Abstract
Background
In sick neonates admitted to the NICU, improper fluid balance can lead to fluid overload. We report the impact of fluid balance in the first postnatal week on outcomes in critically ill near-term/term neonates.
Methods
This analysis includes infants ≥36 weeks gestational age from the Assessment of Worldwide Acute Kidney injury Epidemiology in Neonates (AWAKEN) study (N = 645). Fluid balance: percent weight change from birthweight. Primary outcome: mechanical ventilation (MV) on postnatal day 7.
Results
The median peak fluid balance was 1.0% (IQR: −0.5, 4.6) and occurred on postnatal day 3 (IQR: 1, 5). Nine percent required MV at postnatal day 7. Multivariable models showed the peak fluid balance (aOR 1.12, 95%CI 1.08–1.17), lowest fluid balance in 1st postnatal week (aOR 1.14, 95%CI 1.07–1.22), fluid balance on postnatal day 7 (aOR 1.12, 95%CI 1.07–1.17), and negative fluid balance at postnatal day 7 (aOR 0.3, 95%CI 0.16–0.67) were independently associated with MV on postnatal day 7.
Conclusions
We describe the impact of fluid balance in critically ill near-term/term neonates over the first postnatal week. Higher peak fluid balance during the first postnatal week and higher fluid balance on postnatal day 7 were independently associated with MV at postnatal day 7.
Similar content being viewed by others
Introduction
In sick neonates admitted to the neonatal intensive care unit (NICU), fluid balance depends on multiple factors including fluid provision, insensible losses, and renal function. Furthermore, these neonates have immature renal homeostatic mechanisms which in conjunction with critical illness can predispose to abnormalities in fluid balance including fluid overload (FO) or dehydration. Fluid balance can be adversely impacted by acute kidney injury (AKI), which has been shown to occur commonly in critically ill neonates. Single center studies report an AKI incidence of 15%–70% depending on the population under study, with associated adverse outcomes (increased mortality, length of stay).1,2,3,4,5,6 The Assessment of Worldwide Acute Kidney Injury Epidemiology in Neonates (AWAKEN) study, a 3 month, 24-center retrospective study,7 confirmed these findings and showed that AKI occurred in 30% of NICU admissions. Those with AKI had over 4 times higher adjusted odds of mortality compared to those without AKI.8 Despite our acknowledgement of the impact of neonatal AKI on mortality, there remain no interventions to prevent or treat established AKI. As a result, current neonatal AKI management is focused on preventing further AKI, optimizing nutrition, monitoring electrolytes, and minimizing the development of FO.
Over the past decade pediatricians have been at the forefront of recognizing the deleterious impact of FO on outcomes in a variety of critically ill pediatric populations including septic patients and those who receive continuous renal replacement therapy, mechanical ventilation, and congenital heart surgery.9,10,11,12,13,14,15,16,17 FO represents an attractive metric that can drive clinical practice as a target that is potentially amenable to both prevention or treatment by judicious fluid provision and early renal support therapy. While it is anticipated that well neonates will have a negative fluid balance of up to 5–10% over the first postnatal week, the optimal target for fluid balance in the first postnatal week in critically ill neonates is unknown. Preliminary research in premature infants suggests that abnormalities in fluid balance are associated with the development of chronic lung disease.18 Improving our understanding of the distribution of, and outcomes related to fluid balance in the first postnatal week in critically ill neonates will help delineate the most appropriate fluid provision strategies. To date there has not been a large multicenter evaluation of the epidemiology and impact of fluid balance in any NICU population.
In 2014, the Neonatal Kidney Collaborative with 24 participating institutions from around the world conducted the AWAKEN study, the first multicenter study designed to capture data on AKI, fluid balance, and the impact of other renal-related risk factors on short term outcomes.8 Using data collected in AWAKEN, we sought to1 describe the pattern of changes in fluid balance in sick near-term/ term neonates during the first postnatal week,2 investigate the association of fluid balance with clinical outcomes (mechanical ventilation at postnatal day 7 and NICU mortality), and3 evaluate the association of fluid balance with AKI. We hypothesized that fluid balance during the first postnatal week would be associated with the need for (1) mechanical ventilation at postnatal day 7, (2) hospital mortality, and (3) composite outcome of death or mechanical ventilation at postnatal day 28.
Methods
Study population
The methodology and protocol for the AWAKEN study have previously been published.7 Briefly, the records for all neonates admitted to 24 level 2–4 NICUs between 1 January 2014 and 31 March 2014, were reviewed. Inclusion criteria for enrollment was: (1) receipt of intravenous fluids (IV) for at least 48 h. Exclusion criteria included: (1) admission at ≥14 days after birth, (2) congenital heart disease requiring surgical repair at <postnatal day 7, (3) lethal chromosomal anomaly, (4) death within 48 h of NICU admission, and (5) severe congenital kidney and urinary tract abnormalities. We limited this analysis to those patients with gestational age ≥36 weeks, admission at ≤postnatal day 7, data sufficient to calculate AKI, and recorded weight by postnatal day 2. Weight outliers were reviewed by blinded principal investigators and those with weights that were thought to be invalid (impossible) were excluded. Each center involved in the study had received approval from their Institutional Review Board or Human Research Ethics Committee.
Data collection
A detailed description of data collection for AWAKEN has been previously published.7 Briefly, data were organized in five components: baseline demographics, daily information for week 1, weekly “snapshots” for the remainder of the hospitalization, and discharge data (captured at discharge or 120 days of age, whichever came first). Day of birth was defined as postnatal day 1.
Fluid balance and FO definitions
The AWAKEN study protocol included abstraction of recorded daily weights, intake (intravenous and enteral fluids), and outputs (urine and total output, including all other documented fluid loss) for the first 7 postnatal days, when available. Documentation methods for intake and output were subject to local protocols.
Fluid intake included blood products, intravenous fluids and flushes, medications and all forms of nutritional support. Fluid output included urine output, drain output, blood loss, nasogastric tube output, stool volume, and wound drainage. Not all sites recorded all potential sources of intake and the amount of maternal breast milk ingested at breast could not be quantified. Similarly, quantification of urine output, usually based on diaper weights, as well as other sources of fluid loss were potentially incomplete. Therefore, the decision to use daily weight as a surrogate for fluid balance was made as the most feasible and reliable method in the AWAKEN cohort (analysis outlined below). Fluid balance was thus calculated based on a comparison of daily weight with birthweight: %change = (daily weight − birthweight)/birthweight × 100. We calculated the maximum and minimum weight change during the first postnatal week, %change from birthweight on postnatal days 3 and 7, and the highest and lowest change from birthweight during the first postnatal week.
The following 4 variables to capture fluid balance metrics were calculated: (a) the maximum %weight change that occurred on postnatal days 2–7 of age, (b) the minimum %weight change that occurred on postnatal days 2–7 of age (c) the % weight change at postnatal day 3, (d) the %weight change at postnatal day 7, and (e) negative fluid balance at postnatal day 7. The negative fluid balance at postnatal day 7 was defined as being below birthweight at postnatal day 7.
AKI definition
All serum creatinine (SCr) values obtained during the study period were recorded. A neonatal modification of the kidney disease: improving Global Outcomes workgroup AKI was used to define neonatal AKI.8,19 Based on this criteria AKI was defined as urine output <1 cc/kg/hr in the first 7 days and/or SCr rise ≥0.3 mg/dl or 50% from previous trough. The urinary output threshold for AKI was set at 1 mL/kg per h or less averaged over 24 h normalized for the weight on the day urine output was measured.8 AKI was evaluated as a risk factor for aberrant fluid balance throughout the manuscript
Outcomes
The primary outcome for this study was the need for mechanical ventilation (high frequency ventilation, or conventional ventilation) or ECMO at postnatal day 7. The secondary outcomes were NICU mortality and a composite outcome of death or mechanical ventilation at postnatal day 28.
Statistical analysis
Categorical variables were analyzed by proportional differences with the Chi-square test or Fisher exact test (where appropriate). Continuous variables were tested for normality using the Shapiro–Wilk Test. For normally distributed continuous variables, the mean ± standard deviation (SD) were reported and analyzed using the Student t-test. For non-normally distributed variables, the median and interquartile range (IQR) were reported and groups were compared using the Wilcoxon Rank Sum test. Logistic regression models were used to calculate crude odds ratios (OR) and associated 95% confidence intervals (CI) for the association between maximum fluid balance, minimum fluid balance, fluid balance on Day 3, and fluid balance on Day 7 with the likelihood of mechanical ventilation at day 7. Multivariable logistic regression models were run to account for potential confounding variables, and findings are reported as adjusted OR. Adjusted regression models were constructed using a backwards selection procedure with a significance level of <0.2. Separate regression models were created for each of the four main exposures of interest. In all analyses, a p-value < 0.05 was considered statistically significant. SAS 9.4 (Cary, North Carolina) was used for all analyses.
Results
Patient characteristics
The AWAKEN study screened a total of 4273 neonates, of whom 2162 met the AWAKEN inclusion criteria, and 2022 had the required lab data to diagnose AKI. Of these, 833 infants were ≥36 weeks gestational age, with 148 of these neonates excluded because no weight was available during the first two days of ICU admission. A total of 645 neonates were included in the final analysis (Supplemental Figure S1). A comparison between the study population and those who were excluded showed similar rates of mechanical ventilation at postnatal day 7, but higher mortality in those that who excluded (Supplemental Table S1).
Table 1 summarizes the baseline characteristics of the study population as a whole and dichotomized by need for mechanical ventilation on postnatal day 7. A total of 125 neonates were intubated during resuscitation. The median birthweight was 3140 grams (IQR: 2665, 3530 grams). A total of 530 (82.1%) infants had birthweight ≥2500 grams. The median 1-minute Apgar score was 7 (IQR: 4, 8) and the 5-minute Apgar score was 9 (IQR: 7, 9). The most common admission diagnoses for the infants in this study were respiratory diagnoses (27.9%), hypoxicischemic encephalopathy (12.2%), and/or intrauterine growth restriction (7.9%) and rule-out sepsis (44.8%). During the first postnatal week a total of 51 (7.9%) neonates received vasopressor support, and 403 (62.5%) neonates were treated with antimicrobials. Those who were ventilated at postnatal day 7 were more likely to have received chest compressions during resuscitation, had a lower 1-minute Apgar score, required admission for respiratory failure or congenital heart disease, and received vasopressors in the first postnatal week (Table 1).
Fluid balance
For the cohort there were 3870 potential days of data available for the first 7 postnatal days. On 74.3% of days there were complete intake and output data. A weight was available on 80.5% of the possible days. A description of the peak fluid balance in the first postnatal week stratified by MV at postnatal day 7 is presented in Table 2. The median peak fluid change from birth during the first postnatal week was 1.0% (IQR: −0.5, 4.6) and occurred on postnatal day 3 (IQR: 1, 5). The median lowest fluid balance during the first postnatal week was −3.1% (IQR: −5.6, −0.6) and occurred on postnatal day 3 (IQR: 2, 5). During the first postnatal week, 66.8% of the cohort had at least one day with a peak fluid balance above birthweight; 13.6% of patients developed a peak fluid balance ≥5% and 5.7% of patients developed a peak fluid balance ≥10% (Table 2). The characteristics of fluid balance presented by admission diagnosis during the first postnatal week are summarized in Supplemental Table S2.
Fluid balance by AKI status
AKI occurred in 25.7% (N = 166) of patients during the first postnatal week, of whom 13.9% (N = 90) had stage 1, 5.4% had stage 2 (N = 35), and 6.3% (N = 41) had stage 3 AKI. Eight (1.2%) patients in this cohort received renal replacement therapy. The characteristics of fluid balance in the first postnatal week by AKI status are presented in Table 3. Those with AKI had consistently higher fluid balance irrespective of the day on which fluid status was assessed during the first postnatal week.
Association of fluid balance with outcomes
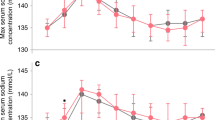
A total of 9% of patients required mechanical ventilation on postnatal day 7. The association between maternal and patient characteristics with need for mechanical ventilation on postnatal day 7 are summarized in Table 1. A description of the distribution of peak fluid balance by outcomes is outlined in Table 2. The associations between measures of fluid balance and the need for mechanical ventilation on postnatal day 7 are summarized in Table 4. Peak fluid balance, lowest fluid balance, fluid balance on postnatal day 3, and fluid balance on postnatal day 7 were all significantly higher among patients who required mechanical ventilation on postnatal day 7 compared to those who did not require mechanical ventilation. The median fluid balance by day in those who did and did not require mechanical ventilation on postnatal day 7 is shown in Fig. 1. The probability of mechanical ventilation on postnatal day 7 as determined by peak fluid balance during the first postnatal week and the fluid balance on postnatal day 7 are presented in Supplemental Figures S2 & S3.
In the unadjusted logistic regression analysis, fluid balance over the first postnatal week, including a negative balance at postnatal day 7, was independently associated with the need for mechanical ventilation on postnatal day 7. After adjusting for confounding variables, each fluid balance measure (peak fluid balance during the 1st postnatal week, lowest fluid balance during the 1st postnatal week, fluid balance on postnatal day 3, fluid balance postnatal day 7, and negative fluid balance at postnatal day 7) remained independently associated with the need for mechanical ventilation on postnatal day 7 (Table 5). The multivariable analyses showed that after adjusting for AKI, admission for respiratory failure or sepsis, for every 1% rise in maximum fluid balance there was a 12% increased odds of receiving mechanical ventilation at postnatal day 7 (adjusted OR 1.12, 95%CI: 1.08–1.17; p < 0.0001). Similarly, after controlling for the same variables, for every 1% increase in the fluid balance nadir in the first postnatal week there was a 14% increased odds of mechanical ventilation at postnatal day 7 (adjusted OR 1.14, 95%CI: 1.07–1.22; p < 0.0001). Furthermore, those with a negative fluid balance on postnatal day 7 had significantly lower odds of being mechanically ventilated on postnatal day 7 (adjusted OR 0.33, 95%CI: 0.16–0.67; p < 0.0001).
Survival to NICU discharge for the cohort was 98.3% (N = 634). The associations of different measures of fluid balance with NICU survival are shown in Supplemental Table S3. Briefly, only the lowest fluid balance in the first week was associated with improved survival on bivariate analysis. This was not significant on multivariable analysis.
In evaluating the composite outcome of interest, 96.6% of the cohort was alive and free of mechanical ventilation at postnatal day 28. The associations of different measures of fluid balance with the composite outcome are shown in Supplemental Table S4 and Supplemental Table S5. Briefly, there were no significant associations with fluid balance and the composite outcome, albeit that the number of patient with composite outcome of death/MV at day 28 was very small (N = 22).
Discussion
In this analysis of the multicenter, multinational, retrospective cohort study of critically ill neonates AWAKEN study we report the epidemiology of fluid balance and its impact on outcomes in the first postnatal week in critically ill term/near-term neonates. In 66.8% of patients there was a positive peak fluid balance in the first postnatal week with 23.5% having a peak fluid balance of ≥5%. Our data confirm that of prior studies; AKI is associated with the development of abnormalities in fluid balance. Furthermore, the current study shows that fluid balance during the first postnatal week is independently associated with the need for mechanical ventilation on postnatal day 7 irrespective of the presence of AKI.
The concept of FO has been well studied in a multitude of pediatric and adult populations and consistently been shown to be associated with adverse outcomes including prolonged mechanical ventilation, increased length of stay, and increased mortality.9,10,11,12,13,14,15,16,17,20,21,22,23,24,25 In critically ill neonates the concept of FO is confounded by the fact that healthy term neonates lose as much as 5–10% of their birthweight over the first postnatal week. Furthermore, altered fluid balance during the first postnatal week may have different etiologies and implications for critically ill neonates of different gestational ages. In a study of 58 critically ill near-term/term neonates, Askenazi et al demonstrated that abnormalities in fluid balance were common, with a median fluid balance excess of 8.2% on postnatal day 3 in those with AKI. However, abnormalities in fluid balance also existed in those without AKI, and suggests that FO may impact patient outcome, even in the absence of AKI.1 This study did not evaluate the independent association of FO with outcomes. We extend these findings by showing that abnormalities in fluid balance are common in a cohort of 645 critically ill near-term/term neonates with over 60% having a positive fluid balance during the first postnatal week. We also show that the peak fluid balance during the first postnatal week is independently associated with the adverse outcome of need for mechanical ventilation on postnatal day 7. Perhaps more importantly we show that the ability to achieve a negative fluid balance at postnatal day 7 is associated with being ventilator free. We hypothesize that the etiology for this aberrant fluid balance is likely multifactorial in nature with contributions from AKI, extensive fluid provision and excess antidiuretic hormone secretion. Despite the likely multifactorial etiology, preventing FO during the first postnatal week may be a potential therapeutic target for improving outcomes, of which further study is warranted.
Over the past decade the epidemiology of AKI and its impact on outcomes in critically ill neonates has become clear. There have been numerous single center studies that have clearly shown that AKI occurs commonly and has consistently been shown to be associated with adverse outcomes. These research efforts have culminated in the publication of the AWAKEN study, which clearly demonstrated the association of AKI with adverse outcomes. An important finding in this study is that those with AKI were more likely to have higher fluid balance at each point evaluated over the first postnatal week. Impressively over 75% of those with AKI had a positive peak fluid balance during the first postnatal week, and 25% had a peak fluid balance of ≥7.4%. Our study also clearly shows that that abnormalities in fluid balance are independently associated with the adverse outcome of needing mechanical ventilation on postnatal day 7, even after adjusting for the presence of AKI. These data suggest that alternative fluid management strategies may be warranted in those who develop AKI and daily fluid balance should be discussed on each patient, particularly those with AKI. In addition, consideration for correction of serum creatinine for fluid balance, which has been proven to be useful in neonates after cardiac surgery by unmasking additional cases of AKI may also be beneficial in other critically ill neonates.26
The measurement of fluid balance in children has classically been performed by a cumulative fluid balance method, or based on changes in weight from a baseline, with both methods demonstrating an association of FO with adverse outcomes.13,14,20,23,24 Utilization of these methods may prove challenging in neonates for several reasons including but not limited to; the need for daily measurement, the presence of insensible losses, difficulty of precisely measuring breast milk intake and urine output. As part of this study we systematically evaluated the recording of daily fluid balance over the first postnatal week and showed that each method had full data recorded on 74.3% of days. While on the surface this would seem equivalent, the absence of accurate in and out data for all days makes an accurate fluid balance impossible to calculate. Weight-based methodology in neonates allows for missed days and still provides the ability to accurately calculate fluid balance on subsequent days despite missed measurements. Our findings are consistent with a previous report in critically ill neonates that showed daily ins and outs were inaccurate.27 The current study emphasizes the need for standardized measurement protocols to accurately assess fluid balance and identifies daily weights as an area for improvement.
The largest strength of this study is the number and variety of centers involved in this international multicenter study providing ample sample size to explore the impact of fluid balance accounting for confounders in the association with outcomes. Despite this strength, we acknowledge several limitations to this study. A limitation inherent to any chart review is the reliance on available data from the medical record including serum creatinine, urine output, and fluid composition. This may have resulted in missed cases of AKI. Furthermore, daily weights were not available for all days in the NICU, which may have impacted the results, although subsequent days’ weights were utilized to account for this. It is also important to note that because of the inclusion and exclusion criteria, the healthiest neonates were not included in this which may limit generalizability to all neonates in the NICU. An important limitation of this study is that the daily data did not extend past postnatal day 7 limiting our detailed evaluation and conclusions. In future studies it will be important to extend the observation period and confirm our findings.
Conclusion
In this retrospective multicenter cohort study, we describe for the first time the distribution and impact of fluid balance in critically ill near-term/term neonates over the first postnatal week. In a cohort that may lose 5–10% of their body weight over the first postnatal week, we show that over 60% of the cohort had a positive peak fluid balance in the first postnatal week. The current study shows that the peak fluid balance and fluid balance on postnatal day 7 were independently associated with the need for mechanical ventilation on postnatal day 7. Furthermore, on adjusted analysis the fluid balance on postnatal day 7 and the inability to achieve a negative fluid balance at postnatal day 7 were the strongest predictors for the need for mechanical ventilation at postnatal day 7. Future prospective studies designed to evaluating different fluid strategies are warranted to determine their impact on outcomes.
Disclaimer
For full disclosure, we provide here an additional list of other author’s commitments and funding sources that are not directly related to this study: D.J.A. serves on the speaker board for Baxter (Baxter, USA) and the Acute Kidney Injury (AKI) Foundation (Cincinnati, OH, USA); he also receives grant funding for studies not related to this manuscript from National Institutes of Health—National Institutes of Diabetes and Digestive and Kidney Diseases (NIH-NIDDK, R01 DK103608 and NIH-FDA (R01 FD005092).
References
Askenazi, D., Griffin, R., McGwin, G., Carlo, W. & Ambalavanan, N. Acute kidney injury is independently associated with mortality in very low birthweight infants: a matched case-control analysis. Pediatr. Nephrol. 24, 991–997 (2009).
Gadepalli, S. K., Selewski, D. T., Drongowski, R. A. & Mychaliska, G. B. Acute kidney injury in congenital diaphragmatic hernia requiring extracorporeal life support: an insidious problem. J. Pediatr. Surg. 46, 630–635 (2011).
Sarkar, S. et al. Relationship between acute kidney injury and brain MRI findings in asphyxiated newborns after therapeutic hypothermia. Pediatr. Res. 75, 431–435 (2014).
Selewski, D. T., Jordan, B. K., Askenazi, D. J., Dechert, R. E. & Sarkar, S. Acute kidney injury in asphyxiated newborns treated with therapeutic hypothermia. J. Pediatr. 162, 725–729 e721 (2013).
Koralkar, R. et al. Acute kidney injury reduces survival in very low birth weight infants. Pediatr. Res. 69, 354–358 (2011).
Zwiers, A. J. et al. Acute kidney injury is a frequent complication in critically ill neonates receiving extracorporeal membrane oxygenation: a 14-year cohort study. Crit. Care. 17, R151 (2013).
Jetton, J. G. et al. Assessment of worldwide acute kidney injury epidemiology in neonates: design of a retrospective cohort study. Front. Pediatr. 4, 68 (2016).
Jetton, J. G. et al. Incidence and outcomes of neonatal acute kidney injury (AWAKEN): a multicentre, multinational, observational cohort study. Lancet Child Adolesc. Health 1, 184–194 (2017).
Arikan, A. A. et al. Fluid overload is associated with impaired oxygenation and morbidity in critically ill children. Pediatr. Crit. Care. Med. 13, 253–258 (2012).
Bhaskar, P., Dhar, A. V., Thompson, M., Quigley, R. & Modem, V. Early fluid accumulation in children with shock and ICU mortality: a matched case-control study. Intensive Care Med. 41, 1445–1453 (2015).
Flori, H. R., Church, G., Liu, K. D., Gildengorin, G. & Matthay, M. A. Positive fluid balance is associated with higher mortality and prolonged mechanical ventilation in pediatric patients with acute lung injury. Crit. Care Res. Pract. 2011, 854142 (2011).
Foland, J. A. et al. Fluid overload before continuous hemofiltration and survival in critically ill children: a retrospective analysis. Crit. Care Med. 32, 1771–1776 (2004).
Goldstein, S. L. et al. Outcome in children receiving continuous venovenous hemofiltration. Pediatrics 107, 1309–1312 (2001).
Goldstein, S. L. et al. Pediatric patients with multi-organ dysfunction syndrome receiving continuous renal replacement therapy. Kidney Int. 67, 653–658 (2005).
Hassinger, A. B., Wald, E. L. & Goodman, D. M. Early postoperative fluid overload precedes acute kidney injury and is associated with higher morbidity in pediatric cardiac surgery patients. Pediatr. Crit. Care. Med. 15, 131–138 (2014).
Hayes, L. W., Oster, R. A., Tofil, N. M. & Tolwani, A. J. Outcomes of critically ill children requiring continuous renal replacement therapy. J. Crit. Care 24, 394–400 (2009).
Hazle, M. A., Gajarski, R. J., Yu, S., Donohue, J. & Blatt, N. B. Fluid overload in infants following congenital heart surgery. Pediatr. Crit. Care. Med. 14, 44–49 (2013).
Schmidt, B. et al. Indomethacin prophylaxis, patent ductus arteriosus, and the risk of bronchopulmonary dysplasia: further analyses from the Trial of Indomethacin Prophylaxis in Preterms (TIPP). J. Pediatr. 148, 730–734 (2006).
Zappitelli, M. et al. Developing a neonatal acute kidney injury research definition: a report from the NIDDK neonatal AKI workshop. Pediatr. Res. 82, 569–573 (2017).
Askenazi, D. J. et al. Fluid overload and mortality are associated with acute kidney injury in sick near-term/term neonate. Pediatr. Nephrol. 28, 661–666 (2013).
Payen, D. et al. A positive fluid balance is associated with a worse outcome in patients with acute renal failure. Crit. Care. 12, R74 (2008).
Seguin, J. et al. Extent, risk factors, and outcome of fluid overload after pediatric heart surgery*. Crit. Care Med. 42, 2591–2599 (2014).
Selewski, D. T. et al. Fluid overload and fluid removal in pediatric patients on extracorporeal membrane oxygenation requiring continuous renal replacement therapy. Crit. Care Med. 40, 2694–2699 (2012).
Selewski, D. T. et al. Weight-based determination of fluid overload status and mortality in pediatric intensive care unit patients requiring continuous renal replacement therapy. Intensive Care Med. 37, 1166–1173 (2011).
Sutherland, S. M. et al. Fluid overload and mortality in children receiving continuous renal replacement therapy: the prospective pediatric continuous renal replacement therapy registry. Am. J. Kidney Dis. 55, 316–325 (2010).
Basu, R. K. et al. Acute kidney injury based on corrected serum creatinine is associated with increased morbidity in children following the arterial switch operation. Pediatr. Crit. Care. Med. 14, e218–e224 (2013).
van Asperen, Y., Brand, P. L. & Bekhof, J. Reliability of the fluid balance in neonates. Acta Paediatr. 101, 479–483 (2012).
Acknowledgements
The authors would also like to thank the outstanding work of the following clinical research personnel and colleagues for their involvement in AWAKEN: Ariana Aimani, Samantha Kronish, Ana Palijan, MD, and Michael Pizzi—Montreal Children’s Hospital, McGill University Health Centre, Montreal, Quebec, Canada. Laila Ajour, BS, Julia Wrona, BS—University of Colorado, Children’s Hospital Colorado, Aurora, Colorado. Melissa Bowman, RN—University of Rochester, Rochester, New York. Teresa Cano, RN, Marta G. Galarza, MD, Wendy Glaberson, MD, Aura Arenas Morales, MD, Denisse Cristina Pareja Valarezo, MD—Holtz Children’s Hospital, University of Miami, Miami, Florida. Sarah Cashman, BS, Madeleine Stead, BS—University of Iowa Children’s Hospital, Iowa City, Iowa. Jonathan Davis, MD, Julie Nicoletta, MD—Floating Hospital for Children at Tufts Medical Center, Tufts University School of Medicine, Boston, Massachusetts. Alanna DeMello—British Columbia Children’s Hospital, Vancouver, British Columbia, Canada. Lynn Dill, RN—University of Alabama at Birmingham, Birmingham, Alabama. Ellen Guthrie, RN—MetroHealth Medical Center, Case Western Reserve University, Cleveland, Ohio. Nicholas L. Harris, BS, Susan M. Hieber, MSQM—C.S. Mott Children’s Hospital, University of Michigan, Ann Arbor, Michigan. Katherine Huang, Rosa Waters—University of Virginia Children’s Hospital, Charlottesville, Virginia. Judd Jacobs, Ryan Knox, BS, Hilary Pitner, MS, Tara Terrell— Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio. Nilima Jawale, MD—Maimonides Medical Center, Brooklyn, New York. Emily Kane—Australian National University, Canberra, Australia. Vijay Kher, DM, Puneet Sodhi, MBBS—Medanta Kidney Institute, The Medicity Hospital, Gurgaon, Haryana, India. Grace Mele—New York College of Osteopathic Medicine, Westbury, New York. Patricia Mele, DNP—Stony Brook Children’s Hospital, Stony Brook, New York. Charity Njoku, Tennille Paulsen, Sadia Zubair—Texas Children’s Hospital, Baylor College of Medicine, Houston, Texas. Emily Pao—University of Washington, Seattle Children’s Hospital, Seattle, Washington. Becky Selman, RN, Michele Spear, CCRC—University of New Mexico Health Sciences Center Albuquerque, New Mexico. Melissa Vega, PA-C—The Children’s Hospital at Montefiore, Bronx, New York, USA. Leslie Walther, RN—Washington University, St. Louis, Missouri. Funding sources: Cincinnati Children’s Hospital Center for Acute Care Nephrology provided funding to create and maintain the AWAKEN Medidata Rave electronic database. The Pediatric and Infant Center for Acute Nephrology (PICAN) provided support for web meetings, for the NKC steering committee annual meeting at the University of Alabama at Birmingham (UAB), as well as support for some of the AWAKEN investigators at UAB (LJB., R.G.). PICAN is part of the Department of Pediatrics at the University of Alabama at Birmingham (UAB), and is funded by Children’s of Alabama Hospital, the Department of Pediatrics, UAB School of Medicine, and UAB’s Center for Clinical and Translational Sciences (CCTS, NIH grant UL1TR001417). Finally, the AWAKEN study at the University of New Mexico was supported by the Clinical and Translational Science Center (CTSC, NIH grant UL1TR001449) and by the University of Iowa Institute for Clinical and Translational Science (U54TR001356). C.L.A. was supported by the Micah Batchelor Foundation. A.A.A. and C.J.R. were supported by the Section of Pediatric Nephrology, Department of Pediatrics, Texas Children’s Hospital. J.R.C. and J.R.S. were supported by a grant from 100 Women Who Care. F.S.C. and K.T.D. were supported by the Edward Mallinckrodt Department of Pediatrics at Washington University School of Medicine. J.F. and A.K. supported by the Canberra Hospital Private Practice Fund. R.G. and E.R. were supported by the Department of Pediatrics, Golisano Children’s Hospital, University of Rochester. P.E.R. was supported by R01 HL-102497, R01 DK 49419. S.S. and D.T.S. were supported by the Department of Pediatrics & Communicable Disease, C.S. Mott Children’s Hospital, University of Michigan. S.S. and R.W. were supported by Stony Brook Children’s Hospital Department of Pediatrics funding.
AWAKEN investigators
The following individuals served as collaborators and site investigators for the AWAKEN study and are collaborators on this manuscript and should be indexed in PubMed as collaborators on this manuscript (named authors above have been removed from this list): Sunny Juul41, Namasivayam Ambalavanan16, Subrata Sarkar17, Alison Kent18, Jeffery Fletcher18, Carolyn L. Abitbol19, Marissa DeFreitas19, Shahnaz Duara19, Jennifer R. Charlton20, Jonathan R. Swanson20, Carl D’Angio21, Ayesa Mian21, Erin Rademacher21, Maroun J. Mhanna22, Rupesh Raina22, Deepak Kumar22, Jennifer G. Jetton23, Patrick D. Brophy23, Tarah T. Colaizy23, Jonathan M. Klein23, Christopher J. Rhee24, Juan C. Kupferman25, Alok Bhutada25, Shantanu Rastogi25, Susan Ingraham26, F. Sessions Cole27, T. Keefe Davis27, Lawrence Milner28, Alexandra Smith28, Mamta Fuloria29, Frederick J. Kaskel29, Danielle E. Soranno30, Jason Gien30, Aftab S. Chishti31, Sangeeta Hingorani32, Michelle Starr32, Craig S. Wong33, Tara DuPont33, Robin Ohls33, Surender Khokhar34, Sofia Perazzo35, Patricio E. Ray35, Mary Revenis35, Sidharth K. Sethi36, Smriri Rohatgi36, Cherry Mammen37, Anne Synnes37, Sanjay Wazir38, Michael Zappitelli39, Robert Woroniecki40, Shanty Sridhar40
Author information
Authors and Affiliations
Consortia
Contributions
All listed authors provided substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data, drafting the article or revising it critically for and final approval of the version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Members of the AWAKEN study group are listed at the end of the paper.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Selewski, D.T., Akcan-Arikan, A., Bonachea, E.M. et al. The impact of fluid balance on outcomes in critically ill near-term/term neonates: a report from the AWAKEN study group. Pediatr Res 85, 79–85 (2019). https://doi.org/10.1038/s41390-018-0183-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-018-0183-9
This article is cited by
-
Use of furosemide in preterm neonates with acute kidney injury is associated with increased mortality: results from the TINKER registry
Pediatric Nephrology (2024)
-
Fluid assessment, fluid balance, and fluid overload in sick children: a report from the Pediatric Acute Disease Quality Initiative (ADQI) conference
Pediatric Nephrology (2024)
-
Improving acute peritoneal dialysis outcome with use of soft peritoneal dialysis catheter (Cook Mac-Loc Multipurpose Drainage catheter®) among infants < 1500 g in a low resource setting
Pediatric Nephrology (2023)
-
Neonatal fluid overload—ignorance is no longer bliss
Pediatric Nephrology (2023)
-
An update on the role of fluid overload in the prediction of outcome in acute kidney injury
Pediatric Nephrology (2023)