Abstract
Background
Extrauterine life is an important factor when considering brain maturation. Few studies have investigated the development of visual evoked potentials (VEP) in extremely preterm infants, and only a minority have taken into consideration the impact of extrauterine life. The aim of this study was to assess the normal maturation of VEP in infants born prior to 29 weeks gestational age (GA) and to explore the potential influence of extrauterine life.
Methods
VEP were prospectively recorded in extremely preterm infants, and principal peaks (N0, N1, P1, N2, P2, N3) were identified. The mean of peak-time and percentages of peak appearances were assessed for three GA groups (23/24, 25/26, 27/28 weeks) and four subgroups of increasing postnatal age (PNA), up to 8 weeks after birth.
Results
A total of 163 VEP recordings in 38 preterm infants were analyzed. With increasing GA at birth, peak-times decreased. When comparing infants with equal GA but longer extrauterine life, those with the highest PNA demonstrated the shortest VEP peak-times. However, this effect was less present in infants born prior to 25 weeks GA.
Conclusion
Provided that a certain maturational threshold is reached, extrauterine life appears to accelerate maturation of the visual system in preterm infants.
Similar content being viewed by others
Introduction
Despite constant improvement in outcomes for infants born prematurely, the issue of neurodevelopmental impairment in this fragile patient population is still a source of grave concern. The identification of preterm infants at risk for impaired neurological development is of great importance to achieve the best possible long-term outcome. Several morphological (cerebral ultrasound, MRI) and neurophysiological (EEG, amplitude-integrated EEG, evoked potentials) methods can be used to assess brain maturation in preterm infants. Visual evoked potentials (VEP), which provide information about the current functional state of the brain (in particular, the visual system), are easily performed at the patient’s bedside and have proven to be a feasible electrophysiological method in young preterm infants.1,2,3,4,5,6,7,8 It is known that one of the most vulnerable regions in the preterm brain is the periventricular white matter, which includes major parts of the optic radiation. Therefore, in case of white matter damage, pathologies in these areas impact VEP results9,10,11 and, hence, VEP are a reasonable method to test brain function in preterm infants.
Identification of pathologic VEP recordings in preterm infants requires knowledge of normal configurations and maturation at different gestational ages (GA). There are some studies available describing waves and peak-times in preterm infants,10, 12, 13 but only a few publications provide reference values for flash VEP in extremely preterm born infants.3, 14 So far, the normal maturation of VEP in extremely preterm born infants is not elucidated completely. Additionally, recent studies suggest that prematurity and experiences of early extrauterine life might alter the brain morphology and function,15,16,17,18 which has not been considered in most previous studies about VEP in preterm infants. Therefore, the aim of this study was to assess normal maturation of flash VEP in extremely preterm infants and to explore the potential influence of extrauterine life on maturation of VEP.
Methods
During July 2010 and December 2012, infants born prior to 29 weeks GA at the Medical University of Vienna (Austria) were consecutively included in the study after informed consent was obtained. Exclusion criteria were birth asphyxia, abnormalities on cranial ultrasound (malformations, intraventricular hemorrhage (IVH) ≥ 3, periventricular leukomalacia (PVL) ≥ 2), retinopathy of prematurity (ROP) ≥ 3, or an impaired neurodevelopmental outcome at 24 months. The study was approved by the local ethics committee (EK 068/2008) and registered in the clinical trials registry (NCT00768586).
Visual evoked potentials
Flash VEP recordings were performed as soon as the clinical condition of the preterm was stable and informed parental consent was obtained. The frequency of recordings was once per week up to a corrected age of 32 weeks, when the intervals were prolonged to 2 weeks. Postnatal age (PNA) was documented at every VEP recording as well head circumference, vital parameters, and medication. None of the patients received medication that potentially impacts brain function at the time point of VEP recording (sedation/analgesia). The time point for measurements was standardized to be immediately after nursing rounds and therefore usually started in a wakefulness state. For the assessment of arousal state, vital signs and behavior of the infants (movements, eye opening) were recorded. A Nihon Koden MEB-9400K Neuropack S1 was used with surface gold electrodes positioned at Oz (+) and at Fz (−), according to the international 10/20-system. The clinical protocol followed the ISCEV standard in its design but was highly adapted to the fragile population of preterm infants.4, 19, 20 Red-light LED goggles (0.4 cd/m2) were held at a distance of 5 cm in front of the infant’s eyes, the impedance was kept below 5 kΩ, the stimulation frequency was 0.7 Hz, the bandpass-filter was 1–100 Hz, and the sweep time was one second. The procedure was performed in the neonatal ward, where the environment routinely is semi-dark (dimmed ceiling illumination; use of spotlight during procedures while infant’s eyes are covered) and the incubator usually covered. Each VEP recording included two sets of 30 averages of the responses to binocular stimulation, with an absolute minimum of ten averages in cases of an extreme fatigue effect. The peaks were analyzed as previously published (Fig. 1).3
Flash VEP in a preterm infant born at a GA of 26 weeks and two days, recorded on postnatal day 7; nomenclature as previously published by Pike et al.3
Neurodevelopment
Neurodevelopmental follow-up was performed for up to 24 months corrected age to avoid a misleading inclusion of seriously impaired infants. Bayley Scales of Infant Development (BSID) were conducted. Owing to an adaptation of the routine neurodevelopmental follow-up protocol at the Medical University Vienna within the study period, the first 27 patients were analyzed using the second edition of the Bayley Scales of Infant Development21 and 11 patients were examined using the German version of the third edition.22 According to BSID-II, Mental Developmental Index (MDI) and Psychomotor Developmental Index (PDI) were obtained. In the third version of BSID the Motor Composite Score (MCS) was assessed and (instead of MDI) the calculated mean value of the Cognitive Composite Score (CCS) and the Language Composite Score (LCS) was used. Also, gross motor function classification scales (GMFCS) were conducted.23 Data of infants with severely abnormal results were excluded (MDI, PDI, CCS, LCS or MCS < 70, GMFCS > II).
Statistical analysis
Statistical calculations were performed using IBM SPSS Statistics 20 (Armonk, New York) and SAS 9.4 (SAS Institute, NC). A basis for reference values was calculated as median with standard deviation and 5th/95th percentile for the three GA groups (I: 23 + 0 to 24 + 6, II: 25 + 0 to 26 + 6, and III: 27 + 0 to 28 + 6). They were subdivided further into four groups according to the 1st/2nd, 3rd/4th, 5th/6th, and 7th/8th week of life. Normal distribution of raw values and regression residuals was determined by visual inspection.
A multivariable linear regression was performed to assess the impact of increasing age on peak-times and a logistic regression model for the impact of increasing age on peak appearances. Multiple observations per patient were taken into account. Calculations were done using the SAS procedure genmod and the GEE model options. GA at birth (GA) and postnatal age at recording (PNA, i.e., corrected GA) were used as independent, N0, N1, P1, N2, P2, N3 as dependent variables. The correlation structure “exchangeable” was used for describing the dependency between repeated measurements. The interaction term GA times PNA was added to the model to look for a dependency of the effect of PNA on GA. As the early components (N0, N1, P1) showed only rare appearances, peak-time analysis was done only for the late components (N2, P2, N3). However, the late components showed very high appearance rates, so that the calculation for peak appearances was done only for the early components. The level of significance was p < 0.05. The initial study design focused on assessment of normal maturation, and the hypotheses of accelerated maturation was not planned in advance. Therefore, no adjustments were made for multiple tests so p-values should be interpreted as exploratory only.
Results
A total of 58 infants were included in the study, of whom 13 had to be excluded due to later developing pathologies (twelve ROP ≥ 3, four IVH ≥ 3, one PVL ≥ 2, one patient with agenesis of the septum pellucidum, and one subject whose Bayley MDI and PDI were <70 at 24 months corrected age). Two patients died and five were lost to follow-up.
Of the remaining 38 infants, 163 VEP were recorded, ranging from one to nine per patient (median 3). The PNA at first recording was 3–19 days (median 9.5 days). Patient characteristics can be taken in detail from Table 1.
Peak-times
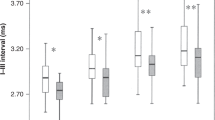
VEP could be recorded in all neonates, independent of their GA. Means of peak-times (in ms) for each GA group and for increasing PNA are given in Table 2. Analysis of the data revealed a significant association between shorter VEP peak-times and both GA and PNA (Table 3 and Supplemental Table S1). However, the impact of PNA on peak-times showed a significant dependency on the initial underlying GA, and this influence rose with increasing GA (Table 3 and Supplemental Table S2). In a subgroup analysis of the three GA groups, infants born at 23/24 weeks showed a significant acceleration with increasing PNA in VEP peak-times only in the N3 component. Those born at 25/26 weeks revealed a significant result for P2 and N3 peak-times, and within the group of infants born at 27/28 weeks peak-times were associated with PNA in all three components (N2, P2, and N3) and showed accelerated results (Fig. 2 and Table 3).
There was no influence of head circumference on the peak-times N2, P2, or N3 in none of the three GA groups (data not shown). A significant impact was seen within infants of different gender since females revealed shorter peak-times for N2 and P2 compared to males (p = 0.014 and p < 0.001, respectively). There was no significant influence of gender on the peak-time of N3.
Peak appearance
In contrast to the late VEP peaks with very high detection rates (N2: 100, P2: 95, and N3: 88%, respectively) the early components N0, N1, and P1 could not be detected reliably in our study cohort (11, 13, and 43%, respectively). Therefore, we primarily studied their manner of appearance, not their peak-time behavior.
In preterm infants of 23/24 weeks GA, P1 was the first to appear after three to four weeks PNA, followed predominately by N1 and N0 (at seven to eight weeks of PNA). The sequence of component appearance in older preterm infants was essentially similar (Fig. 3). There was a significant association between GA at birth only for P1 (p = 0.016), not for N0 or N1 (p = 0.258, respective 0.770). Peak appearances were mainly dependent on PNA in 23/24 weeks and 25/26 weeks, but within the group of infants born 27/28 weeks GA only P1 peak appearance was associated with PNA (see Table 3)
Discussion
In the present study, we were interested in monitoring brain maturation in preterm infants by testing their response to flash VEP. Using flash VEP, we could follow the acceleration in neuronal signal transduction to the visual cortex with increasing age as represented by a decrease in VEP peak-times. What this study adds to previously published data is evidence that extrauterine life impacts the maturation of the visual system in preterm infants. We provide a valid approach for future generation of reference values, considering not only GA at birth but also PNA at the time of recording.
A progressive acceleration of VEP peak-times is observed during early cerebral development with increasing myelination.3, 13, 24 Recent literature, however, suggests that besides the well-known process of genetically driven development, environmental influences also have an impact on brain maturation. Though still debated controversially in the literature,25 it seems that at term equivalent age brain structure and function of preterm infants differ from term born neonates.26, 27 This has been shown for electrophysiology (aEEG), cerebral morphology, and behavioral outcome.15,16,17 It is assumed that neuronal activity and repetitive stimulation lead to consolidation of specific cerebral pathways, which is described as activity-dependent brain development.28 We know from recent publications that it is not only external (mainly postpartum) stimulation but also previously occurring patterns of endogenous neural activation that strengthen certain pathways.29, 30 In preterm born infants precocious visual stimulation is unavoidable and occurs at a developmental stage when visual activation normally is mostly generated endogenously.
In the present study, for example an infant born at 25 weeks GA and recorded at a PNA of 4 weeks revealed shorter peak-times and higher appearance rates of VEP components, compared to an infant born at 28 weeks and recorded one week after birth, although they both have the same corrected age. This indicates that increasing PNA hastens brain maturation in preterm infants and it may be speculated that this is caused by early and additional external visual stimulation added to endogenous stimuli. While we present evidence that VEP peak-times decrease with increasing PNA, other studies revealed different results. Tsuneishi et al.31 reported that in their cohort the absolute peak-time values did not change with increasing age but they observed a change in the VEP waveforms. The difference in the study results may be explained by the different patient population. Since they analyzed two heterogeneous patient groups of infants born below and above 31 weeks GA, we focused on infants born below 28 weeks and analyzed each subgroup (23/24, 25/28, 27/28 weeks GA at birth) separately. However, our results are in accordance with other previously published data presenting evidence that the available extra stimulation time in preterm infants indeed alters maturation of the visual system.3, 13, 24, 32, 33 According to this, lights and also noises on our NICU are reduced to a minimum, to reach an environment similar to intrauterine conditions (at least as far as possible).
However, within our data, the impact of PNA (and therefore external stimulation) seems to depend on the initial GA at birth. The effect was less significant (only for N3) in the most immature infants born at 23 and 24 weeks GA but was increasingly seen in higher GA groups (N2, P2, and N3 in 27/28 weeks). We assume that at the developmental stage of 23/24 weeks GA the (endogenously triggered) maturation of the visual system might have not yet reached the required threshold to be able to adapt to external stimulation.
Taking into consideration the effect of both GA and PNA on maturation of the visual system within our study, we postulate that reference values based on GA at birth alone will not provide reliable and valid data. This is in accordance with a report of Madan et al.34, who question previous VEP studies due to heterogeneous patient populations with too many different gestational and postnatal ages in one cohort. We therefore present a model for reference values that distinguish between GA and PNA, and hence more reliably reflect the maturational status of these infants. Due to the small number of patients in our study, future trials with larger sample sizes will have to confirm our findings.
What remains unclear is what the accelerated VEP maturation in preterm infants implies for their long-term outcome. On the one hand a shortened peak-time might just reflect an early (normal) maturation resulting from adaptation to precocious visual stimulation. However, on the other hand we know from recent studies that sensory overstimulation (environmental conditions in different neonatal intensive care settings) can compromise normal brain development in preterm infants and might lead to neurocognitive and behavioral disturbances.35 Hence, the observed acceleration in VEP maturation could also be an early sign for sensory modulation disturbances resulting in impaired long-term outcome in these infants.
Scherjon and colleagues compared VEP results of preterm infants (26–33 weeks GA) according to the occurrence of brain sparing in prenatal sonography (redistributed perfusion in favor of cerebral blood flow compared to umbilical arterial perfusion) and looked for associations with later cognitive outcome. Interestingly, at 6 months of corrected age the peak-times of the infants with brain sparing were significantly shorter than those in the control group. Moreover, accelerated VEP results were an independent predictor of adverse cognitive outcome (RAKIT-IQ score < 85; Revision of the Amsterdam Children’s Intelligence Test) at 5 years of age.36 Clearly, further studies dedicated to analyzing the association of VEP results with later neurodevelopmental outcome are warranted.
In addition to the acceleration of VEP peak-times with increasing age, we observed that early VEP components appeared in a defined sequence that was closely associated with both GA and PNA. This phenomenon has been previously described in preterm infants of higher GA.3, 13, 24 Additionally, we were able to confirm the specific sequence of component appearance of P1 followed by N1 and N0 in extremely premature infants. Furthermore, we showed a more frequent occurrence of these early peaks with increasing GA and PNA. However, the significance of the appearance of these components in the discrete developmental steps of brain maturation are yet to be elucidated in future studies.
The major limitation of our study is the small number of patients. The intention was to record VEP as early as possible. In these extremely preterm infants, however, study inclusion at that early period of life was hampered by the high vulnerability and clinical instability in many of the patients. Depending on the duration of clinical stabilization, VEP recordings were started on different postnatal days (three to 19; mean 9.5 days), which is another limitation of the study. Additionally, regarding correlation calculations, it has to be taken into account that the calculations were done with multiple measures and therefore a bias towards those infants with a greater number of longitudinal recordings cannot be excluded. Data collection in extremely preterm infants is by itself very challenging and study protocols at some points have to be restricted to a level that would not be appropriate in older children or adults. Although we were able to provide a first step in the generation of reference values, with the small sample size of this study, our results have to be validated in larger studies with higher number of patients.
Another limitation of our study is the lack of identification of arousal state by a standardized electrophysiological method. Identification of arousal state is important for interpretation of VEP results, since waveform, peak-time and amplitudes might be influenced.12, 24, 37 The gold standard hereof is polysomnography. Another accepted technique is (amplitude-integrated) EEG. However, state-classification in this age group is controversial, and a clear wake-state is often difficult to determine in these extremely premature infants. Due to the high vulnerability of our study population, we refrained from further procedures prolonging the examination time and rather tried to restrict interventions to a minimum. Therefore, our approach concerning the issue of arousal state was to standardize the time points of recordings immediately after nursing rounds and to assess vital signs and infant’s behavior during VEP recordings.
VEP results might be influenced by various factors such as gender, nutrition,38, 39 environmental conditions, or head circumference.40 Within our data, there was no significant association between head circumference and peak-times. However, gender seemed to significantly influence the peak-times of N2 and P2. Though this result has to be interpreted with care, since females are overrepresented in our dataset, it nevertheless confirms previously published data with evidence, that females seem to have shorter peak-times compared to males.41, 42 Future studies with higher patient numbers are required to analyze this issue. Also, with increasing GA GABAergic neurotransmitter function in the preterm brain is altered. While excitatory GABA receptors are overexpressed in early gestational weeks, the number of inhibitory GABA receptors rises with increasing age.43 Though no GABAergic medication was given to any of the study participants, and the exact mechanisms of the GABA system is not yet understood completely, the alteration of neurotransmitter function in preterm infants probably also contributes to maturational changes in neurophysiology.
Another factor that has to be considered are the fused eyelids in extremely preterm infants, occurring in infants below 25 weeks GA.44 Since the effect of PNA on VEP results in our study population was observed more significant in infants born above 25 weeks, fused eyelids might be seen as a natural protection against overstimulation in these early maturational stages.
Although both peak-time and amplitude seem to be affected by sleep state, the former has been shown to be more robust and this is why we decided to focus on peak-times in the present study.37 Notwithstanding, simultaneous assessment of sleep state and VEP amplitudes might provide additional information and should therefore be considered when designing future studies.
Conclusion
We were able to show for the first time that in addition to GA, the duration of extrauterine life is critical for the acceleration of stimuli transmission to the visual cortex in extremely premature infants and that this development is subject to a sequential program. Since the decrease in peak-times was less seen in the most immature infants of 23 and 24 weeks GA, we hypothesize that fetal brains must first cross a specific developmental threshold before extrauterine life impacts on their neuronal signal transduction abilities. On these grounds, we think that reference values—also for other electrophysiological methods—should take into consideration not only GA but also PNA and the influence of extrauterine life should be target of future research in preterm infants. Future work designed to link VEP peak appearance with specific developmental milestones in fetal brain development might help to reveal the exact mechanisms of brain maturation, which in turn may improve our ability to provide optimal care for even extremely preterm infants and increase their chances of survival and intact brain development.
References
Chayasirisobhon, S. et al. Evaluation of maturation and function of visual pathways in neonates: role of flash visual-evoked potentials revisited. Clin. EEG Neurosci. 43, 18–22 (2012).
Kraemer, M., Abrahamsson, M. & Sjostrom, A. The neonatal development of the light flash visual evoked potential. Doc. Ophthalmol. 99, 21–39 (1999).
Pike, A. A., Marlow, N. & Reber, C. Maturation of the flash visual evoked potential in preterm infants. Early Hum. Dev. 54, 215–222 (1999).
Taylor, M. J., Saliba, E. & Laugier, J. Use of evoked potentials in preterm neonates. Arch. Dis. Child Fetal Neonatal Ed. 74, F70–F76 (1996).
Tsuneishi, S. Evaluation of the developing human visual system using flash-visual evoked potential. No Hattatsu 34, 141–146 (2002).
Roy, M. S., Barsoum-Homsy, M., Orquin, J. & Benoit, J. Maturation of binocular pattern visual evoked potentials in normal full-term and preterm infants from 1 to 6 months of age. Pediatr. Res. 37, 140–144 (1995).
Mirabella, G., Kjaer, P. K., Norcia, A. M., Good, W. V. & Madan, A. Visual development in very low birth weight infants. Pediatr. Res. 60, 435–439 (2006).
Woods, J. R. Jr & Plessinger, M. A. The fetal visual evoked potential. Pediatr. Res. 20, 351–355 (1986).
Klebermass-Schrehof, K. et al. Can neurophysiological assessment improve timing of intervention in posthaemorrhagic ventricular dilatation? Arch. Dis. Child Fetal Neonatal Ed. 98, F291–F297 (2013).
Taylor, M. J., Murphy, W. J. & Whyte, H. E. Prognostic reliability of somatosensory and visual evoked potentials of asphyxiated term infants. Dev. Med. Child Neurol. 34, 507–515 (1992).
Whyte, H. E. Visual-evoked potentials in neonates following asphyxia. Clin. Perinatol. 20, 451–461 (1993).
Feng, J. J., Wang, W. P., Guo, S. J., Liu, Z. W. & Xu, X. Flash visual evoked potentials in preterm infants. Ophthalmology 120, 489–494 (2013).
Tsuneishi, S. & Casaer, P. Stepwise decrease in VEP latencies and the process of myelination in the human visual pathway. Brain Dev. 19, 547–551 (1997).
Tsuneishi, S., Casaer, P., Fock, J. M. & Hirano, S. Establishment of normal values for flash visual evoked potentials (VEPs) in preterm infants: a longitudinal study with special reference to two components of the N1 wave. Electroencephalogr. Clin. Neurophysiol. 96, 291–299 (1995).
Pineda, R. G. et al. Alterations in brain structure and neurodevelopmental outcome in preterm infants hospitalized in different neonatal intensive care unit environments. J. Pediatr. 164, 52–60.e2 (2014).
Healy, E. et al. Preterm birth and adolescent social functioning-alterations in emotion-processing brain areas. J. Pediatr. 163, 1596–1604 (2013).
Smith, G. C. et al. Neonatal intensive care unit stress is associated with brain development in preterm infants. Ann. Neurol. 70, 541–549 (2011).
Klebermass, K. et al. Intra- and extrauterine maturation of amplitude-integrated electroencephalographic activity in preterm infants younger than 30 weeks of gestation. Biol. Neonate 89, 120–125 (2006).
Odom, J. V. et al. ISCEV standard for clinical visual evoked potentials (2009 update). Doc. Ophthalmol. 120, 111–119 (2010).
Schomer, D. & Lopes da Silva, F. (eds) Niedermeyer’s Electroencephalography 6th edn (Lippincott Williams & Wilkins, Phildelphia, PA, 2012).
Bayley, N. Bayley Scales of Infant Development II (Psychological Corp, San Antonio, TX, 1993).
Reuner, G. R. J., Buschmann, A., Bach, C. & Pietz, J. German version and standardization of the Bayley Scales of infant and toddler development, 3rd edition: first results on clinical validity and criteria validity of the cognitive scale and language scale. Neuropediatrics 46(S01), FV01 (2015).
Rosenbaum, P. L., Palisano, R. J., Bartlett, D. J., Galuppi, B. E. & Russell, D. J. Development of the Gross Motor Function Classification System for cerebral palsy. Dev. Med. Child Neurol. 50, 249–253 (2008).
Taylor, M. J., Menzies, R., MacMillan, L. J. & Whyte, H. E. VEPs in normal full-term and premature neonates: longitudinal versus cross-sectional data. Electroencephalogr. Clin. Neurophysiol. 68, 20–27 (1987).
O’Reilly, M. et al. Ophthalmological, cognitive, electrophysiological and MRI assessment of visual processing in preterm children without major neuromotor impairment. Dev. Sci. 13, 692–705 (2010).
Inder, T. E., Warfield, S. K., Wang, H., Huppi, P. S. & Volpe, J. J. Abnormal cerebral structure is present at term in premature infants. Pediatrics 115, 286–294 (2005).
Soubasi, V. et al. The influence of extrauterine life on the aEEG maturation in normal preterm infants. Early Hum. Dev. 85, 761–765 (2009).
Penn, A. S. C. Principles of Endogenous and Sensory Activity-Dependent Brain Development: The Visual System. The Newborn Brain 147–161 (Cambridge University Press, Cambridge, 2010).
Penn, A. A. Early brain wiring: activity-dependent processes. Schizophr. Bull. 27, 337–347 (2001).
Fulford, J. et al. Fetal brain activity in response to a visual stimulus. Hum. Brain Mapp. 20, 239–245 (2003).
Tsuneishi, S. & Casaer, P. Effects of preterm extrauterine visual experience on the development of the human visual system: a flash VEP study. Dev. Med. Child Neurol. 42, 663–668 (2000).
Tremblay, E. et al. Delayed early primary visual pathway development in premature infants: high density electrophysiological evidence. PLoS ONE 9, e107992 (2014).
Jando, G. et al. Early-onset binocularity in preterm infants reveals experience-dependent visual development in humans. Proc. Natl Acad. Sci. USA 109, 11049–11052 (2012).
Madan, A., Jan, J. E. & Good, W. V. Visual development in preterm infants. Dev. Med. Child Neurol. 47, 276–280 (2005).
Broring, T., Oostrom, K. J., Lafeber, H. N., Jansma, E. P. & Oosterlaan, J. Sensory modulation in preterm children: theoretical perspective and systematic review. PLoS ONE 12, e0170828 (2017).
Scherjon, S., Briet, J., Oosting, H. & Kok, J. The discrepancy between maturation of visual-evoked potentials and cognitive outcome at five years in very preterm infants with and without hemodynamic signs of fetal brain-sparing. Pediatrics 105, 385–391 (2000).
Whyte, H. E., Pearce, J. M. & Taylor, M. J. Changes in the VEP in preterm neonates with arousal states, as assessed by EEG monitoring. Electroencephalogr. Clin. Neurophysiol. 68, 223–225 (1987).
Bauer, I. et al. Omega-3 fatty acids modify human cortical visual processing—a double-blind, crossover study. PLoS ONE 6, e28214 (2011).
Faldella, G. et al. Visual evoked potentials and dietary long chain polyunsaturated fatty acids in preterm infants. Arch. Dis. Child Fetal Neonatal Ed. 75, F108–F112 (1996).
Kothari, R., Singh, R., Singh, S. & Bokariya, P. Effect of head circumference on parameters of pattern reversal visual evoked potential in healthy adults of central India. Nepal Med. Coll. J. 14, 75–79 (2012).
Malcolm, C. A., McCulloch, D. L. & Shepherd, A. J. Pattern-reversal visual evoked potentials in infants: gender differences during early visual maturation. Dev. Med. Child Neurol. 44, 345–351 (2002).
Sharma, R., Joshi, S., Singh, K. D. & Kumar, A. Visual evoked potentials: normative values and gender differences. J. Clin. Diagn. Res. 9, CC12–CC15 (2015).
Sanchez Fernandez, I., Loddenkemper, T., Peters, J. M. & Kothare, S. V. Electrical status epilepticus in sleep: clinical presentation and pathophysiology. Pediatr. Neurol. 47, 390–410 (2012).
Duerksen, K., Barlow, W. E. & Stasior, O. G. Fused eyelids in premature infants. Ophthal. Plast. Reconstr. Surg. 10, 234–240 (1994).
Acknowledgements
We are very grateful to all patients and parents for participating in this study, and we thank Aner Gurvitz, who advised us in English writing and critically revised the manuscript. This work was supported by the OeNB (Oesterreichische Nationalbank Anniversary Fund Nr. 13232). The sponsor was not involved in any part of this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Schwindt, E., Giordano, V., Rona, Z. et al. The impact of extrauterine life on visual maturation in extremely preterm born infants. Pediatr Res 84, 403–410 (2018). https://doi.org/10.1038/s41390-018-0084-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-018-0084-y