Abstract
Background
Early identification of infants at risk for complications from patent ductus arteriosus (PDA) may improve treatment outcomes. The aim of this study was to identify biochemical markers associated with persistence of PDA, and with failure of pharmacological treatment for PDA, in extremely preterm infants.
Methods
Infants born at 22–27 weeks’ gestation were included in this prospective study. Blood samples were collected on the second day of life. Fourteen biochemical markers associated with factors that may affect PDA closure were analyzed and related to persistent PDA and to the response of pharmacological treatment with ibuprofen.
Results
High levels of B-type natriuretic peptide, interleukin-6, -8, -10, and -12, growth differentiation factor 15 and monocyte chemotactic protein 1 were associated with persistent PDA, as were low levels of platelet-derived growth factor. High levels of erythropoietin were associated with both persistent PDA and failure to close PDA within 24 h of the last dose of ibuprofen.
Conclusions
High levels of inflammatory markers were associated with the persistence of PDA. High levels of erythropoietin were associated with both the persistence of PDA and failure to respond to pharmacological treatment.
Similar content being viewed by others
INTRODUCTION
Prolonged patency of the ductus arteriosus continues to be a challenging problem in the care of extremely preterm infants.1 The decision to treat a patient with patent ductus arteriosus (PDA) is usually based on the perceived hemodynamic significance of the PDA; the general first option of management is pharmacological treatment. However, without clear evidence of a reduction in morbidity or mortality, the indications for treatment of PDA remain controversial.2,3
Clinical signs do not reliably assert the hemodynamic significance or the risk for complications of PDA.4 Echocardiography is the current reference standard for evaluating the hemodynamics of the ductus arteriosus, but there is no consensus regarding criteria for determining the hemodynamic significance, and evidence of any association with outcome in extremely preterm infants is scant.5,6 Plasma natriuretic peptides and cardiac troponin T have previously been correlated with echocardiographic measures of ductal shunt volume and to morbidity.7,8 Nevertheless, the ability to quickly and accurately identify infants with prolonged patency and risk for complications from PDA, and to predict the probability of success of pharmacological treatment, is limited.9
The use of multiplex proximity extension assay (PEA) technology allows for the analysis of an extensive range of serum protein markers from the limited blood volume that can be collected from preterm infants.10 In the current literature, we have identified several potential markers for persistence of PDA, known to be associated with either ductal constriction, pulmonary circulatory regulation, vascular remodeling, or inflammation (Table 1).
As previously recognized, echocardiographic signs of hemodynamic changes during the second and third day of life are associated with persistent PDA; therefore, analyses of biochemical markers during this same period could potentially identify alternative indicators for persistent PDA or treatment-resistant PDA in extremely preterm infants.11,12 The aim of this exploratory study was to assess potential associations between the serum protein markers outlined above and PDA outcome, including persistence and treatment response, in premature infants born at 22–27 weeks’ gestational age (GA).
METHODS
Study Population
This prospective observational study was approved by the Swedish Central Ethical Review Board. Infants eligible for inclusion were born at Uppsala University Children’s Hospital between November 2012 and May 2015 with a GA of less than 28 weeks, and without heart defects or major congenital anomalies. Infants were enrolled after informed, written consent was obtained from the parents.
Echocardiography
Infants underwent echocardiographic examinations during the first, second, and third day of life as a part of the study protocol. Additional examinations were made within 24 h of treatment completion, if the infant’s clinical condition called for it, and before discharge. All examinations were conducted and interpreted by the same examiner (A.J.). The assessments for hemodynamically significant PDA were made available to the treating physicians, but records of the specific measurements for the study were kept separate and were not available to the clinical teams.
Internal diameter of the ductus arteriosus was measured at its narrowest point from the parasternal short axis view in 2-dimensional and color Doppler mode. Ductal flow velocity was assessed with pulsed and continuous Doppler directly in line with the ductal flow from the same position. Left atrium to aortic root (LA/Ao) ratio was measured in M-mode from the parasternal long axis. Absent or reversed flow in the descending aorta was assessed using color and pulsed Doppler above and below the ductal orifice.
Treatment of ductus arteriosus
PDA was considered hemodynamically significant if shunting across the ductus arteriosus was predominantly left-to-right, and at least one of the following criteria were met: (1) ductal diameter of >1.5 mm, (2) LA/Ao of >1.5, or (3) an appropriate view of the descending aorta showing absent or reversed flow during diastole.
In accordance with routine clinical policy, pharmacological treatment was initiated after the first day, but before the seventh day of life, when a hemodynamically significant PDA was identified and none of the following contraindications were present: renal failure (serum creatinine >120 µmol/L or serum urea >12 mmol/L); thrombocytopenia (platelets <50 × 109/L); recent intraventricular hemorrhage grade II–IV; or necrotizing enterocolitis. Surgical ligation was considered if pharmacological treatment was contraindicated and a hemodynamically significant PDA continued to persist.
Ibuprofen (5 mg/mL, Pedea, Orphan Europe Nordic, Stockholm, Sweden) was administered in a three-dose regimen with the first intravenous infusion of 10 mg/kg over 20 min and subsequent doses of 5 mg/kg at 24 and 48 h after the initial dose. No additional pharmacological treatment for PDA was administered after this initial three-dose course. Surgical ligation was carried out if a hemodynamically significant PDA persisted or if the ductus arteriosus reopened after pharmacological treatment.
Persistence of PDA
Iterating echocardiographic examinations were performed after the first seven days of life in (1) infants with an open but hemodynamically insignificant PDA during the first seven days of life, (2) infants that did not receive pharmacological treatment because of contraindications, and (3) infants that did not respond to pharmacological treatment. PDA was defined as persistent if a hemodynamically significant PDA was found or remained, and the infant was consequently subjected to surgical ligation. For infants that died, PDA was defined as persistent if an autopsy revealed an open ductus arteriosus. For all other infants, PDA was considered hemodynamically insignificant.
Response to pharmacological treatment
Possible associations between biochemical markers and responsiveness to pharmacological treatment, defined as functional constriction allowing for the anatomical closure of the ductus arteriosus, were explored. PDA was considered responsive to treatment if echocardiography within 24 h of pharmacological treatment could not identify any ductal flow. PDA was otherwise considered unresponsive.
Biochemical markers
Blood samples were collected from indwelling umbilical arterial catheters at the time of the echocardiographic examination during the second day of life. A minimum volume of 20 µL was required for the PEA analyses. After centrifugation at 2400 × g for 7 min, the supernatant serum was obtained and stored at −80 °C until further analysis.
Biochemical markers BNP, NT-proBNP, EGF, PDGF, VEGF, IL-6, IL-8, IL-10, IL-12, EPO, GDF-15, and MCP-1, were analyzed with PEA technique using the Proseek Multiplex 96 × 96 CVD I, Oncology I, and Inflammation I biomarker panels (Olink Bioscience, Uppsala, Sweden) as earlier described.10 All data from the PEA analyses are presented as arbitrary units (AU) in linear values. ET-1 was analyzed by a sandwich ELISA (catalog number DET100, R&D Systems, Inc., Minneapolis, Minnesota). TXB2 was analyzed using the Parameter Thromboxane B2 Assay (catalog number KGE011, R&D Systems, Inc.). Data from the two analyses are presented in pg/mL. Measurements were performed without knowledge of clinical data, and treating physicians were blinded to results of the biochemical marker analyses for this study.
Statistical analysis
To compile and analyze data, custom software was developed using MatLab (The Mathworks Inc., Natick, Massachusetts). Data for each group and period is presented as median values and interquartile range (IQR), or number and percentage. The Mann–Whitney test was used to compare non-parametric continuous data and the Fisher’s exact test was used to compare categorical data. A receiver operating curve was constructed for each biochemical marker and the area under curve (AUC) is presented with 95% confidence interval and p value. All p values presented are two-tailed. To compensate for multiple tests, significances for all parallel analyses of biochemical markers (n = 14) were determined using the Benjamini–Hochberg method to reduce the risk of false discoveries to an expected rate < 10%.13 Due to the strong association between EPO levels and persistent PDA, as well as treatment-resistant PDA, Spearman’s rank-order correlations were used for secondary examination of relationships between EPO and blood gas parameters, which could influence EPO levels.
RESULTS
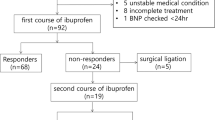
Between 1 November 2012 and 1 May 2015, 122 infants were born at 22–27 weeks’ GA at Uppsala University Children’s Hospital. Twelve infants were not eligible for this study because of major congenital malformations. Eight infants died in the first hours after birth without any echocardiographic examinations conducted, or any blood sampling. Forty-two infants were not included because the parents did not wish to take part in the study, or because of the inability to obtain written consent from the parents prior to the first echocardiographic investigation. Thirteen infants did not have an umbilical catheter during the second day of life, or for other reasons could not provide a valid blood sample. Valid samples were obtained from 47 infants at a median postnatal age of 1.7 days (IQR: 1.5–2.0 days). Information on perinatal characteristics for the included cohort of newborns is presented in Table 2.
Twenty-five (53%) of the infants were pharmacologically treated. Median postnatal age at treatment initiation was 2.9 days (IQR: 2.2–3.4 days) in the responsive group and 3.9 days (IQR: 2.6–4.4 days, p = 0.235) in the non-responsive group. There were no significant differences in perinatal parameters or outcomes between pharmacologically treated and untreated infants. PDA was ultimately considered closed or clinically insignificant in 17 (68%) treated infants and in 16 (73%) untreated infants (p = 0.760). Nine infants (19%) were later subjected to surgical closure of PDA.
Seven infants (35%) closed their PDA within 24 h of the last dose of ibuprofen, while 13 infants (65%) did not respond to treatment. Five pharmacologically treated infants had no echocardiographic examination within 24 h of treatment and were hence not included in the analysis. Of the 13 infants who did not respond to pharmacological treatment, six infants later had spontaneous closure of their PDA.
Biochemical markers for persistence of PDA
Levels of BNP and NT-proBNP, as well as all studied inflammatory biomarkers except for IL-12, were higher in infants with persistent PDA (Table 3). Of the growth factors studied, low PDGF was the only one associated with persistent PDA.
Biochemical markers for treatment response
Information on perinatal parameters and outcomes for infants that did and did not respond to treatment are presented in Table 4. EPO levels were higher in infants that did not respond to pharmacological treatment, but no other biomarker significantly differed between the groups (Table 5).
Post hoc analysis
The correlation between EPO and blood gas levels during the 24 h before biomarker sampling were examined as a post hoc analysis. EPO levels correlated moderately with median pH (ρ = −0.450, p = 0.005), poorly with median pO2 (ρ = −0.386, p = 0.018), and not at all with median pCO2 (ρ = 0.278, p = 0.083).
DISCUSSION
Clinical and echocardiographic signs are routinely used to estimate the hemodynamic significance of PDA shunting, but implications for the clinical impact of PDA in extremely preterm infants remain uncertain. The current study explores potential associations between serum protein markers and the persistence of PDA, irrespective of pharmacological treatment. Additionally, possible associations between serum protein markers and unresponsiveness to pharmacological treatment for PDA were examined.
In line with known associations to hemodynamically significant PDA, we found BNP and NT-proBNP associated with persistence of PDA. The natriuretic peptide and the N-terminal part of its precursor are released mainly by the cardiac ventricles in response to myocardial stretch and volume overload; previous studies have found these markers to be associated with ductal diameter and LA/Ao from 24 h postnatally, thus indicating that the extra cardiac load related to PDA is manifested during the early postnatal period.14,15 In the current study, the association between both markers and PDA outcome supports evidence of early effects of PDA shunting.
High levels of inflammatory markers IL-6, IL-8, IL-10, GDF-15, and MCP-1 were associated with persistence of PDA in our study. From what is known from previous studies in preterm infants, this finding might indicate a relationship to early pulmonary morbidity; although, covariation with other inflammatory conditions could also explain the associations.16,17,18,19,20,21,22,23
High concentrations of EPO were associated with persistent PDA and with failure of pharmacological treatment in our study. This is encouraging as EPO could potentially become a useful tool to guide decisions on whether to initiate or not initiate pharmacological treatment of PDA. The association between persistent PDA and EPO, and the lack of correlation between other inflammatory factors and treatment respons, suggests that the association to EPO may be different from a general inflammatory activity.
It has previously been proposed that intrauterine hypoxia may affect the ability of the ductus arteriosus to close, and that markers for erythropoiesis therefore could be useful in predicting the outcome of PDA.24 In adults, the median half-life of endogenous EPO is 6–8 h and similar half-lives have been reported in preterm infants receiving recombinant human EPO.25 This implies that EPO-levels on the second day of life relate to postnatal conditions, rather than prenatal ones. A study by Holm et al., found no correlation between high EPO concentrations and the lowest pH and pO2 levels during the first three postnatal days.26 Because of its association to PDA, we performed additional analyses of the correlations between EPO and blood gases in the current study, and confirmed significant negative correlations between EPO concentration and median pH during the day before blood sampling. We believe that these correlations support the notion that EPO-levels relate mainly to postnatal hypoxia.
Recombinant EPO also induces increased airway resistance and has been found to stimulate inflammation in the lungs in animal studies.27 Holm et al. identified a dose–response relationship between endogenous EPO blood concentrations in the first two postnatal weeks and later lung disease in infants born before 28 weeks’ GA.26 This correlation between EPO and pulmonary dysfunction is in line with the association between low systolic ductal flow velocity and persistent PDA, conceivable as a sign of elevated pulmonary artery pressure.28
Screening for high levels of inflammatory markers during the second day of life could be used to recognize asymptomatic infants with PDA with a low chance of ductal closure. This would allow for earlier identification of infants that may benefit from pharmacological treatment and could spare infants who are more likely to spontaneously close ductus arteriosus from repeated echocardiographic examinations during their initial postnatal period. Additionally, the association between early high levels of EPO and failure of ibuprofen treatment might allow for more specific identification of infants with a low chance for successful pharmacological treatment. This may lead to earlier selection of extremely preterm infants that require surgical intervention, without the need for additional echocardiographic examinations.
This study is limited by its single center design and the risk for selection bias due to the exclusion of infants without valid blood samples. The limited number of enrolled infants prevents multivariable logistic regression analysis without a major risk of overfitting, but the biochemical analyses have been adjusted for multiple testing.
Although the study aimed to explore additional methods to assess for persistent PDA in extremely preterm infants, the outcomes were influenced by the echocardiographic definition of hemodynamically significant PDA, which is still the method in clinical use for selecting infants for surgical treatment. As indicated above, this potential bias is not unique to our study, but further underlines the need for future studies of PDA with complete omission of treatment or with randomization to groups receiving pharmacological treatment or not receiving treatment at all. Analytical outcomes of treatment response were not influenced by the estimation of hemodynamic significance, but the subgroup of pharmacologically treated infants was small and the results from the treatment response analysis must be cautiously interpreted. The AU in the PEA analysis precludes comparison to other studies, but data on several of the presented markers have, to our knowledge, not been previously reported in relation to PDA in vivo.
CONCLUSIONS
High levels of inflammatory markers in the first days of life were associated with persistence of PDA. High levels of EPO were associated with both the persistence of PDA and failure to respond to pharmacological treatment. Further studies are needed to investigate the biological background for these associations, and to evaluate the clinical usefulness of these biochemical markers in the treatment of extremely preterm infants at risk for adverse outcomes related to PDA.
References
Benitz, W. E. Patent ductus arteriosus in preterm infants. Pediatrics 137, 1–6 (2016).
Clyman, R. I., Couto, J. & Murphy, G. M. Patent ductus arteriosus: are current neonatal treatment options better or worse than no treatment at all? Semin Perinatol. 36, 123–129 (2012).
Gudmundsdottir, A., Johansson, S. & Håkansson, S. et al. Timing of pharmacological treatment for patent ductus arteriosus and risk of secondary surgery, death or bronchopulmonary dysplasia: a population-based cohort study of extremely preterm infants. Neonatology 107, 87–92 (2015).
Thankavel, P. P., Rosenfeld, C. R., Christie, L. & Ramaciotti, C. Early echocardiographic prediction of ductal closure in neonates < 30 weeks gestation. J. Perinatol. 33, 45–51 (2012).
Zonnenberg, I. & de Waal, K. The definition of a haemodynamic significant duct in randomized controlled trials: a systematic literature review. Acta Paediatr. 101, 247–251 (2012).
Evans, N. Preterm patent ductus arteriosus: a continuing conundrum for the neonatologist?. Semin Fetal Neonatal Med 20, 272–277 (2015).
El-Khuffash, A., Davis, P. G., Walsh, K. & Molloy, E. J. Cardiac troponin T and N-terminal-pro-B type natriuretic peptide reflect myocardial function in preterm infants. J. Perinatol. 28, 482–486 (2008).
Mine, K., Ohashi, A., Tsuji, S., Nakashima, J. I., Hirabayashi, M. & Kaneko, K. B-type natriuretic peptide for assessment of haemodynamically significant patent ductus arteriosus in premature infants. Acta Paediatr. 102, 347–352 (2013).
Heuchan A.M. & Clyman R.I. Managing the patent ductus arteriosus: current treatment options. Arch. Dis. Child Fetal Neonatal Ed. 99, F431–F436 (2014).
Assarsson, E. & Lundberg, M. & Holmquist, G. et al. Homogenous 96-plex PEA immunoassay exhibiting high sensitivity, specificity, and excellent scalability. PloS ONE 9, e95192 (2014).
Olsson, K.W., Jonzon, A. & Sindelar, R. A high ductal flow velocity is associated with successful pharmacological closure of patent ductus arteriosus in infants 22–27 weeks gestational age. Crit. Care Res Pract. 2012, 1–6 (2012).
Van Overmeire, B., Van de Broek, H., Van Laer, P., Weyler, J. & Vanhaesebrouck, P. Early versus late indomethacin treatment for patent ductus arteriosus in premature infants with respiratory distress syndrome. J. Pediatr. 138, 205–211 (2001).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B (Methodol.) 57, 289–300 (1995).
El-Khuffash, A., Barry, D., Walsh, K., Davis, P. G. & Molloy, E. J. Biochemical markers may identify preterm infants with a patent ductus arteriosus at high risk of death or severe intraventricular haemorrhage. Arch. Dis. Child Fetal Neonatal Ed. 93, F407–F412 (2008).
Lee, J. H., Shin, J. H., Park, K. H., Rhie, Y. J., Park, M. S. & Choi, B. M. Can early b-type natriuretic peptide assays predict symptomatic patent ductus arteriosus in extremely low birth weight infants? Neonatology 103, 118–122 (2013).
Sorokin, Y., Romero, R. & Mele, L. et al. Umbilical cord serum interleukin-6, C-reactive protein, and myeloperoxidase concentrations at birth and association with neonatal morbidities and long-term neurodevelopmental outcomes. Am. J. Perinatol. 31, 717–726 (2013).
D’Angio C.T., Ambalavanan N., Carlo W.A., et al. Blood cytokine profiles associated with distinct patterns of bronchopulmonary dysplasia among extremely low birth weight infants. J Pediatr. 174, 45-51 (2016).
Paananen R., Husa A.-K., Vuolteenaho R., Herva R., Kaukola T., Hallman M. Blood cytokines during the perinatal period in very preterm infants: J Pediatr. 154, 39–43.e3 (2009).
Lusyati, S., Hulzebos, C. V., Zandvoort, J. & Sauer, P. J. J. Levels of 25 cytokines in the first seven days of life in newborn infants. BMC Res Notes 6, 547 (2013).
Bose, C. L., Laughon, M. M. & Allred, E. N. et al. Systemic inflammation associated with mechanical ventilation among extremely preterm infants. Cytokine 61, 315–322 (2013).
Lohani, O., Colvin, K. L. & Yeager, M. E. Biomarkers for pediatric pulmonary arterial hypertension: challenges and recommendations. Paediatr. Respir. Rev. 16, 225–231 (2015).
Colvin, K. L., Dufva, M. J., Delaney, R. P., Ivy, D. D., Stenmark, K. R. & Yeager, M. E. Biomarkers for pediatric pulmonary arterial hypertension—a call to collaborate. Front Pediatr. 2, 7 (2014).
Duncan, M., Wagner, B. D. & Murray, K. et al. Circulating cytokines and growth factors in pediatric pulmonary hypertension. Mediat. Inflamm. 2012, 1–7 (2012).
Bin-Nun, A., Mimouni, F. B., Fink, D., Sela, H. & Hammerman, C. Elevated nucleated red blood cells at birth predict hemodynamically significant patent ductus arteriosus. J. Pediatr. 177, 10–12 (2016).
Krishnan, R., Shankaran, S., Krishnan, M., Kauffman, R. E., Kumar, P. & Lucena, J. Pharmacokinetics of erythropoietin following single-dose subcutaneous administration in preterm infants. Biol. Neonate 70, 135–140 (1996).
Holm, M., & Skranes, J. & Dammann, O. & Fichorova R.N. & Allred E.N. & Leviton A. Systemic endogenous erythropoietin and associated disorders in extremely preterm newborns. Arch Dis Child Fetal Neonatal Ed 101, F458–F463 (2016).
Polglase, G. R., Barton, S. K. & Melville, J. M. et al. Prophylactic erythropoietin exacerbates ventilation-induced lung inflammation and injury in preterm lambs. J. Physiol. 592, 1993–2002 (2014).
Smith, A., Maguire, M., Livingstone, V. & Dempsey, E. M. Peak systolic to end diastolic flow velocity ratio is associated with ductal patency in infants below 32 weeks of gestation. Arch. Dis. Child Fetal Neonatal Ed. 100, F132–F136 (2015).
Occhipinti, F., De Carolis, M. P. & De Rosa, G. et al. Correlation analysis between echocardiographic flow pattern and N-terminal-pro-brain natriuretic peptide for early targeted treatment of patent ductus arteriosus. J. Matern Fetal Neonatal Med 27, 1800–1804 (2014).
Grass, B., Baumann, P. & Arlettaz, R. et al. Cardiovascular biomarkers pro-atrial natriuretic peptide and pro-endothelin-1 to monitor ductus arteriosus evolution in very preterm infants. Early Hum. Dev. 90, 293–298 (2014).
Letzner, J., Berger, F. & Schwabe, S. et al. Plasma C-terminal pro-endothelin-1 and the natriuretic pro-peptides NT-proBNP and MR-proANP in very preterm infants with patent ductus arteriosus. Neonatology 101, 116–124 (2012).
Hong, Z., Cabrera, J. A., Mahapatra, S., Kutty, S., Weir, E. K. & Archer, S. L. Activation of the EGFR/p38/JNK pathway by mitochondrial-derived hydrogen peroxide contributes to oxygen-induced contraction of ductus arteriosus. J. Mol. Med 92, 995–1007 (2014).
Waleh, N., Seidner, S. & McCurnin, D. et al. Anatomic closure of the premature patent ductus arteriosus: the role of CD14 + /CD163 + mononuclear cells and vegf in neointimal mound formation. Pediatr. Res 70, 332–338 (2011).
Clyman, R. I., Seidner, S. R. & Kajino, H. et al. VEGF regulates remodeling during permanent anatomic closure of the ductus arteriosus. Am. J. Physiol. Regul. Integr. Comp. Physiol. 282, R199–206 (2002).
Sood, B. G., Delaney-Black, V., Glibetic, M., Aranda, J. V., Chen, X. & Shankaran, S. PGE2/TXB2 imbalance in neonatal hypoxemic respiratory failure. Acta Paediatr. 96, 669–673 (2007).
Chen, J.-X., O’Mara, P. W. & Poole, S. D. et al. Isoprostanes as physiological mediators of transition to newborn life: novel mechanisms regulating patency of the term and preterm ductus arteriosus. Pediatr. Res 72, 122–128 (2012).
Wells Logan, J., Allred, E. N., Fichorova, R. N., Engelke, S., Dammann, O. & Leviton, A. Endogenous erythropoietin varies significantly with inflammation-related proteins in extremely premature newborns. Cytokine 69, 22–28 (2014).
Acknowledgements
The authors would like to thank Viktoria Nelin for the suggestions regarding the English language, and the nurses at the Neonatal Intensive Care Unit, Uppsala University Children’s Hospital, Uppsala, and especially the research nurse Cecilia Ewald for her assistance in the collection of blood samples. This study was supported by grants from the Royal Society of Arts and Sciences of Uppsala; Födelsefonden/Perinatalmedicinska Forskningsfonden, Uppsala; the Samariten Foundation for Paediatric Research, Stockholm; Crown Princess Lovisa’s Foundation for Children’s Health Care, Stockholm; the Center for Clinical Research Dalarna; and the Uppsala-Örebro Regional Research Council.
Author information
Authors and Affiliations
Contributions
K.W.O.: conceptualized and designed the study, supervised data collection, designed the custom data handling software, drafted the initial manuscript, and approved the final manuscript as submitted. A.L.: conceptualized and designed the study, carried out analyses of endothelin-1 and thromboxane B2, reviewed and revised the manuscript, and approved the final manuscript as submitted. A.J.: conceptualized and designed the study, carried out the echocardiographic examinations, supervised data collection, reviewed and revised the manuscript, and approved the final manuscript as submitted. R.S.: conceptualized and designed the study, coordinated and supervised data collection, reviewed and revised the manuscript, and approved the final manuscript as submitted. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Olsson, K.W., Larsson, A., Jonzon, A. et al. Exploration of potential biochemical markers for persistence of patent ductus arteriosus in preterm infants at 22–27 weeks’ gestation. Pediatr Res 86, 333–338 (2019). https://doi.org/10.1038/s41390-018-0182-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-018-0182-x
This article is cited by
-
New frontiers in neonatal red blood cell transfusion research
Journal of Perinatology (2023)
-
The relationship between platelet indices and patent ductus arteriosus in preterm infants: a systematic review and meta-analysis
European Journal of Pediatrics (2021)
-
Insights image for exploration of potential biochemical markers for persistence of patent ductus arteriosus in preterm infants at 22–27 weeks’ gestation
Pediatric Research (2019)