Abstract
Background
This study aims to study prospectively specific sleep patterns and risk of ADHD after adjusting for potential confounders such as obstructive sleep apnoea (OSA) and methylphenidate use.
Methods
A population-representative sample of 514 Chinese preschool children was recruited when in kindergarten (K3). Parents reported on their socioeconomic status and children’s sleep duration. The cohort was reassessed 3 years later when the children were in Grade 3 (P3). Parents reported on children’s sleep patterns and ADHD symptoms. Information on OSA diagnosis and methylphenidate use was retrieved from health records.
Results
Among the 514 parent–child dyads (mean [SD] age, 5.52 [0.33] years), 411 were reassessed (80.0% retention; 9.35 [0.33] years) at follow-up. There were no significant baseline differences between follow-up and drop-out groups. A gradient relationship was observed between probable ADHD in P3 and sleep duration in K3. The risk of probable ADHD was 15.5 per 100 for children with <8 h of sleep in K3, whereas it was 1.1 per 100 for children with 11–12 h of sleep in K3. The adjusted risk ratio was 14.19 (p = 0.02).
Conclusions
Sleep deprivation in early childhood is associated with higher risk of ADHD in middle childhood.
Similar content being viewed by others

Introduction
Attention-deficit/hyperactivity disorder (ADHD) is a neurodevelopmental disorder common among school-aged children in both western and Chinese populations.1 The worldwide prevalence of ADHD was estimated to be stable at 5–10%,2 while that in Hong Kong Chinese was at 8.9%.3 It is characterised by a persistent pattern of inattention, hyperactivity, and impulsivity, which is pervasive across different settings,4 and usually manifests in middle childhood. ADHD is associated with adverse health, social, and educational outcomes and imposes a huge burden on the patient, family, and society.5
The aetiology of ADHD appears to be complex with interactions between genetic, neurological, and environmental factors.6 It is closely associated with neurotransmitter disturbances in the frontal lobe and impairments in early executive function development.7 The symptoms of ADHD, particularly those related to executive functions, inattention, and lack of self-inhibition, usually become more apparent at primary school age. Emerging evidence suggest environmental influences on frontal lobe development are important during early preschool years when executive functions develop rapidly.8
Sleep deprivation among preschoolers has been associated with lower school readiness and impaired executive functions.9 Whether the deleterious implications of sleep deprivation on early childhood development (approximately <6 years) would extend to heighten the risk of ADHD in middle childhood (6–11 years) is an important question that has yet to be explored.
Extant evidence of the association between sleep deprivation and ADHD has been mainly derived from cross-sectional studies and it is not clear whether the relations is bidirectional.10 While sleep problem may contribute to the development of ADHD, ADHD children, particularly those receiving medication treatment, were often found to have comorbid sleep problems.11 This hypothesis has been supported in a recent cohort study showing a reciprocal association between sleep difficulties and externalising behaviours (hyperactivity and conduct problems). Touchette’s cohort study conducted in Quebec, Canada found increased hyperactivity symptoms in young children with short sleep duration, but the assessments of hyperactivity and sleep patterns were conducted only in early childhood and not beyond.5 The Avon Longitudinal Study of Parents and Children in South West England showed ADHD to be associated with abnormal sleep pattern and short sleep duration with small effect sizes.12 Previous studies did not address potential confounders including obstructive sleep apnoea (OSA) and methylphenidate use in children with ADHD symptoms. OSA can cause inattention, hyperactivity symptoms, and sleep disturbances,13 whereas methylphenidate, the first-line medication for children with ADHD, may result in short sleep duration.14
This is the first prospective cohort study that examines the relationship between sleep deprivation in early childhood and the development of ADHD 3 years later, taking into account the potential effects of OSA and methylphenidate use. We hypothesised that sleep deprivation in early childhood would increase the risk for developing ADHD in middle childhood. In addition, since ADHD symptom severity differ by sex,15 the study also examined whether sex moderate the association between sleep and ADHD.
Methods
This study followed a sample of Chinese children from kindergarten to primary school. Between 2011 and 2012, children in the third year of kindergarten (K3; 5–6-year old) were recruited from 20 kindergartens in Hong Kong using stratified random sampling. Kindergartens were randomly selected within the selected districts. One class of K3 was then randomly selected. The detailed sampling framework can be found in previous publications.9,16 Parents of 514 K3 children consented to participate in the longitudinal study. They completed a questionnaire on family sociodemographics and children’s sleep duration. These parent–child dyads were reassessed between 2014 and 2015 when the children were in the third year of primary school (P3; 8–9-year old). They completed another set of questionnaires on their children’s ADHD symptoms, sleep duration, pattern, and quality.
Sleep duration and quality
Sleep duration of preschool children in K3 was measured as the average number of sleeping hours per day in the past week (including naps) and subsequently categorised into four groups (≤8, 9–10, 11–12, and ≥13 h per day). According to international guidelines, the recommended number of sleeping hours for preschool children is 11–12 h per day, thus this was used as reference.17 The selection of grouping was based on the previous findings that sleep duration and child development displayed a dose–response relationship.9
Sleep duration and quality in primary school children in P3 were measured using the Chinese Childhood Sleep Quality Index, which was adapted from the Children’s Sleep Habits Questionnaire and the Hong Kong Adolescent Sleep Questionnaire.18,19 This tool consists of five items on sleep duration (average bedtime and wake time on weekdays and weekends, average sleep latency), three items on regular sleep patterns (bedtime, wake time, sleep duration), three items on sleep disturbance (restlessness, nightmares, sudden awakening), and five items on sleep-related daytime dysfunction (unable to wake up independently, negative mood after waking up, extended time before becoming alert, tiredness after waking up, sleepiness on commute). The sleep duration subscale was the weighted average of sleep durations on weekdays and weekends minus the average sleep latency. The categorisation of sleep duration in P3 was changed according to the recommendation for this age group. The regular sleep subscale was the number of days that the child had regular bedtimes, wake times, and sleep durations. The sleep quality subscale was the number of days that the child was free from both sleep disturbance and daytime sleepiness. All variables were based on 1-week recall.
ADHD symptoms
Presence of ADHD symptoms in children in P3 was measured using the parent-rated Chinese Strengths and Weaknesses of ADHD-Symptoms and Normal-Behaviors (SWAN) questionnaire. The Chinese SWAN questionnaire was developed based on the Diagnostic and Statistical Manual of Mental Disorders criteria for ADHD20 and has very high sensitivity and specificity in distinguishing clinically diagnosed ADHD children from a healthy community sample. It has been widely used for the assessment of ADHD symptoms among children in Hong Kong.21 The instrument consists of 18 items, with each item scored on a 7-point response scale ranging from +3 (far below average) to −3 (far above average). The ADHD-Combined (ADHD-C) score was the mean scores of all 18 items, the ADHD-Inattentive (ADHD-I) score was the mean of items 1–9, and the ADHD-Hyperactivity/Impulsivity score was the mean of items 10–18. Children with probable ADHD were identified according to the published SWAN cut-off values.21 These cut-off values were reported to screen out about 16% of children with probable ADHD and had 83% sensitivity and 75% specificity comparing with clinical diagnosis.21 Probable ADHD (binary variable) and ADHD symptoms (continuous variable) were the primary outcome measures in this study. To minimise the possibility of reverse causation, participants’ ADHD symptoms at K3 was adjusted in analysis.
The SWAN questionnaire was not administered at baseline because it was only validated among school-aged children. Instead, the ADHD symptom at K3 was measured by the inattention and hyperactivity subscale in the Chinese Early Development Instrument (CEDI). The CEDI is a validated teacher-report tool for assessing 5-year-olds’ holistic early child development. The inattention and hyperactivity subscale was found to have good reliability.22 All the ADHD measurements did not contain items on sleep duration or quality.
Potential confounders
A family socioeconomic status (SES) index was computed from baseline family socioeconomic indicators at recruitment, including maternal and paternal education, maternal and paternal occupation, family assets, and adjusted family monthly household income. These measurements were aggregated into a single SES index using principal component analysis, which has been previously validated as a method to characterise SES variability within a population.9,22
Hong Kong has a comprehensive computerised record and management system in 42 public hospitals (~80% of all hospitalisations) since 2000. All patient information, medical diagnoses, and drug prescription details are routinely entered into the Clinical Data Analysis Reporting System (CDARS).1 Using the CDARS, we identified children diagnosed with OSA, sleep disturbance, and/or taking oral methylphenidate. These variables could be potential confounders in the relationship between sleep and ADHD yet have not been included in previous cohort studies.13,14 To supplement the potential underdiagnoses of OSA, parent-reported persistent snoring (night-time snoring in >5 days of a week) was also controlled in the analysis. Methylphenidate is the only approved psychostimulant for the treatment of ADHD in children in Hong Kong public hospitals.
Data analysis
Descriptive statistics were calculated for all participants. Independent t test (for continuous variables) and Fisher exact test (for categorical variables) were used to quantify any baseline differences between the remaining cohort and the drop-outs. Absolute risk and risk ratio (RR) of probable ADHD associated with early sleep duration were estimated using modified Poisson regression.23 Probable ADHD (binary variable) was the dependent variable, while sleep duration at K3 were independent variables. Similarly, the prevalence and RR between probable ADHD and concurrent sleep duration/quality were estimated with probable ADHD as the dependent variable and concurrent sleep duration/quality as the independent variable. The associations between ADHD symptom severity and sleep variables were studied using multiple linear regressions. In all regression models, baseline SES index, OSA and sleep disturbance diagnosis, persistent snoring, and methylphenidate use were controlled as covariates. Age was not adjusted because all participants were 5 years at baseline. The effect of sex was explored in subgroup analysis. Sensitivity analysis was conducted for using ADHD diagnosis as the dependent variable. Statistical analyses were performed using the R Statistical Software version 3.2.5. All statistical tests were two tailed and p values <0.05 were regarded as statistically significant.
Results
Among the 514 parent–child dyads recruited in the longitudinal study, 411 of them (80.0% retention) agreed to be reassessed when the child was at primary school in P3 between 2014 and 2015. Three parents did not complete the sleep duration item in K3 and their data were excluded from this analysis. The follow-up and attrition groups did not significantly differ in baseline demographics and sleep duration (Table 1). In the initial assessments at K3, there were 264 girls (51.7%) and 247 boys (48.3%), with a mean (SD) age of 5.52 (0.33) years. In the reassessments at P3, there were 210 females (51.5%) and 198 males (48.5%), with a mean age of 9.35 (0.33) years.
Among the 408 children at follow-up, 4 children (1.0%) were diagnosed with OSA, 26 (6.4%) had persistent snoring, and 20 (4.9%) received methylphenidate medication. All OSA diagnoses and methylphenidate prescriptions occurred after the first assessment at K3. The children with OSA have not been surgically treated. Only 43 (10.5%) had the recommended sleep duration of 11–12 h per day during preschool (K3).17 In primary school (P3), 126 (30.9%) had the recommended sleeping duration of ≥10 h per day,17 38 (9.3%) had regular sleep patterns, and 176 (43.1%) had good sleep quality. At follow-up in P3, 61 (15.0%) children had probable ADHD, 70 (17.2%) had probable ADHD with predominantly inattentive symptoms, and 66 (16.2%) had probable ADHD with predominantly hyperactive symptoms (Table 1).
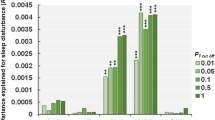
Figure 1 shows the association between probable ADHD in children in P3 and their sleep patterns. The risk of probable ADHD among children with <8 h sleep duration in K3 was 0.16 (95% confidence interval (CI) 0.05–0.48; Fig. 1a), whereas the risk among children with 11–12 h of sleep duration in K3 was 0.01 (95% CI 0.002–0.08). The adjusted RR between the two groups was 14.19 (95% CI 1.54–130.95, p = 0.02). Sensitivity analysis has also confirmed a significant association between ADHD diagnosis and sleep duration (RR for each hour 0.72, 95% CI 0.54–0.97, p < 0.001).
Relationship between probable attention-deficit/hyperactivity disorder (ADHD) and sleep Adjusted for family socioeconomic status and ADHD symptoms at K3, obstructive sleep apnoea diagnosis, persistent snoring, and methylphenidate use. Ref Reference group, RR risk ratio compared with the reference group
The prevalence of probable ADHD among children with 0–3 days of good quality sleep at P3 was 0.17 (95% CI 0.11–0.25; Fig. 1b) compared to those with 6–7 days of good quality sleep (prevalence 0.07, 95% CI 0.05–0.11). The adjusted RR between the two groups was 2.31 (95% CI 1.32–4.03, p = 0.003). Sleep duration and regular sleep habit at P3 was not significantly associated with the risk of probable ADHD (Fig. 1c, d).
The relationships between sleep and ADHD symptom severity are summarised in Table 2. There was a gradient relationship between sleep duration in K3 and subsequent ADHD symptom severity in P3. Children in K3 with sleep duration of <8 h had the highest ADHD symptom severity scores in P3 (β 0.76, 95% CI 0.12–1.40, p < 0.05). However, sleep duration in P3 was not associated with ADHD symptom severity scores. Children in P3 with 0–3 days of good quality sleep and those with the lowest number of days with regular sleep patterns had the highest ADHD-C symptom severity and the highest ADHD-I symptom severity scores (Table 2).
Table 3 shows the influence of sex on the relationship between children’s sleep duration/quality and ADHD symptoms. Boys with sleep deprivation in K3 had significantly increased ADHD symptoms in P3, and the effect sizes were much larger than in girls. Girls with shorter sleep duration in K3 had higher ADHD symptom scores in P3, but this association was not significant. Fewer days with regular sleep patterns and good quality sleep in P3 was associated with increased ADHD symptoms in only boys.
Discussion
This is the first Chinese population-based cohort study to report an association between sleep patterns during early childhood and elevated risk of ADHD in middle childhood. Assessments of OSA and methylphenidate use were also included. Most longitudinal studies conducted over several years tend to be limited by attrition and a high proportion of missing data, particularly among socioeconomically deprived groups. The current study had a relatively high retention rate and there were no significant differences in the sociodemographic between the follow-up and drop-out groups. Prospective findings between early sleep deprivation and subsequent ADHD risk, with a large effect size, provide more robust evidence that sleep patterns in young children are a plausible risk factor for ADHD.
In line with the literature on sleep patterns and ADHD, we found that sleep-deprived preschool children had increased risk of ADHD.4,21,22 However, most previous empirical links were derived from cross-sectional studies, and any causal effects of sleep deprivation on ADHD risk were not ascertained. Moreover, other studies have suggested that children with ADHD were more vulnerable to sleep problems,24 which makes interpreting the relationship between sleep duration and occurrence of ADHD symptoms even more challenging. Thus rigorous longitudinal studies are necessary to delineate this relationship. However, previous longitudinal studies had various limitations. The Canadian cohort study by Touchette et al. focussed only on the relationship between short sleep duration and hyperactive behaviours in early childhood and did not examine the long-term risk of ADHD.5 The Avon Longitudinal Study of Parents and Children in South West England showed that children with ADHD had abnormal sleeping patterns and slept less than children without ADHD, but the absolute differences were small.12 The current study documented a prospective effect between sleep deprivation and later ADHD risk that was relatively large (Fig. 1a).
As mentioned by the investigators of the Avon Longitudinal Study,12 almost all previous studies did not capture sufficient health data to address the potential influence of psychostimulant medication and OSA on sleep disturbances among children with ADHD symptoms. This study sought to address the identified deficits by extracting information on methylphenidate use and OSA diagnoses from children’s health records. The prevalence of OSA and methylphenidate prescriptions were comparable with previous studies.1
Evidence for a relationship between sleep patterns and ADHD has been demonstrated not only in human subjects but also in animal models. For example, an experimental study showed that sleep deprivation impaired attention function in rats25 and increased response latencies on a five-point reaction time task, which were compatible with human findings on the effects of sleep deprivation on attention, psychomotor, and behavioural control.26 A laboratory study also found that melanin-concentrating hormone receptor knockout mice slept less and expressed marked hyperactivity.27 These animal models demonstrate the biological plausibility of sleep deprivation giving rise to ADHD.
Although the exact biological pathway between sleep deprivation and ADHD remains unconfirmed, we speculate that neurological dysfunction may be a key mediator. Previous clinical and laboratory studies have found that dopamine dysfunction played a crucial role in the development of ADHD.28 Emerging evidence that insufficient sleep downregulated dopaminergic receptors in the brain29 may account for increased ADHD symptoms in children with early childhood sleep deprivation in this study. In addition, sleep deprivation has been shown to decrease the functional connectivity and synchronisation of brain networks, especially in prefrontal regions.30 Chronic sleep deprivation may impair functional and structural neural networks within prefrontal regions during the critical phase of brain development in preschool years, leading to the development of ADHD symptoms in middle childhood.
It should be noted that environmental factors, such as psychological stressors, may contribute to ADHD through shorter sleep duration or unhealthy sleep habit. Previous studies have identified the unique role of psychological stress on suboptimal sleep.31 The mediation role of sleep between environmental factors and ADHD warrant further investigation.
Sex differences in the prevalence and occurrence of ADHD have been reported, but the exact causes are still unknown. Population studies in Europe and U.S. found that boys were two to four times more likely to have ADHD than girls,32 and boys tended to manifest ADHD symptoms at a younger age.33 A previous study reported that male adults with ADHD displayed more seriously impaired cerebellar–prefrontal–striatal brain networks than females.34 Neurophysiological studies demonstrated that girls with ADHD exhibited abnormal brain activities in localised regions, whereas boys exhibited more widespread dysfunction.35,36 In line with these observations, results from the current study indicated that sleep deprivation had more prominent detrimental effects on boys than girls in middle childhood. This sex difference in sleep-related neurobehaviour may be linked to underlying epigenetic or neuroendocrine changes37 and warrants further investigation.
Sleep deprivation in children in P3 did not appear to increase ADHD risk, suggesting that children may be less vulnerable to insufficient sleep-related damage during middle childhood.38 Early childhood is a critical stage for the rapid development of executive functions. As such, sleep deprivation may be particularly harmful to early brain development, resulting in an increased ADHD risk. Results from this study provides strong evidence for the importance of sufficient sleep during preschool years, a critical stage for early brain development. Emerging evidence highlights the role of environmental influences on frontal lobe development during early childhood,8 and additional attention should be devoted to examining the early environmental context that provoke vulnerable sleep patterns. For example, disruptions in family emotional climate, routines, and agendas may directly shape children’s sleep patterns.39,40 Furthermore, it is biologically plausible that sleep disturbance is transdiagnostic with implications across multiple psychiatric disorders,41 revealing that vulnerable ecological patterns in early childhood can provide viable targets for early intervention.
In clinical practice, because ADHD is one of the most common neurodevelopmental disorders with significant morbidities across the life course, parents and health-care professionals should be informed about sleep as a key behavioural risk factor and appropriate parenting practices should be advised. When assessing a child with developmental or behavioural concerns, it should be routine practice for paediatric providers to screen for sleep problems and give advice on sleep hygiene. Children with sleep disorders should be referred for evaluation and management by a sleep specialist early on to prevent detrimental effects on their attention and behaviours. A previous study had shown that many primary care paediatricians did not feel confident in counselling patients with sleep issues,42 highlighting the importance of formal education on sleep disorders for all paediatric providers.
Furthermore, better child-friendly policies should be established to ensure young children have sufficient sleep and be optimally protected from developing ADHD and other mental health conditions.
The findings from this study should be interpreted with the following caveats. First, children’s sleep patterns and quality of sleep were based on parental reports rather than objective assessments. Although subjective reporting can be prone to recall bias, this information was collected directly from parents or caregivers who were familiar with the child’s sleep habits. Previous studies also showed that parental reporting of children’s sleep duration were quite reliable.43 Second, the presence of ADHD symptoms was measured using the parent-rated SWAN questionnaire and was not based on clinician assessments. The Chinese SWAN has been validated as a reliable tool with very high sensitivity and specificity and has been widely used in previous population and clinical studies.21 Third, although there were some missing data due to attrition, this cohort study had a relatively high retention rate and there were no significant differences between the follow-up and drop-out groups. Fourth, only parent-report ADHD symptoms is available. Impaired parent–child relationship may confound the association found. Last but not least, the sample size is relatively limited in detecting the moderation effect of sex, despite the observed effect size difference.
In conclusion, insufficient sleep during preschool years increases the risk of ADHD in middle childhood. One potential way to tackle early insufficient sleep is to extend existing effective sleep education44 to parents and caregivers and alert them of the deleterious impact of early childhood sleep deprivation on long-term development. Paediatricians and other health-care providers should beware of the potential adverse effect of sleep deprivation on ADHD and apply relevant treatment and advices for children with sleep problems.
References
Man K. K. et al. ADHD drug prescribing trend is increasing among children and adolescents in Hong Kong. J. Atten. Disord. 21, 1161–1168 (2014).
Polanczyk, G. V., Willcutt, E. G., Salum, G. A., Kieling, C. & Rohde, L. A. ADHD prevalence estimates across three decades: an updated systematic review and meta-regression analysis. Int. J. Epidemiol. 43, 434–442 (2014).
Leung, P. W. et al. The diagnosis and prevalence of hyperactivity in Chinese schoolboys. Br. J. Psychiatry 168, 486–496 (1996).
Biederman, J. & Faraone, S. V. Attention-deficit hyperactivity disorder. Lancet 366, 237–248 (2005).
Touchette, E. et al. Associations between sleep duration patterns and behavioral/cognitive functioning at school entry. Sleep 30, 1213–1219 (2007).
Ficks, C. A. & Waldman, I. D. Gene-environment interactions in attention-deficit/hyperactivity disorder. Curr. Psychiatry Rep. 11, 387–392 (2009).
Garon, N., Bryson, S. E. & Smith, I. M. Executive function in preschoolers: a review using an integrative framework. Psychol. Bull. 134, 31 (2008).
Pauli-Pott, U. & Becker, K. Time windows matter in ADHD-related developing neuropsychological basic deficits: a comprehensive review and meta-regression analysis. Neurosci. Biobehav. Rev. 55, 165–172 (2015).
Tso, W. et al. Sleep duration and school readiness of Chinese children. J. Pediatr. 169, 266–271 (2016).
Corkum, P., Moldofsky, H., Hogg-Johnson, S., Humphries, T. & Tannock, R. Sleep problems in children with attention‐deficit/hyperactivity disorder: impact of subtype, comorbidity, and stimulant medication. J. Am. Acad. Child Adolesc. Psychiatry 38, 1285–1293 (1999).
Stein, M. A. Unravelling sleep problems in treated and untreated children with ADHD. J. Child Adolesc. Psychopharmacol. 9, 157–168 (1999).
Scott, N. et al. Sleep patterns in children with ADHD: a population-based cohort study from birth to 11 years. J. Sleep. Res. 22, 121–128 (2013).
O’Brien, L. M. & Gozal, D. Behavioural and neurocognitive implications of snoring and obstructive sleep apnoea in children: facts and theory. Paediatr. Respir. Rev. 3, 3–9 (2002).
Tirosh, E., Sadeh, A., Munvez, R. & Lavie, P. Effects of methylphenidate on sleep in children with attention-deficit hyperactivity disorder: an activity monitor study. Am. J. Dis. Child. 147, 1313–1315 (1993).
Arnett, A. B., Pennington, B. F., Willcutt, E. G., DeFries, J. C. & Olson, R. K. Sex differences in ADHD symptom severity. J. Child Psychol. Psychiatry 56, 632–639 (2015).
Ip, P. et al. Socioeconomic gradients in school readiness of Chinese preschool children: the mediating role of family processes and kindergarten quality. Early Child. Res. Q. 35, 111–123 (2016).
U.S. National Institutes of Health. Your Guide to Healthy Sleep (National Institutes of Health, Bethesda, Maryland, 2011).
Li, A. M. et al. Validation of a questionnaire instrument for prediction of obstructive sleep apnea in Hong Kong Chinese children. Pediatr. Pulmonol. 41, 1153–1160 (2006).
Liu, X., Liu, L., Owens, J. A. & Kaplan, D. L. Sleep patterns and sleep problems among school children in the United States and China. Pediatrics 115, (Supplement 1), 241–249 (2005).
Swanson, J. M. et al. Categorical and dimensional definitions and evaluations of symptoms of ADHD: history of the SNAP and the SWAN rating scales. Int. J. Educ. Psychol. Assess. 10, 51 (2012).
Lai, K. Y. et al. Validation of the Chinese strengths and weaknesses of ADHD-symptoms and normal-behaviors questionnaire in Hong Kong. J. Atten. Disord. 17, 194–202 (2013).
Ip, P. et al. Validation study of the Chinese early development instrument (CEDI). BMC Pediatr. 13, 1 (2013).
Zou, G. A modified poisson regression approach to prospective studies with binary data. Am. J. Epidemiol. 159, 702–706 (2004).
Youssef, N. A., Ege, M., Angly, S. S., Strauss, J. L. & Marx, C. E. Is obstructive sleep apnea associated with ADHD? Ann. Clin. Psychiatry 23, 213–224 (2011).
do Lago Godoi, F. R., Oliveira, M. G. M. & Tufik, S. Effects of paradoxical sleep deprivation on the performance of rats in a model of visual attention. Behav. Brain Res. 165, 138–145 (2005).
Córdova, C. A. et al. Sleep deprivation in rats produces attentional impairments on a 5-choice serial reaction time task. Sleep 29, 69 (2006).
Willie, J. T., Sinton, C. M., Maratos-Flier, E. & Yanagisawa, M. Abnormal response of melanin-concentrating hormone deficient mice to fasting: hyperactivity and rapid eye movement sleep suppression. Neuroscience 156, 819–829 (2008).
Solanto, M. V. Dopamine dysfunction in AD/HD: integrating clinical and basic neuroscience research. Behav. Brain Res. 130, 65–71 (2002).
Volkow, N. D. et al. Sleep deprivation decreases binding of [11C] raclopride to dopamine D2/D3 receptors in the human brain. J. Neurosci. 28, 8454–8461 (2008).
Koenis, M. M. et al. Does sleep restore the topology of functional brain networks? Hum. Brain Mapp. 34, 487–500 (2013).
Hall, M. et al. Psychological stress is associated with heightened physiological arousal during NREM sleep in primary insomnia. Behav. Sleep Med. 5, 178–193 (2007).
Catala-Lopez, F. et al. Prevalence of attention deficit hyperactivity disorder among children and adolescents in Spain: a systematic review and meta-analysis of epidemiological studies. BMC Psychiatry 12, 168 (2012).
Nussbaum, N. L. ADHD and female specific concerns: a review of the literature and clinical implications. J. Atten. Disord. 16, 87–100 (2012).
Valera, E. M. et al. Sex differences in the functional neuroanatomy of working memory in adults with ADHD. Am. J. Psychiatry 167, 86–94 (2010).
Barry, R. J., Clarke, A. R., McCarthy, R. & Selikowitz, M. Age and gender effects in EEG coherence: III. Girls with attention-deficit/hyperactivity disorder. Clin. Neurophysiol. 117, 243–251 (2006).
Hermens, D. F., Kohn, M. R., Clarke, S. D., Gordon, E. & Williams, L. M. Sex differences in adolescent ADHD: findings from concurrent EEG and EDA. Clin. Neurophysiol. 116, 1455–1463 (2005).
Romero-Martinez, A., Polderman, T. J., Gonzalez-Bono, E. & Moya-Albiol, L. Masculinization in parents of offspring with autism spectrum disorders could be involved in comorbid ADHD symptoms. J. Atten. Disord. 21, 938–943 (2017).
Rice, D. & Barone, S. Jr. Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ. Health Perspect. 108, (Suppl 3), 511–533 (2000).
Brown, E. D. & Ackerman, B. P. Contextual risk, maternal negative emotionality, and the negative emotion dysregulation of preschool children from economically disadvantaged families. Early Educ. Dev. 22, 931–944 (2011).
Boles, R. E. et al. Family chaos and child functioning in relation to sleep problems among children at risk for obesity. Behav. Sleep Med. 15, 114–128 (2017).
Harvey, A. G., Murray, G., Chandler, R. A. & Soehner, A. Sleep disturbance as transdiagnostic: consideration of neurobiological mechanisms. Clin. Psychol. Rev. 31, 225–235 (2011).
Faruqui, F., Khubchandani, J., Price, J. H., Bolyard, D. & Reddy, R. Sleep disorders in children: a national assessment of primary care pediatrician practices and perceptions. Pediatrics 128, 539–546 (2011).
Sadeh, A. A brief screening questionnaire for infant sleep problems: validation and findings for an Internet sample. Pediatrics 113, e570–e577 (2004).
Wing, Y. K. et al. A school-based sleep education program for adolescents: a cluster randomized trial. Pediatrics 135, e635–e643 (2015).
Acknowledgments
We thank the schools, children, and their parents for participating in this study. The cohort study was fully supported by two research grants from the Research Grants Council of the Hong Kong Special Administrative Region, China (Project Nos. 743413 and 746111).
Author contributions
W.T. interpreted the data and wrote the first draft of the manuscript. F.K.W.H. analysed the data, drafted a part of the manuscript, and critically revised the manuscript. M.C., K.L.C., A.M.L., A.T., Y.K.W., I.C.K.W., B.V.V., S.L.L. and W.H.S.G. interpreted the data and critically revised the manuscript. P.I. and N.R. conceptualised and designed the study, interpreted the data, and critically revised the manuscript. All authors have read and approved the final version of the manuscript.
Funding disclosure
The cohort study was supported by two research grants from the Research Grants Council of the Hong Kong Special Administrative Region, China (Project Nos. 743413 and 746111). The funder has no role in (1) study design; (2) the collection, analysis, and interpretation of data; (3) the writing of the report; and (4) the decision to submit the paper for publication.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Co-first authors: Winnie Tso, Meanne Chan.
Rights and permissions
About this article
Cite this article
Tso, W., Chan, M., Ho, F.K. et al. Early sleep deprivation and attention-deficit/hyperactivity disorder. Pediatr Res 85, 449–455 (2019). https://doi.org/10.1038/s41390-019-0280-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-019-0280-4
This article is cited by
-
Gut microbial genetic variation modulates host lifespan, sleep, and motor performance
The ISME Journal (2023)
-
Comorbidity of ADHD and allergic diseases in early adolescence: The role of parental smoking at home
Current Psychology (2023)
-
Exploring the role of family communication time in the association between family dinner frequency and adolescent psychological distress
Current Psychology (2023)
-
Vulnerability and resilience in children during the COVID-19 pandemic
European Child & Adolescent Psychiatry (2022)
-
Treatment with Methylphenidate for Attention Deficit Hyperactivity Disorder (ADHD) and the Risk of All-Cause Poisoning in Children and Adolescents: A Self-Controlled Case Series Study
CNS Drugs (2021)