Abstract
Background
Opsoclonus-myoclonus syndrome (OMS) is a rare neurological disorder, usually accompanied by neuroblastoma (NB). There is no targeted treatment and animal model of OMS. We aimed to investigate whether insulin-like growth factor 1 (IGF-1)/phosphoinositide 3-kinase (PI3K) signaling alleviates neuronal cytolysis in pediatric OMS.
Methods
Cultured rat cerebral cortical neurons and cerebellar neurons were incubated with sera or IgG isolated from sera of children with OMS and NB. Cytolysis and PI3K expression were measured by the lactate dehydrogenase assay and enzyme-linked immunosorbent assay, respectively. Using inhibitors and activators, the effects of IGF-1 and PI3K on cytolysis were investigated.
Results
The incubation of sera or IgG from children with OMS and NB increased cytolysis in not only cerebellar neurons, but also cerebral cortical neurons. Furthermore, the IGF-1 receptor antagonist NVP-AEW541 exaggerated cytolysis in children with OMS and NB. IGF-1 alleviated cytolysis, which was blocked by the PI3K inhibitor LY294002. Additionally, sera or IgG from children with OMS and NB compensatively elevated PI3K expression. LY294002 exacerbated cytolysis; whereas, the PI3K activator 740 Y-P suppressed cytolysis.
Conclusion
IGF-1/PI3K signaling alleviates the cytolysis of cultured neurons induced by serum IgG from children with OMS and NB, which may be innovation therapy targets.
Similar content being viewed by others
Introduction
Opsoclonus-myoclonus syndrome (OMS) is a rare neurological disorder with an estimated annual incidence of 0.27–0.40 cases in children per million.1 The typical symptoms of OMS are opsoclonus, myoclonus, ataxia, and most of the patients also have persistent cognitive, neurological, or behavioral-developmental deficits.2 Neuroblastoma (NB) is found in 50% of children with OMS. Mechanisms underlying OMS are still largely unknown, resulting in no targeted treatment and even no animal model of OMS.
Sera and the IgG fraction isolated from sera of OMS patients have cell toxicity to NB cell lines3,4 and cultured cerebellar cells.5,6 Besides the cerebellum and brainstem, the cerebrum is also affected by the pathogenesis of OMS, for patients with OMS have neurological deficits in multiple cerebral functions,7,8 and brain images show morphological changes in the cerebrum.9,10,11 Moreover, the IgG fraction of sera contains autoantibodies,3 which are expressed in OMS patients.2,12,13 Therefore, we hypothesized that both cerebral cortical and cerebellar neurons are affected by sera or IgG from children with OMS and NB.
Insulin-like growth factor 1 (IGF-1) takes part in various cellular processes via activation of phosphoinositide 3-kinase (PI3K), such as cell survival, apoptosis, and differentiation. Especially, IGF-1/PI3K signaling alleviates neurotoxicity in some diseases.14,15 For instance, the activation of PI3K and downstream IGF-1 suppresses N-methyl-D-aspartate (NMDA)-induced excitotoxicity in cultured hippocampal neurons against autophagy.16 Furthermore, sera from pediatric OMS patients induces cytolysis in cultured cerebellar neurons, which specifically dependents on extracellular signal-regulated kinase (ERK).5 A previous report showed that ERK can have a negative crosstalk with PI3K, thus having opposite effects on cell death.17 However, whether IGF-1 is involved in the cytolysis of cultured neurons induced by serum IgG from children with OMS and NB through PI3K is still unknown.
In this study, we found that the incubation of sera or IgG from children with OMS and NB induces cytolysis in cultured rat cerebral cortical and cerebellar neurons. Moreover, neuronal cytolysis is exaggerated by the IGF-1 inhibitor, whereas cytolysis is alleviated by IGF-1, which is blocked by the PI3K inhibitor. Additionally, sera or IgG from children with OMS and NB compensatively elevates PI3K expression. Neuronal cytolysis is exaggerated by the PI3K inhibitor, while it is alleviated by the PI3K activator. Our results suggest that IGF-1 suppresses neuronal cytolysis of cultured neurons induced by serum IgG from children with OMS and NB via PI3K.
Methods
Participants
All procedures in this study were approved by the Clinical Research Ethics Committees of Beijing Children’s Hospital, Capital Medical University (No. IEC-C-028-A10-V.05). Written informed consent was obtained from all the legal guardians of children enrolled in our study. All the childrens were enrolled between January 2015 and December 2017 from the Beijing Children’s Hospital and were Han Chinese. Sera of 8 children with OMS and NB (OMS + NB) were collected. Sera of 17 children with NB without OMS (NB), sera of 10 healthy children were used as control. Moreover, 10 children with juvenile idiopathic arthritis (JIA) were enrolled to reveal whether effects on neurons are special for OMS or common for all IgG-related diseases. All the sera were stored at −80 °C until further detection.
Clinical data
The age and gender in healthy children, NB, OMS + NB and JIA groups, and also tumor stage in NB and OMS + NB groups were not significantly different. More than half of blood samples in NB, OMS + NB and JIA groups were collected before any treatment (Table 1). All the children in the JIA group suffered from systemic onset JIA. Electroencephalogram results were clinically normal in the OMS + NB group.
For patients with OMS, the degree of ataxia, opsoclonus, ataxia/gait, ataxia/stance, and sleep/mood disturbance was graded from 0 to 3, respectively, and OMS score was the summary of the 5 aspects. The OMS evaluation criteria have been previously described.18
The IgG fraction isolated from sera
Sera were applied to 100 µl protein G Agarose (Thermo Fisher Scientific, California) as described before.3 After centrifugation at 10,000×g for 10 min, the IgG-free fraction in supernatants was removed. After washing with 0.01 M phosphate buffer saline (PBS), IgG was eluted using 0.1 M glycine-HCl (pH 2.7), followed by neutralization buffer PBS. The concentration of IgG was determined using the BCA assay (Pierce, Rockford).
Primary cultures of cerebral cortical and cerebellar neurons
Primary cultures of cerebral cortical and cerebellar neurons were prepared from the cerebral cortex and cerebellum of Sprague Dawley rat embryos (16–18 days old) as previously described.6,19 The cerebral cortex and cerebellum were cut and incubated at 37 °C for 30 min in calcium–magnesium free PBS containing 0.01% trypsin, respectively. After triturating, cells were centrifuged for 5 min at 800 rpm and resuspended in Eagle’s Basal Medium prepared without glutamine, with twice the usual concentration of other amino acids and four times the usual concentration of vitamins (MEM-Pak; University of California, San Francisco Cell Culture Facility, San Francisco, CA). The culture was supplemented on the day of plating with 2 mM glutamine, 15 mM HEPES, pH 7.4, and glucose to a final concentration of 30 mM. Cell suspensions were filtered, supplemented with 10% horse serum and 10% fetal calf serum, and seeded on poly-l-lysine coated plates (Corning Inc., Corning). After 24 h, the media were replaced with serum free Neurobasal growth medium containing B27 (Gibco, Boston), 0.5 mM l-Glutamax (Sigma-Aldrich, St Louis), 2.5 M cytosine arabinoside, 100 U/ml penicillin, and 100 g/ml streptomycin. Cultures were fed daily with a medium containing B27. To prepare neuron-enriched cultures, 24 h after seeding the cells, cytosine arabinoside at 10 μM was added.
Lactate dehydrogenase (LDH) assay
LDH is expressed in the cytoplasm, which can be detected in the supernatant of cultured neurons only when the plasma membrane is damaged, therefore LDH in supernatant is an indicator of cytolysis.3,5 Neurons were seeded onto 6-well plates (Corning Inc., Corning) at 3.0 × 105 cells/well to get both media for the LDH assay and neurons for the enzyme-linked immunosorbent assay (ELISA) parallelly, for the purpose to calculate the correlations between each other (Figs. 1a–d and 2), while in other experiments (Figs. 1g, h, and 3–6) neurons were seeded onto 96-well plates (Corning Inc., Corning) at 1.0 × 104 cells/well. Neurons were cultured for 16 h, and then the medium was replaced with a fresh medium containing sera or IgG. In 6-well plates, 40 µl of sera and 1960 µl of medium were added in each well, 1:50 diluted. Also IgG at 100–200 µg in a 2000-μl volume, at the final concentration of 0.05–0.1 µg/µl, and the IgG-free fraction at 1000–1400 µg were used for each well. In 96-well plates, the volume was 1/10 of that in 6-well plates with the same final concentration. In other words, 4 µl of sera or IgG at 10–20 µg in a 200-μl volume was used for each well of 96-well plates.3 Additionally, commercially available human IgG (Sigma-Aldrich) was used to confirm whether effects on cytolysis are simply related to higher concentration of IgG. Commercially available IgG was dissolved in sterile normal saline, which was added into each well with medium (10 µg/well) at the final concentration of 0.1 µg/µl, the largest concentration in the OMS + NB group. Sera or IgG was incubated for 48 h. Then 100 μl of neuron culture supernatants were measured by the Cytotoxicity Detection Kit (Roche, Indianapolis). Maximum LDH release was induced by 1% Triton X-100, whereas background control was untreated cells. The percentage of specific cytolysis was determined by the following formula: [(experimental value-background control)/(maximum value-background control)] × 100. Each sera of a child parallelly treated three wells of neurons.
Sera or IgG isolated from sera of children with OMS and NB induces cytolysis of rat cerebral cortical and cerebellar neurons compared with that of NB patients and healthy controls. Note that neuronal cytolysis was increased in cerebral cortical neurons a and cerebellar neurons b incubated with sera from children with OMS and NB. The IgG fraction in the OMS + NB group increased the cytolysis of rat neurons compared with that of the NB or healthy control group c, d, but not the IgG-free fraction e, f. No alteration was observed in the cytolysis of rat neurons incubated with IgG isolated from sera of children with juvenile idiopathic arthritis (JIA) or commercially available human IgG g, h. ***P < 0.001, one-way ANOVA, n = 10 (Health control), n = 17 (NB), n = 8 (OMS + NB), n = 10 (JIA), n = 15 (IgG)
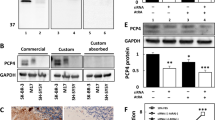
The expression of PI3K is increased by sera or IgG isolated from sera of children with OMS and NB in rat cerebral cortical and cerebellar neurons, compared with that of NB patients and healthy controls. Note that the expression of PI3K in cerebral cortical a, c and cerebellar neurons b, d were increased after preincubation with sera or IgG from children with OMS and NB, however incubation with sera or IgG from children with NB had no such effect. **P < 0.01, ***P < 0.001, one-way ANOVA, n = 10 (Health control), n = 17 (NB), n = 8 (OMS + NB)
NVP-AEW541, an antagonist of IGF-1 receptor, exacerbates the cytolysis of cultured neurons induced by sera or IgG of children with OMS and NB. Note that the cytolysis of cerebral cortical neurons a and cerebellar neurons b induced by sera in the OMS + NB group was exaggerated by NVP-AEW541, and the cytolysis of cerebral cortical c and cerebellar neurons d induced by IgG in the OMS + NB group was aggravated by NVP-AEW541. *P < 0.05, **P < 0.01, ***P < 0.001, one-way ANOVA, n = 17 (DMSO, NB; LY294002, NB), n = 8 (DMSO, OMS + NB; LY294002, OMS + NB)
IGF-1 alleviates the cytolysis of cultured neurons induced by sera or IgG of children with OMS and NB, which is attenuated by the PI3K inhibitor LY294002. Note that the cytolysis of cerebral cortical a and cerebellar neurons b induced by sera in the OMS + NB group was depressed by IGF-1, and the cytolysis of cerebral cortical c and cerebellar neurons d induced by IgG in the OMS + NB group was inhibited by IGF-1, however IGF-1 did not affect cytolysis in neurons incubated with sera or IgG from NB patients under our experimental design. The effects of IGF-1 in cultured rat neurons were suppressed by pretreatment with LY294002 a–d. *P < 0.05, **P < 0.01, ***P < 0.001, one-way ANOVA, n = 17 (PBS, NB; IGF-1, NB), n = 8 (PBS, OMS + NB; IGF-1, OMS + NB; DMSO, IGF-1, OMS + NB; LY294002, IGF-1, OMS + NB)
The PI3K pharmacological inhibitor LY294002 aggravates the cytolysis of cultured neurons induced by sera or IgG from children with OMS and NB. Note that the cytolysis of cerebral cortical a and cerebellar neurons b induced by sera in the OMS + NB group was exaggerated by LY294002, and the cytolysis of cerebral cortical c and cerebellar neurons d induced by IgG in the OMS + NB group was aggravated by LY294002, whereas LY294002 had no effect on cytolysis in neurons incubated with sera or IgG of NB patients. **P < 0.01, ***P < 0.001, one-way ANOVA, n = 17 (DMSO, NB; LY294002, NB), n = 8 (DMSO, OMS + NB; LY294002, OMS + NB)
Exposure to 740 Y-P, a cell-permeable phosphopeptide activator of PI3K, alleviates the cytolysis of cultured rat neurons induced by sera or IgG of children with OMS and NB. Note that the cytolysis of cerebral cortical a and cerebellar neurons b induced by sera in the OMS + NB group was blocked by 740 Y-P, and the cytolysis of cerebral cortical c and cerebellar neurons d induced by IgG in the OMS + NB group was attenuated by 740 Y-P, whereas 740 Y-P had no effect on cytolysis in neurons incubated with sera or IgG of NB patients. ***P < 0.001, one-way ANOVA, n = 17 (Saline, NB; 740 Y-P, NB), n = 8 (Saline, OMS + NB; 740 Y-P, OMS + NB)
To explore the mechanisms of cytotoxicity induced by sera and IgG from pediatric OMS patients, we focused on the IGF-1 cascade, which is mainly secreted from microglia in the brain and is involved in cell survival and differentiation.14,15,16 The IGF-1 receptor inhibitor NVP-AEW541 (Sellect, China) dissolved in sterile 5% dimethyl sulfoxide (DMSO, Sigma-Aldrich) was added into the culture medium to make a final concentration of 8 µM.20 Recombinant rat IGF-1 (R & D systems, Minneapolis) dissolved in sterile 0.01 M PBS was added at 10 nM.15 To investigate the effects of the PI3K inhibitor LY294002 on cytolysis, LY294002 (Sigma-Aldrich) dissolved in sterile 5% DMSO was added at 20 µM.15 The final concentration of DMSO was 0.01%. Finally, 740 Y-P (R & D systems), the phosphopeptide activator of PI3K, was dissolved in sterile normal saline and was added at 1.53 µM.21 Cultured neurons were serum-starved for 24 h before IGF-1 treatment to exclude the affections of insulin. All the drugs were used 30 min before treatment with sera or IgG and neurons were incubated with LY294002 30 min prior to the addition of IGF-1.
ELISA
Neurons used for the ELISA and culture media used for the LDH assay were from the same well of 6-well plates (Figs. 1a–d and 2). Neurons were lysed as we previously described and commercially available ELISA kits (CUSABIO, Wuhan, China) were applied to detect the protein expression of PI3K following manufacturer’s instructions.22 PI3K concentration in cultured neurons was normalized to the amount of total protein detected by a BCA protein assay kit.
Statistical analysis
All data were represented as mean ± SEM. All statistical analyses were carried out using GraphPad Prism (GraphPad Software Inc., San Diego). Statistical differences of groups were analyzed by one-way analysis of variance (ANOVA). Spearman’s correlation coefficient was used for correlation tests. There were 8 childrens in the OMS + NB group, and the average of cells in 3 wells to carry out parallel experiments for each child was used to calculate correlations. P < 0.05 was fixed as statistically significant.
Results
Sera or the IgG fraction from children with OMS and NB induces cytolysis in not only cultured cerebellar neurons but also cerebral cortical neurons
Rat cerebral cortical neurons and cerebellar neurons were incubated with sera or the IgG fraction from children with OMS and NB. As shown in Fig. 1a, neuronal cytolysis was increased in cerebral cortical neurons incubated with sera from children with OMS and NB compared with that of NB patients or healthy children (25.35 ± 2.23% OMS + NB, 14.21 ± 1.62% NB, 7.97 ± 1.86% Healthy control, P < 0.001), while cytolysis was not significantly changed in rat neurons treated with sera of children with only NB at least under our experimental design. Similarly, IgG isolated form sera of children with OMS and NB also gave rise to cytolysis in rat cerebral cortical neurons in contrast to that of NB patients or healthy children (24.22 ± 1.62% OMS + NB, 10.47 ± 1.39% NB, 5.66 ± 1.63% Healthy control, P < 0.001, Fig. 1c). Moreover, sera and IgG in the OMS + NB group elevated the cytolysis of cerebellar neurons compared with the NB or healthy control group (Fig. 1b, d). In contrast, the IgG-free fraction from sera of children with OMS and NB showed no impact (Fig. 1e, f). Additionally, no alteration was observed in the cytolysis of cultured neurons incubated with IgG isolated from sera of children with JIA or commercially available human IgG (Fig. 1g, h), suggesting that cytolysis mediated by IgG from OMS is special, rather than common for all IgG-changed diseases, and is not simply related to higher concentration of IgG. In order to be more precise, all the 4 groups were tested in the same plate, cytolysis in neurons treated with IgG from healthy controls and OMS + NB patients was measured again (Fig. 1g, h), not the same data in Fig. 1c, d.
Children with OMS have multiple symptoms, which were valued by OMS score (Table 2). Correlation analyses revealed that cytolysis in both cerebral cortical and cerebellar neurons induced by OMS + NB sera or IgG was positively correlated with OMS scores, except cerebral neurons incubated with sera (Table 3). These results suggested that serum IgG from children with OMS and NB induces lysis in cultured cerebral cortical and cerebellar neurons, which is correlated with symptoms of pediatric OMS.
The IGF-1 inhibitor NVP-AEW541 exaggerates the cytolysis of cultured neurons caused by sera or IgG from children with OMS and NB, whereas IGF-1 suppresses neuronal cytolysis, which is blocked by the PI3K inhibitor LY294002
To investigate whether IGF-1 takes part in IgG-mediated neuronal cytolysis in OMS, the IGF-1 receptor antagonist NVP-AEW541 was preincubated with cultured neurons before sera or IgG. Our results showed that cytolysis in cerebral cortical neurons was increased pretreated with NVP-AEW541 before OMS + NB sera or IgG (Sera: 39.39 ± 1.57% NVP-AEW541, OMS + NB vs. 26.69 ± 2.80% DMSO, OMS + NB, P < 0.01, IgG: 39.94 ± 2.52% NVP-AEW541, OMS + NB, vs. 28.40 ± 3.46% DMSO, OMS + NB, P < 0.05, Fig. 3a, c). Similarly, cytolysis in cerebellar neurons was elevated by NVP-AEW541 (Fig. 3b, d), suggesting that IGF-1 alleviates the IgG-induced neuronal cytolysis in pediatric OMS and NB.
Furthermore, our results showed that cytolysis in cerebral cortical neurons was suppressed by incubation with IGF-1 before OMS + NB sera (13.27 ± 2.78% IGF-1, OMS + NB vs. 27.01 ± 1.39% PBS, OMS + NB, P < 0.001, Fig. 4a), and also, the elevated level of cytolysis in cerebral cortical neurons was partly blocked by treatment with IGF-1 before OMS + NB IgG (14.15 ± 2.05% IGF-1, OMS + NB vs. 28.66 ± 1.99% PBS, OMS + NB, P < 0.001, Fig. 4c). Coincidently, cytolysis was also relieved by IGF-1 in cerebellar neurons incubated with sera or IgG from the OMS + NB group (Fig. 4b, d).
Additionally, we investigated whether PI3K, the downstream target of IGF-1, is affected by the incubation of sera or IgG. The results showed that the effects of IGF-1 were blocked by pretreatment with the PI3K inhibitor LY294002 in cerebral cortical and cerebellar neurons incubated with sera or IgG from children with OMS and NB (Fig. 4a–d), suggesting that the protective effect of IGF-1 dependents on PI3K.
The expression of PI3K is increased in cerebral cortical and cerebellar neurons incubated with sera or IgG from children with OMS and NB
PI3K expression in cerebral cortical neurons was increased after incubation with sera of children with OMS and NB (322.4 ± 26.64 pg/mg total protein OMS + NB, 217.9 ± 18.01 pg/mg total protein NB, 207.5 ± 7.79 pg/mg total protein Healthy control, P < 0.01, Fig. 2a). Furthermore, PI3K concentration in cerebral cortical neurons was enhanced after incubation with IgG in children with OMS and NB (350.8 ± 19.34 pg/mg total protein OMS + NB, 172.1 ± 11.30 pg/mg total protein NB, 188.4 ± 10.84 pg/mg total protein Healthy control, P < 0.001, Fig. 2c). Consistently, PI3K expression was also upregulated in cerebellar neurons incubated with sera or IgG from children with OMS and NB (Fig. 2b, d). Correlation analyses revealed that PI3K expression was positively correlated with cytolysis in both cerebral cortical and cerebellar neurons, except cerebral neurons incubated with IgG (Table 3). Taken together, these results indicated that PI3K participates in the lysis of cultured neurons incubated with sera or IgG from children with OMS and NB.
The PI3K inhibitor LY294002 aggravates the cytolysis of cultured neurons caused by sera or IgG from children with OMS and NB, whereas the PI3K activator 740 Y-P suppresses cytolysis
We found that the upregulation of PI3K in cerebral cortical neurons was exaggerated after preincubation with LY294002 before OMS + NB sera (31.03 ± 1.02% LY294002, OMS + NB vs. 23.20 ± 1.79% DMSO, OMS + NB, P < 0.01), however no effect of LY294002 in the NB group was observed (Fig. 5a). Moreover, the elevated level of PI3K in cerebral cortical neurons was exaggerated after preincubation with LY294002 before OMS + NB IgG (Fig. 5c). Similarly, the increased expression of PI3K was also exacerbated by LY294002 in cerebellar neurons incubated with sera or IgG from the OMS + NB group (Fig. 5b, d).
In order to further confirm that PI3K positively regulates cytolysis induced by sera or IgG from OMS patients, we used the PI3K activator 740 Y-P. The upregulation of PI3K in cerebral cortical neurons was alleviated by incubation with 740 Y-P before OMS + NB sera (7.62 ± 0.67% 740 Y-P, OMS + NB vs. 27.14 ± 1.68% Saline, OMS + NB, P < 0.001), however preincubation with 740 Y-P had no such effect in the NB group (Fig. 6a). Furthermore, PI3K upregulation in cerebral cortical neurons was attenuated by incubation with 740 Y-P before OMS + NB IgG (Fig. 6c). Consistently, PI3K upregulation was also blocked by 740 Y-P in cerebellar neurons incubated with OMS + NB sera or IgG (Fig. 6b, d). Combined with the aforementioned results, these results suggested that the activation of PI3K alleviates cytolysis in cultured neurons incubated with sera or IgG from children with OMS and NB.
Discussion
Sera and the IgG fraction from children with OMS and NB induce cytolysis in cultured cerebral cortical and cerebellar neurons
Previous reports have shown that sera and the IgG fraction isolated from sera of patients with OMS and NB are cytotoxic to NB cell lines and cerebellar granular neurons.3,4,5 Consistently, our results suggested that sera or IgG from children with OMS and NB induces cytolysis in cultured neurons, but not the IgG-free fraction, indicating that cytolysis induced by sera mainly dependents on the IgG fraction. Cytolysis induced by sera and IgG is highly correlated with OMS grades of patients. Children with OMS have multiple symptoms,2 which may be affected by whether timely visiting to hospitals, genetic background, age to developmental and social factors contained in irritableness, and also, children with minimal symptoms and their parents are more likely to agree to participate in the study. Actually, cytolysis may be more relevant to OMS grades in patients with more significant symptoms. Additionally, static and exercise ataxia may occur separately, for they are regulated by different regions of the cerebellum and molecular mechanisms, similar as previously reported paroxysmal gait ataxia induced by walking.23
A growing body of evidence suggests that besides the cerebellum and brainstem, the cerebrum is also involved in the pathogenesis of OMS. Approximately 70–80% OMS patients have persistent neurological impairments, including deficits in intelligence, attention, processing speed, memory, language, visuospatial, and visuoconstructive skills, fine motor skills and executive functions.7,8 Additionally, brain imaging of OMS patients showed changes in the cerebrum. Magnetic resonance imaging of a patient showed significant nodular enhancing lesions at gray–white junction of bilateral cerebral hemispheres.11 Cerebral cortical thickness was reduced across the motor and visual areas in patients with pediatric OMS.10 A patient with OMS revealed decreased metabolism in the bilateral occipital lobes and increased functional connectivity, including the primary and motion-sensitive visual cortex.9 In order to investigate changes in the brain comprehensively, we incubated both cerebral cortical and cerebellar neurons with sera or IgG of OMS pediatric patients. Expectedly, our results showed that sera and IgG from OMS pediatric patients have approximately similar degrees of cytolysis in both cerebral cortical and cerebellar neurons.
Although 50% of children with OMS have NB, and patients with non-paraneoplastic OMS have better outcomes with fewer relapses compared with those having paraneoplastic OMS,2 the relationship of paraneoplastic and non-paraneoplastic childhood OMS is still not clearly known. In our study, we found that sera and IgG from children with OMS and NB cause the lysis of cultured neurons compared with children with only NB, indicating that IgG may be specifically involved in the pathogenesis of OMS. However, we did not obtain sera from children with OMS and without NB for its rare incidence, therefore we cannot completely exclude the possibility that cytolysis induced by serum IgG from patients with OMS and NB may be the synergistic effects of OMS and NB. IgG from NB patients does not induce cytolysis in cultured neurons under our experimental design, however it may have cytotoxic effects with longer incubation time or higher dose.
Moreover, no alternation was observed in the cytolysis of neurons treated with IgG from sera of children with JIA or commercial available human IgG, suggesting that IgG-mediated lysis is special for pediatric OMS and is not simply due to higher concentration of IgG, consistent with previous study that IgG-induced cytolysis only occurs in pediatric OMS, but not adult OMS.5 Certainly, JIA IgG at higher dose may have neurotoxic effects. In fact, sera cannot confirm what actually happens in the brain, cerebrospinal fluid (CSF) will be tested in our future research, although for the difficulty to collect CSF from patients, sera are used in numerous studies of neurological diseases.3,4,5,24,25
IGF-1 alleviates the cytolysis of cultured cerebral cortical and cerebellar neurons induced by sera and IgG from children with OMS and NB via PI3K
The IGF-1/PI3K cascade is involved in cell survival and differentiation in various kinds of cells.14,15,16 In line with previous reports, our results showed that IGF-1 attenuates the lysis of cultured neurons induced by sera and IgG from children with OMS and NB. In addition, recombinant human IGF-1 has been clinically used in patients with growth failure26 and may be a possible treatment for patients with central nervous system disorders.27 Accordingly, the administration of IGF-1 might be an efficient way to treat pediatric OMS.
PI3K is a downstream target of IGF-1, which regulates a variety of cellular functions, such as survival, apoptosis, and migration, thus participates in multiple pathological diseases.14,15,16,22 In spite of the fact that PI3K increases cell death in several kinds of tumor cells and normal cells,28,29 accumulative evidence has documented that in neurons PI3K positively regulate neuronal survival and alleviates the cytotoxicity.30,31 Moreover, the phosphorylation of ERK is specifically necessary for IgG-mediated cytotoxicity of OMS patients in cerebellar neurons.5 However ERK and PI3K can have negative crosstalk, thus having opposite effects in cell death.17 Consistently, our results showed that PI3K reduces the lysis of cultured neurons induced by OMS and NB, and the upregulation of PI3K may be a compensative mechanism to lysis. Despite previous reports that IGF-1 and PI3K take part in NB cell line,32 we did not observe changes of neuronal cytolysis induced by IgG from children with NB in cultured brain neurons, the possible reason may be different mechanisms in NB cells and brain neurons.
Our results showed that the alleviation of cytolysis by IGF-1 was blocked by the PI3K inhibitor in cultured neurons, suggesting that the direct neuroprotective effect of IGF-1 on cultured neurons against IgG from OMS children dependents on PI3K. Our finding is consistent with previous reports that the IGF-1/PI3K cascade and following downstream signaling, such as Akt and NF-kappa B, protect against neuronal loss,33,34 and alleviate neuronal loss-induced impaired memory and cognitive functions in neurodegenerative diseases. Thus IGF-1/PI3K signaling may serve as potential therapy targets to both OMS and neurodegenerative diseases, such as Alzheimer disease.27 Of course, besides PI3K, there may be other downstream molecules of IGF-1 included in the alleviation of cytolysis.
The IgG fraction in sera of pediatric OMS patients may be autoantibodies
Both autoantibodies targeted intracellular and extracellular antigens are found in patients with OMS, such as autoantibodies against neuronal nuclear antigen, glutamic acid decarboxylase in the cytoplasm and receptors in the neuronal surface.2,12,13 Autoantibodies in sera from children with OMS bind to antigens in the cytoplasm and nucleus of fixed neurons, although surface epitopes may be altered. On the other hand, autoantibodies in patients with OMS bind to non-synaptic puncta on the surface of living neurons.2,6,35
Furthermore, extracellular targets-binding autoantibodies are pathogenic in many diseases, while autoantibodies to intracellular antigens may also contribute to the development of diseases. Autoantibodies to extracellular targets cause defective presynaptic vesicle36 and neuron loss,24 leading to impaired brain function.25 On the other hand, autoantibody targeted to α-internexin, an intermediate filament protein expressed in the cytoplasm or nucleus, results in pronounced cognitive dysfunction that mimics features of neuropsychiatric systemic lupus erythematosus,37 possibly caused by the impairment of neuronal membrane integrity, similar as Alzheimer disease.38
Additionally, in line with previous results that autoantibodies in OMS patients inhibit cell proliferation and induce apoptosis in human NB cell lines and cultured cerebellar neurons,3,4,5 our results showed that IgG from pediatric OMS patients induces cytolysis in cultured cerebral cortical and cerebellar neurons using the same method to isolate IgG. Hence, it is reasonable that the IgG fraction in our study may be autoantibodies to both extracellular and intracellular targets, although we did not identify these autoantibodies, and autoantibodies include IgG and IgM antibodies.2
In summary, we demonstrated that the incubation of sera or the serum IgG fraction from children with OMS and NB induces cytolysis in cultured cerebral cortical and cerebellar neurons. Moreover, the inhibition of IGF-1 exacerbates cytolysis, whereas IGF-1 alleviates neuronal cytolysis induced by sera or IgG of children with OMS and NB, which depends on downstream PI3K. Additionally, sera or IgG from children with OMS and NB elevates PI3K expression. Cytolysis in neurons is exaggerated by the PI3K inhibitor, while it is alleviated by the PI3K activator. These data suggest that IGF-1 has a protective role in neuronal cytolysis induced by serum IgG from children with OMS and NB through PI3K. Hence, IGF-1 and PI3K activators may serve as promising therapeutic interventions in OMS patients.
References
Hasegawa, S. et al. A nationwide survey of opsoclonus-myoclonus syndrome in Japanese children. Brain Dev. 37, 656–660 (2015).
Armangue, T. et al. Clinical and immunological features of opsoclonus-myoclonus syndrome in the era of neuronal cell surface antibodies. JAMA Neurol. 73, 417–424 (2016).
Korfei, M. et al. Functional characterisation of autoantibodies from patients with pediatric opsoclonus-myoclonus-syndrome. J. Neuroimmunol. 170, 150–157 (2005).
Blaes, F., Pike, M. G. & Lang, B. Autoantibodies in childhood opsoclonus-myoclonus syndrome. J. Neuroimmunol. 201, 221–226 (2008).
Fuhlhuber, V. et al. Autoantibody-mediated cytotoxicity in paediatric opsoclonus-myoclonus syndrome is dependent on ERK-1/2 phophorylation. J. Neuroimmunol. 289, 182–186 (2015).
Panzer, J. A., Anand, R., Dalmau, J. & Lynch, D. R. Antibodies to dendritic neuronal surface antigens in opsoclonus myoclonus ataxia syndrome. J. Neuroimmunol. 286, 86 (2015).
Bravo, J., Lopez-Almaraz, R., Mateos, M., Diaz, L. & Hernandez-Exposito, S. Neuropsychological profile in opsoclonus-myoclonus-ataxia syndrome presenting as neuroblastic tumours. Rev. Neurol. 62, 249–257 (2016).
Pranzatelli, M. R., Tate, E. D. & McGee, N. R. Demographic, clinical, and immunologic features of 389 children with opsoclonus-myoclonus syndrome: a cross-sectional study. Front Neurol. 8, 468 (2017).
Oh, S. Y. et al. Longitudinal multi-modal neuroimaging in opsoclonus-myoclonus syndrome. J. Neurol. 264, 512–519 (2017).
Anand, G. et al. Cerebellar and cortical abnormalities in paediatric opsoclonus-myoclonus syndrome. Dev. Med. Child Neurol. 57, 265–272 (2015).
Kanjanasut, N., Phanthumchinda, K. & Bhidayasiri, R. HIV-related opsoclonus-myoclonus-ataxia syndrome: report on two cases. Clin. Neurol. Neurosurg. 112, 572–574 (2010).
Markakis, I., Alexiou, E., Xifaras, M., Gekas, G. & Rombos, A. Opsoclonus-myoclonus-ataxia syndrome with autoantibodies to glutamic acid decarboxylase. Clin. Neurol. Neurosurg. 110, 619–621 (2008).
Berridge, G. et al. Glutamate receptor δ2 serum antibodies in pediatric opsoclonus myoclonus ataxia syndrome. Neurology 91, e714–e723 (2018).
Kim, W. et al. miR-126 contributes to Parkinson’s disease by dysregulating the insulin-like growth factor/phosphoinositide 3-kinase signaling. Neurobiol. Aging 35, 1712–1721 (2014).
Laurino, L. et al. PI3K activation by IGF-1 is essential for the regulation of membrane expansion at the nerve growth cone. J. Cell Sci. 118, 3653–3662 (2005).
Wang, Y. S. et al. IGF-1 alleviates NMDA-induced excitotoxicity in cultured hippocampal neurons against autophagy via the NR2B/PI3K-AKT-mTOR pathway. J. Cell Physiol. 229, 1618–1629 (2014).
Dai, R. Y., Chen, R. & Li, H. Cross-talk between PI3K/Akt and MEK/ERK pathways mediates endoplasmic reticulum stress-induced cell cycle progression and cell death in human hepatocellular carcinoma cells. Int J. Oncol. 34, 1749–1757 (2009).
Matthay, K. K. et al. Opsoclonus myoclonus syndrome in neuroblastoma a report from a workshop on the dancing eyes syndrome at the advances in neuroblastoma meeting in Genoa, Italy, 2004. Cancer Lett. 228, 275–282 (2005).
Cao, G. D. et al. Caspase-activated DNase/DNA fragmentation factor 40 mediates apoptotic DNA fragmentation in transient cerebral ischemia and in neuronal cultures. J. Neurosci. 21, 4678–4690 (2001).
Garcia-Echeverria, C. et al. In vivo antitumor activity of NVP-AEW541-A novel, potent, and selective inhibitor of the IGF-IR kinase. Cancer Cell 5, 231–239 (2004).
Williams, E. J. & Doherty, P. Evidence for and against a pivotal role of PI 3-kinase in a neuronal cell survival pathway. Mol. Cell Neurosci. 13, 272–280 (1999).
Ding, X. et al. BDNF contributes to the development of neuropathic pain by induction of spinal long-term potentiation via SHP2 associated GluN2B-containing NMDA receptors activation in rats with spinal nerve ligation. Neurobiol. Dis. 73, 428–451 (2015).
Synofzik, M. et al. Acetazolamide-responsive exercise-induced episodic ataxia associated with a novel homozygous DARS2 mutation. J. Med. Genet 48, 713–715 (2011).
Kowal, C. et al. Human lupus autoantibodies against NMDA receptors mediate cognitive impairment. Proc. Natl Acad. Sci. USA 103, 19854–19859 (2006).
Bravo-Zehnder, M. et al. Anti-ribosomal P protein autoantibodies from patients with neuropsychiatric lupus impair memory in mice. Arthritis Rheumatol. 67, 204–214 (2015).
Chatterjee, S. et al. Phenotypic spectrum and responses to recombinant human IGF1 (rhIGF1) therapy in patients with homozygous intronic pseudoexon growth hormone receptor mutation. Eur. J. Endocrinol. 178, 481–489 (2018).
Costales, J. & Kolevzon, A. The therapeutic potential of insulin-like growth factor-1 in central nervous system disorders. Neurosci. Biobehav. Rev. 63, 207–222 (2016).
Soletti, R. C. et al. Peptide gomesin triggers cell death through L-type channel calcium influx, MAPK/ERK, PKC and PI3K signaling and generation of reactive oxygen species. Chem. -Biol. Interact. 186, 135–143 (2010).
Sarro, E. et al. Phosphoinositide 3-kinase inhibitors protect mouse kidney cells from cyclosporine-induced cell death. Kidney Int. 73, 77–85 (2008).
Park, H. H. et al. L-DOPA-induced neurotoxicity is reduced by the activation of the PI3K signaling pathway. Toxicology 265, 80–86 (2009).
Xie, Z. Y. et al. Exendin-4 attenuates neuronal death via GLP-1R/PI3K/Akt pathway in early brain injury after subarachnoid hemorrhage in rats. Neuropharmacology 128, 142–151 (2018).
Gao, Q. G., Xie, J. X., Wong, M. S. & Chen, W. F. IGF-I receptor signaling pathway is involved in the neuroprotective effect of genistein in the neuroblastoma SK-N-SH cells. Eur. J. Pharmacol. 677, 39–46 (2012).
Yamaguchi, A. et al. Akt activation protects hippocampal neurons from apoptosis by inhibiting transcriptional activity of p53. J. Biol. Chem. 276, 5256–5264 (2001).
Heck, S., Lezoualc’h, F., Engert, S. & Behl, C. Insulin-like growth factor-1-mediated neuroprotection against oxidative stress is associated with activation of nuclear factor kappa B. J. Biol. Chem. 274, 9828–9835 (1999).
Blaes, F. et al. Surface-binding autoantibodies to cerebellar neurons in opsoclonus syndrome. Ann. Neurol. 58, 313–317 (2005).
Werner, C. et al. Human autoantibodies to amphiphysin induce defective presynaptic vesicle dynamics and composition. Brain 139, 365–379 (2016).
Lu, X. Y. et al. Anti-alpha-internexin autoantibody from neuropsychiatric lupus induce cognitive damage via inhibiting axonal elongation and promote neuron apoptosis. PLoS ONE 5, e11124 (2010).
Gunn, A. P. et al. Amyloid-β peptide Aβ3pE-42 induces lipid peroxidation, membrane permeabilization, and calcium influx in neurons. J. Biol. Chem. 291, 6134–6145 (2016).
Funding
The present work was supported by grants from the Beijing Talents Fund (2015000021469G204) and the Basic Medicine and Clinical Medicine Cooperation Fund of Capital Medical University (15JL70, 16JL20).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Ding, X., Han, W., Wang, J. et al. IGF-1 alleviates serum IgG-induced neuronal cytolysis through PI3K signaling in children with opsoclonus-myoclonus syndrome and neuroblastoma. Pediatr Res 85, 885–894 (2019). https://doi.org/10.1038/s41390-018-0251-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-018-0251-1