Abstract
Background
To evaluate the efficacy of stoss therapy using fortified biscuit for vitamin D-deficient children.
Methods
A total of 108 children aged 30–72 months with vitamin D deficiency were studied in a randomized single-blind clinical trial. The deficient children were assigned to three groups, namely, vitamin D-fortified biscuit (BG), capsule vitamin D (CG), and ampoule vitamin D (AG). Capsules and biscuits containing 50,000 IU of cholecalciferol were consumed twice per week for 3 consecutive weeks. Ampoules with 300,000 IU of cholecalciferol were injected intramuscularly in a single dose. Three weeks after treatment, serum 25(OH)D concentrations were measured, and the three groups were compared.
Results
Each method of treatment could increase the mean serum 25(OH)D concentration to optimal level. Serum 25(OH)D concentrations ≥100 ng/mL were observed in six children, including four from AG and two from CG (P = 0.09). The comparison of the mean serum 25(OH)D concentrations after treatment showed between ampoule and capsule (P = 0.3) and capsule and biscuit (P = 0.62) were insignificant; however, the ampoule and biscuit groups differed significantly (P = 0.012).
Conclusion
Stoss therapy using fortified biscuit may be an effective way to improve compliance in children who cannot take capsules without adverse effects and may also be recommended for prevention purposes.
Similar content being viewed by others
Introduction
Vitamin D deficiency remains a public health concern1,2,3 since adequate vitamin D concentrations are essential in maintaining health and skeletal, muscular, immune, metabolic, and respiratory system functions.2,4 Despite the international vitamin D supplementation programs, many children are still at a high risk for vitamin D deficiency due to the parents’ poor adherence to supplementation regimens. As the signs and symptoms of vitamin D deficiency without rickets are vague or nonspecific, the condition often remains unrecognized and untreated.4,5 According to some studies, the prevalence of vitamin D deficiency was 65.3% in Chinese children aged 12–24 months,6 3% in children aged 6–7 years from Iran,7 and 12.1% among infants aged 8–24 months from the United States.8
Vitamin D-deficient children should be aggressively treated to prevent rickets.9,10,11 Sunlight is the main source of vitamin D. Regular sunlight exposure can prevent vitamin D deficiency, but the safe time of exposure for children is unknown.4 When sunlight exposure is limited, the oral intake of vitamin D via diet or supplements becomes essential.12 Natural dietary sources of vitamin D are limited.1,13 Studies have shown that the fortification of food products increases serum vitamin D levels. The fortification of dairy products with vitamin D, which began in the 1930s,14 is not enough, and additional foods that can be consumed by all populations should be fortified with vitamin D. The fortification of bread and orange juice with 400 and 1000 IU were as effective in maintaining the vitamin D status in young and older adults as oral supplementation.4,12,13 Although food fortification with vitamin D is a strategy to improve the vitamin D status in populations and prevent vitamin D deficiency, it is not used to treat vitamin D deficiency.
Stoss therapy is a cost-effective way of treating vitamin D deficiency.15 Stoss therapy is the oral or intramuscular administration of high-dose vitamin D in the short term. In this method, the total dose of vitamin D administered is 300,000 IU (7500 µg) to 500,000 IU (12,500 µg), as a single dose or two to four divided doses, given at intervals of days to several weeks.4 Intramuscular administration is an unpleasant route, especially in children. The administration of oral vitamin D capsules (50,000 IU) is also difficult. It is thus important to find a safe and effective treatment for vitamin D deficiency. The food fortification strategy was introduced to meet this need; however, the type of food used for fortification is an important issue as well, and fortified biscuits might be the most appropriate. The present study was therefore conducted to evaluate the efficacy of stoss therapy using fortified biscuit for vitamin D-deficient children.
Methods
Study population and data collection
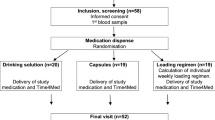
A total of 108 children vitamin D deficiency (25-hydroxyvitamin D (25(OH)D) < 20 ng/mL) were selected via an epidemiologic study. The eligibility criteria consisted of age 30–72 months, resident of Babol area (north of Iran) for at least the past year, the parents’ or guardians’ written informed consent, no chronic illness according to the medical history and physical examination by a physician, no acute illness during the course of the treatment, no use of medications known to affect bone metabolism, and no use of vitamin D supplements during the past 6 months.
The demographic details, anthropometric measurements, medical history, and examination results (performed by a pediatric gastroenterologist) were recorded in the screening visit. The child’s wrist and hand were radiographed. One pediatric radiologist from Amirkola Children’s Hospital observed the radiographs to determine the presence of rickets. Rickets was defined as cupping of epiphysis, widening of the wrist, and fraying of the epiphyseal edges.16 A fasting blood sample was taken to detect calcium (Ca), phosphorus (P), parathyroid hormone (PTH), aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), total protein, fasting blood glucose, blood urea nitrogen, and creatinine (Cr) levels using standard methods. Serum 25(OH)D and PTH levels were measured using the enzyme-linked immunosorbent assay (IDS and EUROIMMUN Kits, manufactured in England). The intra- and inter-assay coefficients of variation were 5.3% and 4.6% for 25(OH)D level and 9.5% and 11% for PTH, respectively. All the measurements were performed at Amirkola Children’s Hospital laboratory.
Interventions and outcome measures
This study was a randomized single-blind clinical trial. The intervention started in mid-April and ended in the second week of August 2016. Children with vitamin D deficiency were randomly divided into three study groups using a computer-generated randomization code. Group 1 (BG) was given vitamin D-fortified biscuit, Group 2 (CG) received vitamin D capsules manufactured by Dana Pharmaceuticals Company, and Group 3 (AG) was administered intramuscular vitamin D, manufactured by Osve Pharmaceuticals Company. Each capsule and biscuit contained 50,000 IU of cholecalciferol and was advised to be consumed twice per week for 3 consecutive weeks. The content of the vitamin D capsules could be dissolved in water or mixed with food.10,17 Each ampoule contained 300,000 IU of cholecalciferol and was intramuscularly injected in a single dose. The medications and biscuits were placed in similar packages with instructions for use, and the research team was not aware of the content of the packages.
A 50-mg/kg calcium supplement (in two separate doses per day) was administered for 2 consecutive weeks along with vitamin D in the case of the children who showed radiographic evidence of rickets, hyperparathyroidism (PTH > 40 ng/mL), and/or hypocalcemia (Ca ≤ 8.5 mg/dL).
The primary outcome was the comparison of serum 25(OH)D concentrations among the three groups. To measure this outcome, the subjects’ blood samples were taken at least 3 weeks after completing the treatment regimen.
The secondary outcomes were the comparison of serum 25(OH)D concentrations between the single dose (intramuscular vitamin D) and intermittent (CG and BG) methods and to assess the treatment side effects, acceptance and compliance. To identify side effects from the beginning of the treatment until a month later, a list was prepared of the symptoms of vitamin D excess or hypervitaminosis, and the parents were asked to visit or call the therapist if their children experienced any side effects, and a special biochemical analysis was performed for them that included serum K (Potassium), Na (Sodium), Ca, P, ALT, AST, 25(OH)D, PTH, ALP, and spot urine calcium/creatinine ratios (UCa/UCr). As recommended by the Endocrine Society, serum concentrations exceeding 100 ng/mL and 150 ng/mL were taken as cases of vitamin D excess and intoxication.18,19 In children, serum 25(OH)D concentrations ≤20 and between 20 and 30 ng/mL are considered cases of vitamin D deficiency and insufficiency, respectively,19 and serum 25(OH)D concentrations between 30 and 100 ng/mL are defined as the optimal range.17,19 Vitamin D replacement therapy is not given to children for vitamin D levels between 20 and 30 ng/mL, unless there are other signs of vitamin D deficiency or important risk factors such as perinatal risk factors.17
To assess treatment acceptance, the parents were trained to use the visual analog scale (VAS) to assess their children’s acceptance of the treatment. The scores obtained ranged from 0 (children not happy to take treatment) to 100 (taking the medications or biscuits comfortably).20 Also, to assess treatment compliance, the number of children who did not complete the treatment was compared between the three groups.
The study was approved by the ethics committee of Babol University of Medical Sciences (MUBABOL.REC.1394.31) and pre-registered at the Iranian Registry of Clinical Trials (IRCT) (IRCT:201504296705N3). The parents of all the selected children signed an informed consent form.
Fortification of biscuits
The biscuits were fortified using a powdered vitamin D (dry vitamin D3 type 100), cold water soluble and DSM nutritional products (Roch company) designed for pharmaceutical and food products in the amount of 100,000 IU or 2500 µg/g. The goal was to produce a biscuit (10 g) that contained up to 50,000 IU of vitamin D3. The powder was dispersed into wheat flour and then added to an oil and sugar mixture. The biscuit was then baked at 250–260 °C for 15 min and high-performance liquid chromatography (HPLC) was then used to determine the stability of vitamin D and estimate losses during dough-baking and biscuit-making. HPLC was performed at the pharmacological laboratory of Babol University of Medical Sciences. HPLC equipped with an ultraviolet (UV) detector (Knauer, Germany) was used to measure the concentration of cholecalciferol in the samples. The work conditions included a wave length of 265 nm, C18 (Eurospher column), and a mobile phase with a mixture of acetonitrile and water at the ratio of 67:33. The temperature was set at 25 °C, and the flow rate in the mobile phase was 0.6 mL/min. EZChrom software was used to extract the area and height of the peaks. The biscuit samples dissolved in the mobile phase, which consisted of a mixture of acetonitrile and water, were centrifuged at 3000 rpm for 10 min. The samples were then injected using a syringe-loading injector fitted to a 20-μL loop. The vitamin D3 concentrations in the biscuit were measured by sensitive, specific, and validated HPLC using a UV detector. The HPLC showed a vitamin D loss of about 8% (4000 IU) after baking, and this amount was therefore added in the dough, and after baking was complete, the concentration of vitamin D was 50,000 IU. The HPLC also showed that the biscuit samples remained stable 1, 7, and 30 days after baking and no differences were observed in their vitamin D3 concentrations (Fig. 1).
Sample size
The subjects were selected from an epidemiological study that examined the prevalence of vitamin D deficiency and risk factors in children aged 30–72 months (data not published). Children with a serum 25-(OH)D level <20 ng/mL were selected for this clinical trial (Fig. 2). Of the 406 children, 119 had 25(OH)D levels <20 ng/dL and among them 108 children (90.7%) returned for treatment (Fig. 2).
Statistical analysis
The initial analysis was performed according to intention to treat (ITT) that all subjects randomized to each arm be included in the analysis and a secondary analysis was done via pre-protocol analysis that excluded those who did not complete the treatment.
Statistical analyses were performed in SPSS-19 (SPSS Inc., Chicago, IL, United States). Continuous variables are presented as mean ± standard deviation (SD). Changes in 25(OH)D were assessed using one-way analysis of variance and the independent and paired t tests among and within the groups. Chi-squared test or Fisher’s exact test were performed to compare the percentages. P = 0.05 was considered statistically significant for all the analyses.
Results
The cross-sectional screening led to the identification of 119 (30 .5%) children who were vitamin D deficient [25(OH)D ≤ 20 ng/mL]. After contacting the subjects, 108 children visited for treatment, and 96 (38 boys and 58 girls) ultimately completed the therapy (Fig. 2). The mean age of the children was 50.3 ± 11.78 months. There were no significant differences between the subjects in terms of the baseline characteristics (Table 1).
There were no significant differences between the groups in terms of baseline serum 25(OH)D concentrations. After the intervention, according to ITT analysis, serum 25(OH)D concentrations increased to 57.46 ± 31.06, 47.20 ± 24.86, and 39.52 ± 21.50 in the AG, CG, and BG groups, respectively, that showed significant differences between the three groups (P = 0.016). Based on the post hoc test, the comparison of the mean serum 25(OH)D concentrations between the ampoule and capsule (P = 0.3) and capsule and fortified biscuit (P = 0.61) groups showed no significant differences; however, there was a significant difference between the ampoule and fortified biscuit groups (P = 0.012). The within-group comparison showed a significant mean increase in serum 25(OH)D concentrations (P = 0.012). Also a pre-protocol analysis was performed that shown in table 2 and according to this analysis, serum 25(OH)D concentrations were significantly different between the three groups (P = 0.005). The within-group comparison showed a significant mean increase in serum 25(OH)D concentrations (P < 0.001).
The mean serum 25(OH)D concentrations increased to the optimal range in all the three groups, and all the children achieved 25(OH)D concentrations ≥20 ng/mL, except one child from the AG group.
The mean serum 25(OH)D concentrations were 63.34 ± 28.16 and 45.96 ± 22.90 in the single and intermittent dose groups, respectively (P = 0.002).
Serum concentrations exceeding 100 ng/mL were observed in six children who were clinically asymptomatic, and four participants received intramuscular vitamin D, and the other two received vitamin D capsules (P = 0.09).
To assess treatment acceptance, the mean VAS was calculated as 88.67 ± 1.2, 85.60 ± 1.98, and 85.12 ± 2.1 in the CG, BG, and AG groups, respectively (P = 0.43). There were no complaints about the taste of the biscuits, but three of the parents did not agree to their children consuming fortified biscuit. According to Fig. 2, 4 (10.5%) of the children from the BG group and 1 (2.9%) from the AG group did not complete their treatment (P = 0.2).
Among the treatment groups, 34 children needed calcium supplementation. The increase in serum 25(OH)D concentrations was high in the children with an initially low vitamin D status, but the correlation was insignificant (r = −0.05, P = 0.64). The radiographic data did not show evidence of rickets in the children.
Discussion
The results of this study showed that fortified biscuit with a high dose of cholecalciferol is as effective as vitamin D capsules for the treatment of vitamin D deficiency in children.
The review of literature revealed only a few studies on the bioavailability of vitamin D from foods. In a 12-week double-blind trial on 14 subjects, the daily ingestion of fortified orange juice with 1000 IU of vitamin D increased serum 25(OH)D by 150% compared to baseline.13 Other studies showed that vitamin D was bioavailable from fortified low-fat and regular-fat cheese with 28,000 IU/week of vitamin D1 and bread fortified with 5000 IU21 and 400 IU12 of vitamin D3 per day. The present findings were consistent with the results of these studies. In contrast, an assay on the efficiency of food fortification with vitamin D in Finland showed that, 4 years after starting the fortification regimen, only 7% of the participants had an adequate serum 25(OH)D concentration.22
Vitamin D concentrations up to 100 ng/mL (250 nmol/L) are considered safe both for children and for adults.23,24 The present study showed that biscuits increased serum 25(OH)D concentrations to an optimal level. The change in the concentrations of 25(OH)D from the baseline was significantly greater in the children who were injected with intramuscular vitamin D3 compared to those who consumed fortified biscuits. Nonetheless, the difference was not significant between the biscuit group and the capsule group, which suggests that a high dose of vitamin D is equally bioavailable from fortified biscuits and vitamin D capsules. Although not yet clear, the other content of biscuits, such as oil, appear to balance intestinal absorption and affect the bioavailability of vitamin D, and more research is thus needed to clarify this issue.
The present study did not reveal any symptoms of vitamin D intoxication, such as anorexia, diarrhea, constipation, nausea, and vomiting; bone pain; drowsiness; continuous headaches; irregular heartbeat; loss of appetite; joint pain; frequent urination; excessive thirst; weakness; nervousness; and itching.24
Vitamin D toxicity tends to occur as a result of excessive supplementation, and even fortified foods can lead to vitamin D toxicity; however, there is still no consensus on the best dose of vitamin D for stoss therapy.24 A meta-analysis showed no risk of hypercalcemia or hypercalciuria with stoss therapy in a single oral dose <400,000 IU, while doses >400, 000 IU were associated with a risk of hypercalcemia.25 A study by Shah et al. showed that 600,000 IU of vitamin D in six doses (100,000 IU/2 h) increased the serum 25(OH)D concentrations in 42 children aged 5–109 months and secondary hyperparathyroidism was also observed in 94.9% of the children.11
In a prospective study, 30 children aged 4–19 months with vitamin D nutritional rickets were divided into three groups that received 300,000 IU of vitamin D orally, 300,000 IU intramuscularly, and 600,000 IU orally. The results showed no significant differences between the 300,000 IU administered orally and intramuscularly, and biochemical changes were more prominent in the group that was administered 600,000 IU orally compared to the other two groups. Nevertheless, hypercalcemia was observed in 30% of patients in this group. The authors therefore concluded that a 300,000-IU dose administered intramuscularly is safe in stage-II and -III rickets.26
The therapeutic dose (300,000 IU) used in this study thus seems to be safe, but stoss therapy performed by different means affected serum 25(OH)D concentrations differently.27 For example, the intramuscular vitamin D injection was associated with excess concentrations in four subjects and with deficient concentrations in one subject, while the intermittent route was associated with excess concentrations in two subjects. Although serum 25(OH)D concentrations increased the intramuscular single dose more than the intermittent oral route, and although treatment acceptance was similar between the groups, one study on the clinical effects of vitamin D excess and intoxication showed clinically asymptomatic cases, which has clinical significance,23 and although the single high dose of vitamin D was more effective in rapidly increasing 25(OH)D concentrations, the intermittent doses were also found to improve treatment adherence.28
In the present study, treatment compliance did not differ between the oral and injection groups. A study conducted to assess the safety and acceptability of single-dose oral and parenteral vitamin D found both routes to be effective; however, the injectable form was shown to be more effective, and no side effects were observed and both methods were well tolerated.29 In contrast, in a study conducted on infants and toddlers, the intramuscular route of vitamin D supplementation was selected owing to the poor compliance accomplished with the oral route and also because the parents preferred the injectable route over giving their children daily doses of the vitamin.30
Intramuscular injection is an effective invasive method used to treat secondary vitamin D deficiency due to malabsorption4 but is not an appropriate choice for children without malabsorption problems. Although the present findings showed that the intermittent route is a safe method, administering the oral capsule is more difficult in children. The researchers therefore selected the fortified biscuit method to avoid the poor compliance that is associated with oral medication administration and that has the same effectiveness as oral capsules.
Although some parents did not want their children to take fortified biscuits due to its novelty and uncommon nature, public awareness about the benefits of this method should be gradually increased.
Biscuit is a food product that can be consumed quite easily by children and even during infancy, although fortified dairy products such as milk,13 cheese,1 and yogurt3,13 are also effective. In one study, Asian children were found to prefer other fortified foods due to the high prevalence of lactose intolerance and milk allergies.31 Biscuits are therefore believed to be the best choice for this age group in terms of compliance.
The limitations of this study included the intervention being short term; consequently, the results cannot be generalized to the long-term consumption of vitamin D-fortified foods, especially for prevention cases, and more research is required on the subject. Other limitations of the study include not recording the time of dietary intake and sun exposure, which might have affected serum 25(OH)D concentrations. The study lacked a control group that could help with the comparisons, but depriving one group of vitamin D would have been ethically questionable, and the children were unable to complete the VAS due to their age, and treatment acceptance was thus assessed for the parents.
Conclusion
Vitamin D is bioavailable in fortified biscuits at high doses and in short periods. The fortification of biscuits is therefore a safe option for treating children with vitamin D deficiency and has a high compliance among children and parents. Stoss therapy has not yet been tested for prevention purposes. The fortification of foods that are taken on a daily basis has been carried out with much lower concentrations of vitamin D. Further research is warranted to see whether stoss therapy with biscuits can be used for prevention purposes.
References
Wagner, D. et al. The bioavailability of vitamin D from fortified cheeses and supplements is equivalent in adults. J. Nutr. 138, 1365–1371 (2008).
Biancuzzo, R. M. et al. Fortification of orange juice with vitamin D2 or vitamin D3 is as effective as an oral supplement in maintaining vitamin D status in adults. Am. J. Clin. Nutr. 91, 1621–1626 (2010).
Nikooyeh, B. et al. Daily consumption of vitamin D–or vitamin D+calcium–fortified yogurt drink improved glycemic control in patients with type 2 diabetes: a randomized clinical trial. Am. J. Clin. Nutr. 93, 764–771 (2011).
Munns, C. et al. Prevention and treatment of infant and childhood vitamin D deficiency in Australia and New Zealand: a consensus statement. Med. J. Aust. 185, 268–272 (2006).
Ozkan, B. Nutritional rickets. J. Clin. Res. Pediatr. Endocrinol. 2, 137–143 (2010).
Strand, M. A. et al. Diagnosis of rickets and reassessment of prevalence among rural children in northern China. Pediatr. Int 49, 202–209 (2007).
Salek, M. et al. Is vitamin D deficiency prevalent in healthy 6-year old children in Isfahan city? I. U. M. S. 25, 95–103 (2007).
Gordon, C. M. et al. Prevalence of vitamin D deficiency among healthy infants and toddlers. Arch. Pediatr. Adolesc. Med 162, 505–512 (2008).
Holick, M. F. Resurrection of vitamin D deficiency and rickets. J. Clin. Invest 116, 2062 (2006).
Markestad, T. et al. Plasma concentrations of vitamin D metabolites before and during treatment of vitamin D deficiency rickets in children. Acta Paediatr. 73, 225–231 (1984).
Shah, B. R. & Finberg, L. Single-day therapy for nutritional vitamin D-deficiency rickets: a preferred method. J. Pediatr. 125, 487–490 (1994).
Natri, A.-M. et al. Bread fortified with cholecalciferol increases the serum 25-hydroxyvitamin D concentration in women as effectively as a cholecalciferol supplement. J. Nutr. 136, 123–127 (2006).
Tangpricha, V. et al. Fortification of orange juice with vitamin D: a novel approach for enhancing vitamin D nutritional health. Am. J. Clin. Nutr. 77, 1478–1483 (2003).
Tylavsky, F. A. et al. Strategies to improve vitamin D status in northern European children: exploring the merits of vitamin D fortification and supplementation. J. Nutr. 136, 1130–1134 (2006).
Vesa, T. H., Marteau, P. & Korpela, R. Lactose intolerance. J. Am. Coll. Nutr. 19, 165–175 (2000).
Carpenter, T. Overview of rickets in children. www.update.com. (2016).
Misra, M. Vitamin D insufficiency and deficiency in children and adolescents. www.uptodate.com. (2016).
Vogiatzi, G. M., Dickman, J. E. & DeBoer, D. M. Vitamin D supplementation and risk of toxicity in pediatrics: a review of current literature. J. Clin. Endocrinol. Metab. 99, 1132–1141 (2014).
Holick, M. F. et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 96, 1911–1930 (2011).
Mahdavi, M. et al. The effect of adding synbiotics to polyethylene glycol in childhood functional constipation: a randomized clinical trial study. Int. J. Pediatr. 5, 5357–5367 (2017).
Mocanu, V. et al. Long-term effects of giving nursing home residents bread fortified with 125 lg (5000 IU) vitamin D3 per daily serving. Am. J. Clin. Nutr. 89, 1132–1137 (2009).
Lehtonen-Veromaa, M. et al. Prospective study on food fortification with vitamin D among adolescent females in Finland: minor effects. Br. J. Nutr. 100, 418–423 (2008).
Glenville, Jones Pharmacokinetics of vitamin D toxicity. Am. J. Clin. Nutr. 88, 582–586 (2008).
Alshahrani, F. & Aljohani, N. Vitamin D: deficiency, sufficiency and toxicity. Nutrients 5, 3605–3616 (2013).
McNally, J. D. et al. Rapid normalization of vitamin D levels: a meta-analysis. Pediatrics 135, 152–166 (2015).
Özkan, B. et al. Comparison of different treatment modalities (300,000 U oral, 300,000 U IM, 600,000 U oral vitamin D) in nutritional rickets. Çocuk Sağlığı Hastalık. Derg. 43, 30–35 (2000).
Koçyiğit, C. et al. Can stoss therapy be used in children with vitamin D deficiency or insufficiency without rickets? J. Clin. Res. Pediatr. Endocrinol. 9, 150–15 (2017).
Cipriani, C. et al. Effect of a single oral dose of 600,000 IU of cholecalciferol on serum calciotropic hormones in young subjects with vitamin D deficiency: a prospective intervention study. J. Clin. Endocrinol. Metab. 95, 4771–4777 (2010).
Billoo, A. G. et al. Comparison of oral versus injectable vitamin-D for the treatment of nutritional vitamin-D deficiency rickets. J. Coll. Physicians Surg. Pak. 19, 428–431 (2009).
Soliman, A. T. et al. Clinical responses to a mega-dose of vitamin D3 in infants and toddlers with vitamin D deficiency rickets. J. Trop. Pediatr. 56, 19–26 (2009).
Zeiger, R. S. Dietary aspects of food allergy prevention in infants and children. J. Pediatr. Gastroenterol. Nutr. 30, 77–86 (2017).
Acknowledgements
Hereby, we wish to express our gratitude to the children and their families for participating in this study and the staff at the Non-Communicable Pediatric Diseases Research Center, Health Research Institute, Babol University of Medical Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Moslemi, L., Esmaeili dooki, M., Moghadamnia, A.A. et al. Stoss therapy using fortified biscuit for vitamin D-deficient children: a novel treatment. Pediatr Res 84, 662–667 (2018). https://doi.org/10.1038/s41390-018-0135-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-018-0135-4