700–708 (2013).
Akima, S., Kent, A., Reynolds, G. J., Gallagher, M. & Falk, M. C. Indomethacin and renal impairment in neonates. Pediatr. Nephrol. 19, 490–493 (2004).
Haase, M. et al. The outcome of neutrophil gelatinase-associated lipocalin-positive subclinical acute kidney injury: a multicenter pooled analysis of prospective studies. J. Am. Coll. Cardiol. 57, 1752–1761 (2011).
Allegaert, K., De Hoon, J., Debeer, A. & Gewillig, M. Renal side effects of non-steroidal anti-inflammatory drugs in neonates. Pharmaceuticals (Basel) 3, 393–405 (2010).
Kashani, K. et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit. Care 17, R25 (2013).
Kuribayashi, R. et al. Urinary neutrophil gelatinase-associated lipocalin is an early predictor of acute kidney injury in premature infants. Exp. Ther. Med. 12, 3706–3710 (2016).
Parravicini, E. The clinical utility of urinary neutrophil gelatinase-associated lipocalin in the neonatal ICU. Curr. Opin. Pediatr. 22, 146–150 (2010).
Sellmer, A. et al. Urinary neutrophil gelatinase-associated lipocalin in the evaluation of patent ductus arteriosus and AKI in very preterm neonates: a cohort study. BMC Pediatr. 17, 7 (2017).
Tosse, V. et al. Urinary NT-proBNP, NGAL, and H-FABP may predict hemodynamic relevance of patent ductus arteriosus in very low birth weight infants. Neonatology 101, 260–266 (2012).
Fujiu, K., Manabe, I. & Nagai, R. Renal collecting duct epithelial cells regulate inflammation in tubulointerstitial damage in mice. J. Clin. Invest. 121, 3425–3441 (2011).
Dessing, M. C. et al. The calcium-binding protein complex S100A8/A9 has a crucial role in controlling macrophage-mediated renal repair following ischemia/reperfusion. Kidney Int. 87, 85–94 (2015).
McCullough, P. A. et al. Diagnosis of acute kidney injury using functional and injury biomarkers: workgroup statements from the tenth Acute Dialysis Quality Initiative Consensus Conference. Contrib. Nephrol. 182, 13–29 (2013).
Endre, Z. H. et al. Differential diagnosis of AKI in clinical practice by functional and damage biomarkers: workgroup statements from the tenth Acute Dialysis Quality Initiative Consensus Conference. Contrib. Nephrol. 182, 30–44 (2013).
Lee, B. S. et al. Effect of furosemide on ductal closure and renal function in indomethacin-treated preterm infants during the early neonatal period. Neonatology 98, 191–199 (2010).
Fiseha, T. & Tamir, Z. Urinary markers of tubular injury in early diabetic nephropathy. Int. J. Nephrol. 2016, 4647685 (2016).
Koralkar, R. et al. Acute kidney injury reduces survival in very low birth weight infants. Pediatr. Res. 69, 354–358 (2011).
Parry, G., Tucker, J., Tarnow-Mordi, W. & Group UKNSSC. CRIB II: an update of the clinical risk index for babies score. Lancet 361, 1789–1791 (2003).
Huynh, T. K. et al. Reference values of urinary neutrophil gelatinase-associated lipocalin in very low birth weight infants. Pediatr. Res. 66, 528–532 (2009).
Vinall, J. & Grunau, R. E. Impact of repeated procedural pain-related stress in infants born very preterm. Pediatr. Res. 75, 584–587 (2014).
Acknowledgements
We thank all nurses of the NICU for their diligence in collecting samples. We also thank Simone Voigt for her indefatigable efforts in this study. We are indebted to all parents of our tiny patients who gave their consent to have their children participate in this study. The study was funded by the German Research Foundation (Research Unit FOR1368). NephroCheck® Test Kits and the Astute 140® Meter were kindly provided by Astute Medical (San Diego, CA, USA). ARCHITECT® Urine NGAL assays were kindly provided by Abbott Diagnostics (Abbott Laboratories, Abbott Park, IL, USA).
Author information
Authors and Affiliations
Contributions
A.F., S.W., and J.H.W. conceived and designed the experiments. S.W., A.F., B.B., T.H.W., and J.H.W. performed the experiments. S.W., A.F., T.B., F.S., B.T., J.P., T.H.W., and J.H.W. analyzed the data or interpreted the results. J.H.W. wrote the draft of the article. A.F., S.W., B.T., and T.H.W. edited the manuscript. All other authors approved the manuscript to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Waldherr, S., Fichtner, A., Beedgen, B. et al. Urinary acute kidney injury biomarkers in very low-birth-weight infants on indomethacin for patent ductus arteriosus. Pediatr Res 85, 678–686 (2019). https://doi.org/10.1038/s41390-019-0332-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-019-0332-9
This article is cited by
-
Gut microbiota and neonatal acute kidney injury biomarkers
Pediatric Nephrology (2023)
-
Impact of nephrotoxic drugs on urinary biomarkers of renal function in very preterm infants
Pediatric Research (2022)
-
Acute kidney injury in premature and low birth weight neonates: a systematic review and meta-analysis
Pediatric Nephrology (2022)
-
Serum and urinary biomarkers to predict acute kidney injury in premature infants: a systematic review and meta-analysis of diagnostic accuracy
Journal of Nephrology (2022)
-
The effectiveness of urinary TIMP-2 and IGFBP-7 in predicting acute kidney injury in critically ill neonates
Pediatric Research (2020)