Abstract
Background
Hypoxic–ischemic encephalopathy (HIE) remains a major cause of cerebral palsy. Increasing evidence has suggested that mesenchymal stem cells have a favorable effect on HIE. However, the efficacy of human amniotic fluid stem cells (hAFS) for HIE, especially in the chronic phase, remains unclear. The aim of this study was to determine the neurorestorative effect of hAFS on the chronic phase of HIE.
Methods
hAFS were isolated from AF cells as CD117-positive cells. HI was induced in 9-day-old mice. Animals intranasally received hAFS or phosphate‐buffered saline at 10 days post HI and were harvested for histological analysis after functional tests at 21 days post HI. We also implanted PKH26-positive hAFS to assess their migration to the brain. Finally, we determined gene expressions of trophic factors in hAFS co-cultured with HI brain extract.
Results
hAFS improved sensorimotor deficits in HIE by gray and white matter restoration and neuroinflammation reduction followed by migration to the lesion. Brain-derived neurotrophic factor (BDNF), nerve growth factor (NGF), hepatocyte growth factor (HGF), and stromal cell-derived factor-1 (SDF-1) gene expressions in hAFS were elevated when exposed to HI-induced brain extract.
Conclusion
hAFS induced functional recovery by exerting neurorestorative effects in HIE mice, suggesting that intranasal administration of hAFS could be a novel treatment for HIE, especially in the chronic phase.
Similar content being viewed by others
Introduction
Hypoxic–ischemic encephalopathy (HIE) remains a major cause of brain injury in infants, occurring in approximately 2 per 1000 live births with a birth weight ≧2000 g and gestation ≧33 weeks in Japan.1 It is suggested that up to 25% of the survivors have permanent neurological deficits, including cerebral palsy, mental retardation, learning disability, and chronic epilepsy.2 A sudden onset of severe intrapartum HI insult at birth caused by placental abruption and umbilical cord prolapse is considered to contribute to HIE. In addition, there may also be precipitating obstetric risk factors involved, including preeclampsia, antenatal bleeding, or fetal growth restriction.1,2,3
HIE involves two separate cascades of events in humans, one within 72 h after injury (acute to subacute phase) and one persisting even weeks later (chronic phase).4,5 Although recent clinical trials have shown that hypothermia has modest effects on outcome in the acute to subacute phase,6,7 neurodevelopmental deficits persist in approximately half of patients even after treatment with hypothermia.7 Therefore, the development of novel and efficacious therapies for treating perinatal HI-induced brain injury has long been expected.
Stem cell therapy is a useful treatment for various refractory central nervous system diseases.8 Recently, mesenchymal stem cell (MSC) transplantation in the acute phase was proven successful in a rodent model of HIE.9 However, MSC allotransplantation involves risks when used during the neonatal period. To overcome the issues associated with allotransplantation, autologous stem cell therapy is promising for HIE. Among MSCs, umbilical cord blood cells (UCBCs) have provided autologous stem cells for neonates, and phase I clinical studies in the acute to subacute phase of HIE have shown that UCBCs may be safe, feasible, and potentially efficacious in humans.10 However, UCBCs cannot be used for neonates when a sufficient amount of umbilical cord blood cannot be collected at delivery in a tense obstetric situation, such as placental abruption and umbilical cord prolapse, resulting in HIE.
Amniotic fluid stem cells (AFS) fabricated during pregnancy could also have potential for the treatment of neonates when autologous cell therapy is required, even immediately after birth. Recently, Corcelli et al.,11 using a mouse model of HIE, reported that intracranial injection of hAFS in the acute phase showed neuroprotective effects.11 However, consideration should be given to the neuroprotective phase (acute to subacute phase: <48–72 h of injury) and the neurorestorative phase (chronic phase: >72 h after injury) for the timing of cell delivery.4,5 And, the therapeutic efficacy of hAFS treatment for HIE in the chronic phase remains to be determined. The aim of this study was to determine whether transplantation of hAFS can be used in the chronic phase of neonatal HIE.
Materials and methods
Isolation of hAFS
This study was approved by the institutional review board of Keio University School of Medicine (No. 20140285) and informed consent was obtained from all patients. Five milliliters of amniotic fluid samples were obtained from 15- to 17-week pregnant women who underwent amniocentesis. CD117-positive cells were isolated as hAFS, as previously described.12,13 Briefly, within 2 h, cells were centrifuged at 1500 r.p.m. for 5 min. After the removal of the supernatant, the cell pellet was cultivated in growth medium consisting of 65% α-minimum essential medium (Invitrogen, Ghent, Belgium), 15% fetal bovine serum (FBS) (Invitrogen), 1% l-glutamine (Invitrogen), 1% penicillin/streptomycin (Invitrogen), and 40% AmnioMax-II (Life Technologies, Carlsbad, CA, USA). After the cell population became sub-confluent, spindle-shaped CD117-positive cells were isolated as hAFS with the Magnetic Cell Sorting Kit (Miltenyi Biotec, Auburn, CA, USA), as previously described.12,13
Animals
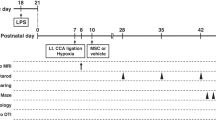
All experiments were approved by the animal committee of Keio University (No. 16014-0). At postnatal day 9 (P9), C57BL/6 male mice (Charles River Laboratories Japan Inc., Kanagawa, Japan) underwent HI by permanent right common carotid artery occlusion under isoflurane anesthesia (4% induction and 1% maintenance in O2: N2O (1:1)) followed by exposure to 8% oxygen in nitrogen for 30 min.14,15,16 Pups were randomly assigned to three experimental groups: (a) sham-operated group (sham-operated animals in which the right common carotid artery was exposed but not ligated); (b) non-treated group (HI insult and phosphate‐buffered saline (PBS) administration as sham treatment); and (c) treated group (HI insult and hAFS administration). At P19, hAFS dissolved in PBS or PBS alone was delivered intranasally in animals. Thirty minutes before administration, two doses of 3 μL hyaluronidase (total 100 U; Sigma-Aldrich, St. Louis, MO, USA) in PBS were applied to each nostril and spontaneously inhaled.15 Subsequently, a total of 5.0 × 105 hAFS dissolved in 12 μL PBS or 12 μL PBS alone were administered as two doses of 3 μL applied to each nostril. We used a total of 45 mice in this study.
Sensorimotor functional test
The cylinder rearing test (CRT) was used to assess forelimb use asymmetry at P19 and P30. The weight-bearing forepaw(s) to contact the wall during a full rear was recorded as left (impaired), right (nonimpaired), or both. Paw preference was calculated as ((nonimpaired − impaired)/(nonimpaired + impaired + both))100%.17,18 Forelimb contacts while rearing were scored with a total of 20 contacts recorded for each animal. To evaluate sensorimotor coordination,19 the rotarod test (ROT) was performed at P19 and P30. The rotation speed of the rod was fixed at 15 r.p.m. at P19 (at 20 r.p.m. at P30). At P19 these tests were applied just before the hAFS administration. The mice rested in their cage for 10 min between each trial. The mean time (maximum 3 min) on the rotarod was determined from three trials. These analyses were performed in a blinded set up by three persons.
Immunohistochemical analysis
At P30 after the functional tests, animals were deeply anesthetized with 5% isoflurane and perfused with 4% paraformaldehyde in PBS via the left ventricle. Brains were embedded in paraffin or cryoprotected in 30% sucrose and embedded in Tissue OCT freezing medium (Tissue-Tec, Sajura Zoeterwoude, The Netherlands) at P30 and sectioned coronally into 2-mm slices using a mouse brain slicer. We then analyzed the ratio of the ipsilateral/contralateral area in each brain section (4, 6, 8, and 10 mm from the anterior pole of the cerebrum) using ImageJ (National Institutes of Health, Bethesda, MD, USA; http://rsb.info.nih.gov/ij/, 1997–2007).
Coronal paraffin sections (6 mm from the anterior pole) were incubated with mouse anti-microtubule-associated protein 2 (MAP2; Sigma-Aldrich, 1:500), rabbit anti-myelin basic protein (MBP; Abcam, Cambridge, UK, 1:500), rabbit anti-glial fibrillary acidic protein (GFAP; Dako, Santa Clara, CA, USA, 1:200), or rabbit anti-ionized calcium binding adapter molecule-1 (Iba-1; Wako, Osaka, Japan, 1:500) and binding was visualized with a Vectastain ABC Kit (Vector Laboratories, Burlingame, CA, USA). Images were captured by BZ9000 (Keyence, Osaka, Japan) and morphometric analysis was performed with ImageJ. The ratio of the ipsilateral to contralateral hemisphere stained with MAP2 or MBP was measured to determine the damage of neurons and oligodendrocytes in the gray and white matters, respectively. To evaluate neuroinflammation including astrogliosis and microglial activation, we counted the number of GFAP-positive or Iba-1-positive cells in the area near the ipsilateral hippocampus (Fig. 3a). Among Iba-1-positive cells, activated microglia were defined as small rod cells with wavy, branching processes.16 We selected the zone where most GFAP-positive or Iba-1-positive cells were found per high power field in the area near the ipsilateral hippocampus. These analyses were performed in a blinded set up by three persons.
hAFS tracking in the brain after nasal application
Mice underwent HI at P9, and PKH-26 (Sigma-Aldrich)-labeled hAFS were administered intranasally at P19. The brains were harvested 12, 24, 48, and 72 h after cell administration and cryoprotected in 30% sucrose and embedded in Tissue OCT freezing medium (Tissue-Tec), and then sectioned coronally into 2-mm slices using a mouse brain slicer in each brain section (4, 6, and 8 mm from the anterior pole of the cerebrum). Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) (Sigma-Aldrich, 1:1000). Fluorescent images were captured with an LSM 710 confocal microscope (Carl Zeiss, Oberkochen, Germany) and BZ9000 (Keyence).
hAFS co-culture with brain extracts harvested from HIE mice in the chronic phase
To determine whether the HI brain environment stimulates hAFS to express neurotrophic and chemotactic factors, we co-cultured hAFS with HI brain extracts from HI-induced mice on P19. Mice were euthanized by pentobarbital overdose and decapitated and their brains were removed. The ipsilateral hemisphere was dissected on ice. Dissected brains were dissolved in knockout Dulbecco's modified Eagle's medium (Thermo Fisher Scientific Inc., Tokyo, Japan) at a final concentration of 150 mg/mL and centrifuged for 10 min at 3000 × g at 4 °C. Supernatants were collected as “brain extract.”20 hAFS were cultured at a concentration of 40,000 cells per well in a 6 well-plate for 24 h before replacing the medium with expansion medium containing 1% brain extract. RNA was isolated from the hAFS 48 h after culture with brain extract.
RNA isolation and RT-qPCR
Total RNA was isolated with the RNeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. The amount of RNA was measured with the NanoDrop 1000 (Thermo Scientific, Waltham, MA, USA). The RNA quality was determined with the optical density 260/280 ratio, ranging from 1.9 to 2.1. Reverse transcription of total RNA to complementary DNA was performed using a Prime Script RT Master Mix (Takara Bio, Shiga, Japan). Quantitative PCRs were performed in triplicate in a volume of 25 µL per reaction in a 96-well Bio-Rad CFX96 RealTime PCR System (Bio-Rad Inc., Hercules, CA, USA). Reaction mixtures included 5 ng of genomic DNA as template, 0.4 mM of each primer (Thermo Fisher Scientific Inc., Tokyo, Japan), SYBR Premix Ex Taq II (Tli RNaseH Plus) (Takara Bio), and sterile H2O. The primer sets are listed in Table 1. PCR was performed as follows: pre-denaturation at 95 °C for 30 s, 45 cycles of denaturation at 95 °C for 5 s, and annealing at 60 °C for 20 s. The relative gene expression level for each sample was calculated using the 2−ΔΔCT method. Genes were normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as an internal control.
Statistical analysis
Values are expressed as mean ± standard error. Statistical differences between groups were analyzed using analysis of variance and Tukey’s honest significant difference. P values <0.05 were considered to be statistically significant.
Results
hAFS improved sensorimotor deficits induced by HI insult
P9 mouse pups underwent HI and were treated with an intranasal administration of hAFS dissolved in PBS or PBS alone at P19 (Fig. 1a). Using the CRT and ROT, we investigated whether hAFS administration could restore deficits in sensorimotor behavior induced by HI insult. At P19, HI induction did not affect the CRT results and significantly shortened the time on the rotarod compared to sham operation. However, no significant difference was observed between the non-treated group and the treated group (Fig. 1b). However, sensorimotor functions were significantly restored in the hAFS group at P30 compared to the non-treated group. After HI induction, forepaw preference in the CRT was significantly improved by hAFS treatment (treated vs. non-treated; 7.5 ± 1.9 vs. 15.0 ± 1.9%; P < 0.01) and the time on the rotarod in the hAFS group was twice as long as in the non-treated group (treated vs. non-treated; 83.6 ± 3.6 s vs. 41.3 ± 3.6 s; P < 0.01) (Fig. 1c). These results indicated that hAFS treatment markedly improved sensorimotor deficits induced by HI insult.
hAFS improved functional outcome in HIE. a Time course of this study. b Sensorimootor functional tests at P19. There were no significant differences in sensorimotor function between the non-treated and treated groups before hAFS administration. c Sensorimotor function tests at P30. HI-induced sensorimotor dysfunction was recovered by hAFS treatment. Non-treated, n = 6; treated, n = 6; sham, n = 5. Results are presented as mean ± SEM. *p < 0.05. hAFS human amniotic fluid stem cells, HIE hypoxic–ischemic encephalopathy
hAFS reduced the brain lesion size induced by HI insult
HI induction in rodents reportedly reduces brain volume in the ipsilateral hemisphere, which causes sensorimotor deficits.15,16 To determine the effect of HI induction on brain lesion size in our study, histological analysis of the injured brain was performed at P30 (Fig. 1a). Although HI induction reduced the ratio of the ipsilateral/contralateral area in brain coronal sections at 6 mm compared to sham operation, there was no significant difference between the sham and non-treated groups at other reference sections. hAFS treatment significantly increased the ratio of the ipsilateral/contralateral area in brain sections at 6 mm compared to the non-treated group (treated vs. non-treated; 0.956 vs. 0.874; p < 0.01) (Fig. 2b), suggesting that hAFS treatment reduced the brain lesion size induced by HI insult.
hAFS reduced the brain lesion size evoked by HI insult. a Representative images of the brain coronal sections. b The ratio of the right (ipsilateral)/left (contralateral) hemisphere area at the coronal sections at (4, 6, 8, and 10 mm) from the anterior pole. S sham, N non-treated, T treated. c Representative images of the brain coronal sections stained with MAP2. The ratio of the right/left MAP2-positive area. d Representative images of the brain coronal sections stained with MBP. The ratio of the right/left MBP-positive area. Non-treated, n = 6; treated, n = 6; sham, n = 5. Results are presented as mean ± SEM. *p < 0.05. hAFS human amniotic fluid stem cells, HIE hypoxic–ischemic encephalopathy
hAFS prevented white and gray matter damage and reduced neuroinflammation induced by HI insult
To determine the potential of hAFS treatment to protect against neuronal and oligodendrocyte damage induced by HI, we evaluated the ratio of the ipsilateral/contralateral MAP2-positive area as a measure of gray matter and the ratio of the ipsilateral/contralateral MBP-positive area as a measure of white matter at P30. Although HI insult induced white and gray matter damage compared to sham operation, intranasal administration of hAFS also significantly reduced gray matter loss (treated vs. non-treated; 0.96 vs. 0.83; P < 0.01) (Fig. 2b) and white matter loss (treated vs. non-treated; 0.94 vs. 0.85; P < 0.01) (Fig. 2c) at P30. We next determined whether hAFS treatment had an effect on the neuroinflammatory response, including astrogliosis and microglial activation, induced by HI insult. To this end, we evaluated GFAP-positive or Iba-1-positive cells in the area near the ipsilateral hippocampus in the coronal section 6 mm from the anterior pole. Although HI insult upregulated GFAP and Iba-1 expressions as previously described,21 hAFS treatment significantly reduced the number of astrocytes (Fig. 3b) and activated microglia (Fig. 3c) in the lesion. These results suggested that hAFS treatment attenuated neuronal and oligodendrocyte damage and neuroinflammation in the ipsilateral hemisphere.
hAFS reduced gliosis and neuroinflammation after HI insult. a Examination field (the area near the ipsilateral hippocampus in the coronal section 6 mm from the anterior pole). b Representative images of GFAP-positive cells (arrow). c Representative images of Iba-1-positive cells. Among Iba-1-positive cells, activated microglia were defined as small rod cells with wavy, branching processes (arrow). Non-treated, n = 6; treated, n = 6; sham, n = 5. Results are presented as mean ± SEM. *p < 0.05. Scale bar, 100 μm. hAFS human amniotic fluid stem cells, HIE hypoxic–ischemic encephalopathy
hAFS migrated to the brain lesion through the intranasal route
The ability of hAFS to migrate to the brain lesion was assessed by hAFS labeled with PKH26. Brain samples harvested after injection of hAFS labeled with PKH26 were examined. Although few PKH26-labeled cells could be detected in brain sections within 12 h after administration, large numbers of hAFS were observed in brain coronal sections mainly at 4 mm from the anterior pole 24 h after administration (Fig. 4). Forty-eight hours after administration, hAFS were present predominantly in the section at 8 mm from the anterior pole, although few hAFS could be detected in brain section at 4 mm. Furthermore, few hAFS could be detected later than 72 h after administration. These results suggested that hAFS could migrate to the brain through the intranasal route and transiently engraft in the brain lesion, resulting in functional recovery by preserving neurons and oligodendrocytes, which is associated with less neuroinflammation.
HI-induced brain extract in the chronic phase induced gene expressions of neurotrophic and chemotactic factors in hAFS
MSCs transplanted at sites of central nervous injury are thought to promote functional recovery by producing trophic factors that induce survival and regeneration of host neurons and oligodendrocytes.22 Therefore, we further examined whether the HI brain environment stimulates hAFS to express neurotrophic and chemotactic factors using hAFS co-cultured with HI brain extracts in the chronic phase. We demonstrated that brain-derived neurotrophic factor (BDNF), nerve growth factor (NGF), hepatocyte growth factor (HGF) and stromal cell-derived factor-1 (SDF-1) mRNA expression significantly increased after co-culture with HI brain extract (Fig. 5), suggesting that hAFS have the potential to produce these trophic factors, which could contribute to hAFS migration to the injured lesion, protect host brain cells, and reduce neuroinflammation.
Gene expression of trophic factors in hAFS co-cultured with or without HIE-induced brain extract. HIE-induced brain extract induced mRNA expression of trophic factors in hAFS determined by RT-qPCR (n = 3). Results are presented as mean ± SEM. *p < 0.05 hAFS human amniotic fluid stem cells, HIE hypoxic–ischemic encephalopathy
Discussion
In the present study, we demonstrated that hAFS migrated to the brain through the intranasal route. hAFS successfully induced functional recovery by preserving neurons and oligodendrocytes, and by reducing neuroinflammation evoked by HI insult. We also found that HI-induced brain extract, harvested in the chronic phase, elevated a gene expression of a chemotactic factor to move the injured brain lesion. It also increased gene expressions of neurotrophic factors to stimulate neurons and oligodendrocytes to enhance their survival in hAFS. These results indicated that intranasal transplantation of hAFS can be used in the chronic phase of neonatal HIE to improve histological and functional outcomes. hAFS treatment effects will provide an experimental basis for further clinical application as a less invasive stem cell treatment.
The present study was designed to elucidate the therapeutic effects of hAFS on the chronic phase of HIE. In this study, we confirmed, using two sensorimotor tests to assess motor coordination and forelimb asymmetry (ROT and CRT), that hAFS treatment significantly attenuated sensorimotor dysfunction. In addition, hAFS treatment also showed restoration of neurons and oligodendrocytes, after HI insult. These favorable effects of hAFS on HIE are considered to be involved in hAFS capacity to secrete neurotrophic factors that prevent neuronal and oligodendrocyte loss subsequent to promoting endogenous neurogenesis and suppressing neuroinflammation after “homing.” Corcelli et al.11 recently reported that hAFS transplanted in the acute phase (at the time of HI insult) reduced the microglial response to neuronal death via modulating the TGFβ1/Smad 3 pathway in a paracrine manner. However, these neuroprotective strategies (i.e., prevention of cell death) could be applied in the acute to subacute phase of HIE, not in the chronic phase, because the brain lesion size is determined within 10 days after HI insult, as previously reported in rodents.17 In contrast, in the chronic phase of HIE, cellular and molecular neurorestorative mechanisms, which include neurogenesis, immunomodulation, and trophic factor secretion, have been implicated in the therapeutic effect of MSCs.23 Our study showed that hAFS induced gene expressions of neurotrophic factors, such as BDNF, NGF, and HGF, after the stimulation of brain extract harvested from the chronic phase of HIE mice in vitro. These neurotrophic factors can directly boost endogenous repair of HI brain injury to promote the neuroregenerative process in neurons and oligodendrocytes,24,25,26 and also suppress neuroinflammation including microglial activation and gliosis evoked by HI insult.5,27 Myelination is induced by blocking microglial activation that directs pro-oligodendrocyte precursors to differentiate into astrocytes, and not oligodendrocytes.27,28 Thus, these neurotrophic factors secreted from hAFS in contact with signals provided by HI-induced brain may play a crucial role in brain repair process in the chronic phase of HIE in our study. Considering the previous findings that hAFS have neuroprotective potential for the acute phase of HIE,11 hAFS could be a novel autologous stem cell treatment for HIE for a wide therapeutic period.
In this study, hAFS migrated to the brain lesion after nasal application and transiently engrafted to the brain. The “homing” to the brain lesion subsequently to the secretion of multiple trophic factors in the host tissue is considered the main mechanism of functional recovery in this study. Consistent with our results, several investigators have found that MSCs transplanted through the nasal route after HI insult could migrate to the brain lesion by crossing the cribiform plate and passing the rostral migratory stream.15,29 SDF-1 and its receptor, chemokine receptor type 4 (CXCR4), play a crucial role in the mechanism responsible for “homing.”30,31,32 In response to SDF-1 expression in the brain lesion after HI insult, which apparently has a longer duration in adult than in neonatal animals,33 hAFS may stimulate the expression of endogenous SDF-1 and also express SDF-1. Our data, obtained in vitro, support the view that hAFS are capable of secreting SDF-1 under the stimulation of brain tissue after HI insult. Given that hAFS have the potential to migrate to lesions via SDF-1 signaling, as shown in several other rodent models,34,35,36 hAFS intranasally transplanted may accumulate in the brain lesion due to the reaction of the SDF-1/CXCR4 axis.
The optimal route and timing for hAFS transplantation must be determined for clinical application. Although intravenous, intracerebral, intraperitoneal, and intranasal administrations of MSC were reported using rodent models of HIE, we selected intranasal application as a less invasive route. Our results were consistent with those of a previous report that intranasal transplantation of bone marrow-derived MSCs after HI insult could reduce brain damage.15 In case of intravenous stem cell administration, the pulmonary passage is considered to be a major obstacle, with a smaller number of stem cells crossing over and the majority of cells being confined inside the lungs.37 Additionally, intracerebral administration may be too invasive to translate into clinical study for neonates, and intraperitoneal route may be less efficacious compared to other routes of stem cell administration.38 Further studies are required to develop a safe and effective route of hAFS treatment for HIE. With respect to the timing of hAFS administration, we provided additional evidence that hAFS were also effective in the chronic phase of HIE, in addition to the acute phase as previously described.11 Hypothermia therapy and UCBC administration have been reported to be effective in the acute to subacute phase of HIE in humans.
Therefore, we should determine whether hAFS administration in the chronic phase could be an additional treatment option over hypothermia therapy and/or UCBC administration in the acute phase and whether hAFS could be also effective for HIE in humans.
For the clinical use of hAFS, we can collect amniotic fluid cells during pregnancies that are at greater risk of HIE, as indicated in cases of previous history of placental abruption, elderly pregnancy, and preterm labor. And then we can fabricate hAFS during pregnancy in advance so that a sufficient amount of autologous stem cells could be administered for HIE. Although a lack of evidence exists on the effectiveness of hAFS for HIE directly compared with other MSCs, including UCBC, hAFS has several definitive advantages for neonates as an autologous stem cell source. First, autologous hAFS can be immediately administered if severe fetal hypoxia occurs at birth as long as they are prepared before delivery. Second, there is adequate time to prepare a sufficient amount of hAFS via cell culture before delivery. Third, preconditioning, including hypoxic and spheroid culture, and drug stimulation could enhance the therapeutic effect of hAFS.39 Thus, hAFS could be a novel stem cell source for neonatal HIE compared to other MSCs, including UCBC, for autologous transplantation during the neonatal period.
In conclusion, this study showed that hAFS can migrate to the brain through the intranasal route and induce functional recovery by preserving the gray and white matter areas. Our results suggest that intranasal administration of hAFS could be a novel autologous stem cell treatment for neonatal HIE to prevent the development of cerebral palsy.
References
Hasegawa, J. et al. Relevant obstetric factors for cerebral palsy: from the nationwide obstetric compensation system in Japan. PLoS ONE 11, e0148122 (2016).
Ferriero, D. M. Neonatal brain injury. N. Engl. J. Med 351, 1985–1995 (2004).
Nabetani, M., Shintaku, H., Hamazaki, T. Future perspectives of cell therapy for neonatal hypoxic–ischemic encephalopathy. Pediatr Res. 83, 356–363 (2017).
Douglas-Escobar, M. & Weiss, M. D. Hypoxic–ischemic encephalopathy: a review for the clinician. JAMA Pediatr. 169, 397–403 (2015).
Gonzales-Portillo, G. S., Reyes, S., Aguirre, D., Pabon, M. M. & Borlongan, C. V. Stem cell therapy for neonatal hypoxic–ischemic encephalopathy. Front Neurol. 5, 147 (2014).
Gluckman, P. D. et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet 365, 663–670 (2005).
Shankaran, S. et al. Whole-body hypothermia for neonates with hypoxic–ischemic encephalopathy. N. Engl. J. Med. 353, 1574–1584 (2005).
Goldman, S. A. Stem and progenitor cell-based therapy of the central nervous system: hopes, hype, and wishful thinking. Cell Stem Cell 18, 174–188 (2016).
Johnston, M. V., Fatemi, A., Wilson, M. A. & Northington, F. Treatment advances in neonatal neuroprotection and neurointensive care. Lancet Neurol. 10, 372–382 (2011).
Cotten, C. M. et al. Feasibility of autologous cord blood cells for infants with hypoxic–ischemic encephalopathy. J. Pediatr. 164, 973–9.e1 (2014).
Corcelli, M. et al. Neuroprotection of the hypoxic–ischemic mouse brain by human CD117(+)CD90(+)CD105(+) amniotic fluid stem cells Sci. Rep. 8, 2425 (2018).
De Coppi, P. et al. Isolation of amniotic stem cell lines with potential for therapy. Nat. Biotechnol. 25, 100–106 (2007).
Ochiai, D. et al. Human amniotic fluid stem cells: therapeutic potential for perinatal patients with intractable neurological disease. Keio J. Med. in press (2018). https://doi.org/10.2302/kjm.2017-0019-IR.
Rice, J. E. III, Vannucci, R. C. & Brierley, J. B. The influence of immaturity on hypoxic–ischemic brain damage in the rat. Ann. Neurol. 9, 131–141 (1981).
van Velthoven, C. T., Kavelaars, A., van Bel, F. & Heijnen, C. J. Nasal administration of stem cells: a promising novel route to treat neonatal ischemic brain damage. Pediatr. Res. 68, 419–422 (2010).
Kidani, Y. et al. The therapeutic effect of CD133(+) cells derived from human umbilical cord blood on neonatal mouse hypoxic–ischemic encephalopathy model. Life Sci. 157, 108–115 (2016).
van Velthoven, C. T., Kavelaars, A., van Bel, F. & Heijnen, C. J. Mesenchymal stem cell treatment after neonatal hypoxic–ischemic brain injury improves behavioral outcome and induces neuronal and oligodendrocyte regeneration. Brain Behav. Immun. 24, 387–393 (2010).
van der Kooij, M. A. et al. Mild neonatal hypoxia–ischemia induces long-term motor- and cognitive impairments in mice. Brain Behav. Immun. 24, 850–856 (2010).
Deacon, R. M. Measuring motor coordination in mice. J. Vis. Exp. 75, e2609 (2013) https://doi.org/10.2302/kjm.2017-0019-IR.
Donega, V. et al. Intranasally administered mesenchymal stem cells promote a regenerative niche for repair of neonatal ischemic brain injury. Exp. Neurol. 261, 53–64 (2014).
Liu, F. & McCullough, L. D. Inflammatory responses in hypoxic–ischemic encephalopathy. Acta Pharmacol. Sin. 34, 1121–1130 (2013).
Li, Y. et al. Human marrow stromal cell therapy for stroke in rat: neurotrophins and functional recovery. Neurology 59, 514–523 (2002).
van Velthoven, C. T., Kavelaars, A., van Bel, F. & Heijnen, C. J. Repeated mesenchymal stem cell treatment after neonatal hypoxia–ischemia has distinct effects on formation and maturation of new neurons and oligodendrocytes leading to restoration of damage, corticospinal motor tract activity, and sensorimotor function. J. Neurosci. 30, 9603–9611 (2010).
Xiao, J. et al. Brain-derived neurotrophic factor promotes central nervous system myelination via a direct effect upon oligodendrocytes. Neurosignals 18, 186–202 (2010).
Aloe, L., Rocco, M. L., Bianchi, P. & Manni, L. Nerve growth factor: from the early discoveries to the potential clinical use. J. Transl. Med. 10, 239 (2012).
Ohya, W., Funakoshi, H., Kurosawa, T. & Nakamura, T. Hepatocyte growth factor (HGF) promotes oligodendrocyte progenitor cell proliferation and inhibits its differentiation during postnatal development in the rat. Brain Res. 1147, 51–65 (2007).
Kaur, C., Rathnasamy, G. & Ling, E. A. Roles of activated microglia in hypoxia induced neuroinflammation in the developing brain and the retina. J. Neuroimmune Pharmacol. 8, 66–78 (2013).
Bain, J. M., Ziegler, A., Yang, Z., Levison, S. W. & Sen, E. TGFbeta1 stimulates the over-production of white matter astrocytes from precursors of the “brain marrow” in a rodent model of neonatal encephalopathy. PLoS ONE 5, e9567 (2010).
Danielyan, L. et al. Intranasal delivery of cells to the brain. Eur. J. Cell Biol. 88, 315–324 (2009).
Bogoslovsky, T. et al. Stromal-derived factor-1[alpha] correlates with circulating endothelial progenitor cells and with acute lesion volume in stroke patients. Stroke 42, 618–625 (2011).
Rosenkranz, K. et al. The chemokine SDF-1/CXCL12 contributes to the “homing” of umbilical cord blood cells to a hypoxic–ischemic lesion in the rat brain. J. Neurosci. Res. 88, 1223–1233 (2010).
Song, M., Mohamad, O., Gu, X., Wei, L. & Yu, S. P. Restoration of intracortical and thalamocortical circuits after transplantation of bone marrow mesenchymal stem cells into the ischemic brain of mice. Cell Transplant. 22, 2001–2015 (2013).
Miller, J. T. et al. The neuroblast and angioblast chemotaxic factor SDF-1 (CXCL12) expression is briefly up regulated by reactive astrocytes in brain following neonatal hypoxic–ischemic injury. BMC Neurosci. 6, 63 (2005).
Garcia, O. et al. Amniotic fluid stem cells inhibit the progression of bleomycin-induced pulmonary fibrosis via CCL2 modulation in bronchoalveolar lavage. PLoS ONE 8, e71679 (2013).
Sun, D. et al. Therapeutic effects of human amniotic fluid-derived stem cells on renal interstitial fibrosis in a murine model of unilateral ureteral obstruction. PLoS ONE 8, e65042 (2013).
Yang, D. Y. et al. Dual regeneration of muscle and nerve by intravenous administration of human amniotic fluid-derived mesenchymal stem cells regulated by stromal cell-derived factor-1alpha in a sciatic nerve injury model. J. Neurosurg. 116, 1357–1367 (2012).
Fischer, U. M. et al. Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect. Stem Cells Dev. 18, 683–692 (2009).
Ohshima, M. et al. Intraperitoneal and intravenous deliveries are not comparable in terms of drug efficacy and cell distribution in neonatal mice with hypoxia–ischemia. Brain Dev. 37, 376–386 (2015).
Dziadosz, M., Chan, M., Basch, R., Young, B. K. Effects of pharmacological agents on human amniotic fluid-derived stem cells in culture. Stem Cells Dev. (2016). https://doi.org/10.1089/scd.2016.0141.
Acknowledgements
Funding
This work was supported by JSPS Grant-in-Aid for Scientific Research (C) Grant Number JP15K09724, JSPS Grant-in-Aid for Scientific Research (B) Grant Number 17H04236, JSPS Grant-in-Aid for Challenging Exploratory Research Grant Number JP16K15536, JAOG Ogyaa Donation Foundation, Japan Spina Bifida and Hydrocephalus Research Foundation, Keio University Academic Development Funds research funding (individual research), and Kawano Masanori Memorial Public Interest Incorporated Foundation for Promotion of Pediatrics.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Otani, T., Ochiai, D., Masuda, H. et al. The neurorestorative effect of human amniotic fluid stem cells on the chronic phase of neonatal hypoxic–ischemic encephalopathy in mice. Pediatr Res 85, 97–104 (2019). https://doi.org/10.1038/s41390-018-0131-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-018-0131-8
This article is cited by
-
Prophylactic administration of human amniotic fluid stem cells suppresses inflammation-induced preterm birth via macrophage polarization
Molecular and Cellular Biochemistry (2023)
-
Insights on the Human Amniotic Membrane in Clinical Practice with a Focus on the New Applications in Retinal Surgery
Regenerative Engineering and Translational Medicine (2022)
-
Microglia and Stem-Cell Mediated Neuroprotection after Neonatal Hypoxia-Ischemia
Stem Cell Reviews and Reports (2022)
-
MSCs derived from amniotic fluid and umbilical cord require different administration schemes and exert different curative effects on different tissues in rats with CLP-induced sepsis
Stem Cell Research & Therapy (2021)
-
Prophylactic therapy with human amniotic fluid stem cells improved survival in a rat model of lipopolysaccharide-induced neonatal sepsis through immunomodulation via aggregates with peritoneal macrophages
Stem Cell Research & Therapy (2020)