Abstract
Rare but recurrent mutations in the fibroblast growth factor receptor (FGFR) pathways, most commonly in one of the four FGFR receptor tyrosine kinase genes, can potentially be targeted with broad-spectrum multi-kinase or FGFR selective inhibitors. The complete spectrum of these mutations in paediatric cancers is emerging as precision medicine programs perform comprehensive sequencing of individual tumours. Identification of patients most likely to benefit from FGFR inhibition currently rests on identifying activating FGFR mutations, gene fusions, or gene amplification events. However, the expanding use of transcriptome sequencing (RNAseq) has identified that many tumours overexpress FGFRs, in the absence of any genomic aberration. The challenge now presented is to determine when this indicates true FGFR oncogenic activity. Under-appreciated mechanisms of FGFR pathway activation, including alternate FGFR transcript expression and concomitant FGFR and FGF ligand expression, may mark those tumours where FGFR overexpression is indicative of a dependence on FGFR signalling. In this review, we provide a comprehensive and mechanistic overview of FGFR pathway aberrations and their functional consequences in paediatric cancer. We explore how FGFR over expression might be associated with true receptor activation. Further, we discuss the therapeutic implications of these aberrations in the paediatric setting and outline current and emerging therapeutic strategies to treat paediatric patients with FGFR-driven cancers.

Similar content being viewed by others
Introduction
Receptor tyrosine kinases (RTKs) are cell surface receptors containing intracellular kinase domains. They regulate fundamental cellular processes including cell survival, proliferation, differentiation, and metabolism [1]. Large-scale genomic studies have shown that many kinase genes, including those that encode RTKs and non-receptor tyrosine kinases (NRTKs), are recurrently altered in cancer [2], and biological studies have characterised many as bona fide cancer driver genes. The most notable example in paediatric cancers being the BCR-ABL1 fusion gene in B-cell acute lymphoblastic leukaemia (B-ALL) [3]. Strikingly, many other kinase-activating fusions that phenocopy BCR-ABL1 are now recognised in B-ALL, and characterise the new Ph-like B-ALL entity [4]. The importance of establishing the oncogenicity of tyrosine kinase (TK) driver genes is exemplified by the remarkable clinical success of ABL-targeted multi-kinase inhibitors, including imatinib [5] and dasatinib [6], in BCR-ABL1 + and Ph-like B-ALL. Another remarkable success in this field is the NTRK-inhibitor larotrectinib, which has demonstrated clinical efficacy in a range of adult and paediatric cancers specifically driven by NTRK fusion genes [7]. There are, however, many more dysregulated TKs and TK pathways that represent potential drivers and novel drug targets in paediatric cancer that warrant further investigation. One that deserves further attention is the Fibroblast Growth Factor Receptor (FGFR) pathway.
An increasing body of data shows that aberrations in FGFR genes are present in many paediatric cancer types. The common FGFR genomic alterations include gene amplifications, gene mutations or single nucleotide variants (SNVs), and structural variants (SVs), which promote stemness, proliferation, angiogenesis, epithelial-mesenchymal transition, invasion and drug resistance in cancer cells [8]. Understanding whether FGFR RNA overexpression, even in the absence of genomic alteration, represents another important but less well understood mechanism of oncogenesis has the potential to expand the therapeutic reach of FGFR inhibitors [9].
Pan-cancer genomic studies and precision medicine clinical trials have enabled greater understanding of the prevalence of FGFR pathway aberrations in paediatric cancer [10,11,12,13,14,15,16]. These studies have demonstrated that FGFR genomic aberrations are rare but recurrent, ranging from 0.3 to 5% in pan-cancer cohorts. However, genomic FGFR alterations are enriched in specific subtypes of difficult-to-treat paediatric brain tumours, sarcomas, and rare subtypes of haematological malignancies [10, 11, 16, 17]. Identifying and understanding the oncogenic function of these alterations and their sensitivity to targeted therapies is critical to improving the dismal outcomes currently facing paediatric patients with these cancers. Like BCR-ABL + ALL and NTRK fusion-driven cancers, the presence of true FGFR driver alterations has the potential to be targeted with an expanding range of novel FGFR inhibitors. Several have recently shown superior efficacy and lower toxicity over first-generation broad-spectrum FGFR-inhibitors in multiple cancer subtypes [18, 19]. It is thus an exciting and prescient time to assess the status of FGFR aberrations, their driver potential, and their targetability with clinically relevant drugs in paediatric cancers.
Normal FGFR-pathway signalling: structure, function, and regulation of FGFRs
FGFR signalling functions in a range of normal physiological processes, including embryonic development, metabolism, and tissue homoeostasis. Studies in mice have demonstrated that Fgfr1 and Fgfr2 are required for normal development, and homozygous disruption of either of these genes results in embryonic lethality [20, 21]. Conversely, Fgfr3-deficient embryos are viable but have skeletal and inner ear defects [22], while disruption of Fgfr4 alone has no overt effects on development [23]. The FGFRs have a conventional RTK structure consisting of an extracellular ligand binding domain, which binds fibroblast growth factor (FGF) ligands, a transmembrane domain, and intracellular kinase domain [1]. In humans, there are four FGFRs (FGFR1-4), which share high sequence homology of 55-72% [24], and 18 FGF ligands that interact with the FGFRs with varying binding affinities. FGFs have been reviewed in detail elsewhere [25], but they are broadly grouped into two classes, (1) canonical FGFs, containing five subfamilies of paracrine FGFs that require heparin/heparan sulphate as co-factors for receptor binding, and (2) endocrine FGFs, consisting of three FGFs that exhibit low heparin-binding affinity and can be released from the extracellular matrix and act as endocrine factors, requiring either αKlotho or βKlotho as co-factors [26]. Binding of these ligands to FGFRs is regulated by the immunoglobulin-like domains 1–3 (IgI, IgII, and IgIII) (Fig. 1). IgII and IgIII, and the linker region between these domains, are required for ligand binding and mediate specificity of FGF ligands and their receptors through alternative splicing of two exons (IIIb and IIIc) that encode the distal portion of the IgIII domain [27]. In addition to the alternative splicing of the IgIII domains of FGFR1-3, splice isoforms lacking the IgI domain (FGFR1 and FGFR2), the IgI-IgII linker region (FGFR3), or both (FGFR2) have also been described (reviewed in [28]). Notably, the IgIII domain of FGFR4 is not alternatively spliced [29]. Functionally, the IgI domain and the IgI-IgII linker region, containing an “acid box” consisting of eight acidic residues, are thought to inhibit ligand binding [24, 30]. In keeping with this, FGFR1 transcript variants without the IgI-coding domain (termed FGFR1β) display increased FGF2 ligand binding affinity [31].
Binding of FGF triggers FGFR dimerisation and autophosphorylation at specific tyrosine residues in the activation loop of the kinase domain, for example Y653 and Y654 in FGFR1 [32]. Activation of FGFR kinase leads to subsequent activation of several notable downstream cellular signalling pathways, including RAS/MAPK and PI3K/AKT (Fig. 2). There are two important mediators of this process, FGFR substrate 2 (FRS2), an adaptor protein that is constitutively associated with the FGFR and is phosphorylated upon kinase activation, and PLCγ, which binds to phosphorylated Y766 at the C-terminal of FGFR and activates protein kinase C (PKC) [33, 34].
Overview of the normal regulation of FGFR kinase activation and consequences of mutant FGFR expression in cancer. Under normal conditions, binding of FGF ligands, including 18 canonical and three endocrine FGFs, mediates receptor dimerisation and transphosphorylation resulting in activation of the kinase. Mutation of FGFR, commonly the result of gene amplification, mutation or gene fusion, results in constitutive activation of the kinase domain and aberrant downstream signalling, leading to activation of multiple pathways, notably PI3K/AKT and RAS/MAPK.
Abnormal FGFR signalling
Aberrant FGFR activation in cancer is commonly associated with one of several genomic events including gene amplification, mutation, and SVs, including gene fusions and other rare structural events such as internal tandem duplication (ITD). In addition, less well explored mechanisms of FGFR activation include FGFR alternative splicing and alternate transcript expression, and FGF ligand mediated activation. FGFR RNA overexpression in the absence of a genomic event remains unexplained in most instances, although there is some evidence to suggest that this might be a secondary phenomenon related to other oncogenic fusions [35]. The circumstances in which FGFR RNA expression is truly associated with FGFR activation remains an important conundrum to solve.
Gene amplification
Gene amplification of FGFRs is thought to lead to increased protein expression and a functional dependency on FGFR signalling (Fig. 3A). The greatest functional evidence supporting this mechanism of activation comes from adult cancers, including FGFR1-amplified lung cancer and FGFR1- and FGFR2-amplified breast cancer [36, 37]. For example, FGFR1-amplified lung cancer cell lines exhibit increased FGFR1 protein expression compared to non-FGFR1-amplified cell lines and are sensitive to shRNA-mediated knockdown or pharmacological inhibition of FGFR1 [36]. Further, in breast cancer, FGFR1-amplified cell lines express higher levels of FGFR1 protein and are more sensitive to FGFR inhibition with the pan-kinase inhibitor, brivanib, compared to cell lines with normal FGFR1 or FGFR1 deletion [37]. Despite these observations, it is notable that ectopic overexpression of wild-type (WT) FGFR1, which in essence recapitulates copy number gain, is not sufficient to transform cytokine dependent cell lines (Ba/F3) and requires addition of exogenous FGF2 (also known as bFGF) ligand and heparin to stimulate proliferation [38]. This contrasts with FGFR1-activating mutations or fusion genes that transform these same cytokine-dependent cell lines [39, 40]. It is likely then, that cooperation between FGFR amplification and ligand overexpression, or another co-operating activating mechanism, results in FGFR activation. This highlights the importance of interrogating the genomic and transcriptomic context of FGFR amplification to determine if specific FGF ligands might be driving FGFR signalling.
A Gene amplification is predicted to result in an increase in FGFR protein expression and increased FGFR signalling. It is likely other events, including an increase in FGF ligand expression, cooperate with FGFR amplification to drive receptor activation. B Mutations in FGFRs most commonly occur in the ligand binding or kinase domain which result in increased ligand-binding affinity or ligand-independent receptor activation through a disruption of normal kinase autophosphorylation, respectively, leading to FGFR activation. C There are two classes of fusion genes which involve FGFRs, Type I –fusion of an N-terminal partner, or Type II—fusion of a C-terminal partner. Both N and C-terminal partners commonly contribute dimerisation domains that result in ligand-independent dimerisation and phosphorylation and activation of the FGFR kinase. D FGFR1 internal tandem duplication (ITD) results in a receptor with containing two tyrosine kinase domains (TKD1 and TKD2) separated by a linker region of variable length, which has been shown to promote kinase activation. E The functional result of increased FGF ligand or FGFR RNA or protein overexpression is currently unclear. It is possible that it is the combination of both ligand and receptor overexpression that will promote FGFR activation. F FGFR ligand binding domains are subject to splicing under normal conditions, but alternate isoforms lacking regions of the ligand binding domains have been shown to be upregulated in specific cancers. The common functional result of FGFRs that lack these domains is an increase in either ligand or heparin binding affinity which results in increased FGFR activation.
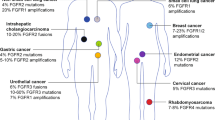
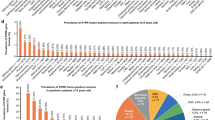
In paediatric cancers, FGFR amplification is rare but predominately identified in sarcomas, particularly osteosarcoma (OS), and less frequently in brain tumours, with gain of FGFR1 being most common (Fig. 4 and Supplementary Table 1). In the Ma et al and Grobner et al pan-cancer genomic studies [10, 11], comprising 2670 paediatric tumour samples of 24 cancer types combined, FGFR amplification was identified in only two cases (0.075%), both involving FGFR1, in one rhabdomyosarcoma (RMS) and one OS sample. When studies selecting for high-risk patients (relapsed, recurrent/refractory, or high-risk subtype diagnosis) are analysed alone: MOSCATO-01 [14]; the Zero Childhood Cancer Program (ZERO) [12]; the Paediatric Precision Oncology INFORM Registry [15]; the MAPPYACTS Trial [16], the NCI-COG Paediatric MATCH Trial [41], and the GAIN/iCAT Study [42], FGFR amplification is identified at a higher frequency, in 28 of a combined 3451 samples (0.81%). Recent interrogation of two paediatric cancer cohorts (The Dana-Faber/Boston Children’s Profile Study and the GAIN/iCAT2 study) comprising a combined total of 1395 patients, identified FGFR amplification in 11 tumours (0.8%). This included FGFR1 amplification in RMS (n = 3), Ewing sarcoma (ES; n = 2), OS (n = 1) and a sarcoma other (n = 1); FGFR3 amplification in a CNS tumour (n = 1); and FGFR4 amplification in RMS (n = 3). In a cohort unselected by age of 275 osteosarcomas arising in the bone (age range: 4–64 years), FGFR1 amplification was detected in 24 (~10%) patients, including paediatric patients, and was disproportionately present in the rarer histological variants and associated with poor response to chemotherapy [43]. While pre-clinical data is limited, one study demonstrated that FGFR1 amplification in 1/17 primary osteosarcoma samples predicted sensitivity to the selective FGFR-inhibitor NVP-BGJ398 [44]. Notably, FGFR amplification is almost never identified in paediatric haematological maliganancies, including the most common paediatric tumour type, B-ALL.
Schematic of protein outcomes of FGFR genomic and transcriptomic aberrations in paediatric cancer. Functional protein domains and mutation types are coloured according to the key. Single cases of HOOK3-FGFR1 and FOXO1-FGFR1 gene fusions have also been reported in paediatric cancers but the molecular structure of these fusions is either unknown or unreported and as such are not included in this figure. Figures were generated using Protein Paint [128]. A list of genomic variants used to generate this figure, and the specific paediatric cancer subtypes they have been identified in is included as Supplementary Table 1.
Gene mutation
Gene mutations, or SNVs, in FGFRs typically occur in the ligand binding or kinase domains, and functionally result in increased ligand binding affinity or ligand independent receptor activation (Fig. 3B) [45]. The FGFR1 N546K kinase domain mutation has been shown to disrupt the sequential three-step autophosphorylation of normal FGFR1[32], inducing phosphorylation at the active site, Y653, at a rate 25 times that of WT FGFR1 [46]. This change in autophosphorylation has also been observed for the FGFR3 K650E mutation, which promotes an overall increase in phosphorylation on the activation loop residues (Y647 and Y648) and broad phosphorylation of additional sites compared to WT FGFR3 [47]. Functionally, mutations in the active site, including the FGFR3 K650E mutation, and its analogous mutations in FGFR1 (K656E) and FGFR4 (K645E), induce FGFR activation and transformation of NIH3T3 cells [48]. FGFR4 N546K and V550E mutants have been shown to increase FGFR4 autophosphorylation, STAT3 signalling, proliferation, and metastatic potential when expressed in a murine RMS cell line [49]. A recent high-throughput functional analysis of FGFR aberrations (largely gene mutations and a small number of fusions) assessed the transforming activity of 158 FGFR mutations, in comparison to the respective WT FGFR. Importantly, of 122 variants of unknown significance (VUS) tested, 25 were determined to be oncogenic or likely oncogenic (Table 1), highlighting the breadth of functionally important mutations in these genes [50].
Most FGFR1/2/3 SNVs are sensitive to second generation FGFR-selective inhibitors (for example, AZD4547 and erdafitinib) that reversibly bind to the ATP-binding pocket of the kinase and thus prevent phosphorylation at the active site. However, mutations in the active site, including FGFR1 N546K and FGFR2 N549D/K, confer resistance to most FGFR-selective inhibitors, with the exception of moderate responses observed towards irreversible FGFR inhibitors (E7090 and futibatinib), which covalently bind to the kinase and cannot be displaced by ATP [50]. In the context of gatekeeper mutations, including FGFR1 V561M, resistance is most commonly attributed to steric hinderance, caused by the replacement of the gatekeeper residue with a hydrophobic amino acid that stabilises the active conformation and reduces affinity to inhibitors [51]. However, analysis of the interaction between FGFR1 V561M and various ATP-competitive inhibitors has demonstrated that inhibitors with binding flexibility can still bind to this active conformation (AZD4547), while for others the mechanism of resistance remains unexplained (ponatinib and dovitinib) [51, 52]. Functional analysis of the FGRF1 N546K specifically has demonstrated that this mutation does not affect the affinity of the mutant for binding ATP-competitive inhibitors (ponatinib, dovitinib, PD173074 and BGJ-398), but instead increases affinity of FGFR1 to ATP [52]. However, as with the gatekeeper mutation, it is likely that the mechanism of resistance is also dependent on the structure of the inhibitor. Indeed, it has been suggested that FGFR1-activating mutations may also prevent binding of specific inhibitors, as they are fixed in the active “closed” conformation and are often complexed with ATP analogues, while FGFRs are commonly in “open” conformation when complexed to ATP-competitive inhibitors [50]. Due to the structural differences between FGFR4 and the other FGFRs, targeting FGFR4 SNVs, including the V550L gatekeeper mutation, with pan-FGFR inhibitors is largely ineffective [50]. However, RMS cell lines expressing these mutants have been shown to be more sensitive to the FGFR-selective inhibitor PD173074 and pan-kinase inhibitor ponatinib, compared to cell lines expressing WT FGFR4 [49, 53].
Mutations in FGFR genes have been identified in a wide range of paediatric cancer types. These include established activating FGFR mutations, FGFR1 N546K and K656E in low-grade (LGG) and high-grade glioma (HGG), neuroblastoma, medulloblastoma, glioblastoma, and Wilms tumour, and FGFR4 N535K/D and V550L/E in RMS [11, 12, 41, 42, 49, 54,55,56,57]. Whole genome sequencing of 149 paediatric LGG patients demonstrated that FGFR aberrations are recurrent in LGG but predominately consist of SVs (including gene fusions and ITDs), only reporting the FGFR1 N546K mutation in one patient [54]. Molecular profiling of 91 uncommon cerebral low-grade neuroepithelial tumours (LGNTs), identified FGFR1, FGFR2, and FGFR3 aberrations in 30 (33%), two (2%), and one (1%) patient/s, respectively [58]. The authors demonstrated that FGFR1 aberrations were characteristic of LGNTs with an oligodendroglial phenotype (dysembryoplastic neuroepithelial tumours (DNETs) and oligodendrogliomas), identified in 62% of patients, but were a less common feature of astrocytic tumours and gangliomas. Of note, the authors described four cases of two FGFR1 mutations occurring on the same allele and one case of FGFR1 mutations occurring on different alleles in single patients. Further, the authors also identified co-occurrence of FGFR1 mutations with other driver mutations (BRAF or NF1) in two patients suggesting that while this does occur its relatively rare (2/30 patients). A more recent study of over 1000 paediatric LGGs identified FGFR1 hotspot mutations, primarily consisting of N546K and K656E, in 1.5% of their cohort, suggesting that they may be more frequent in LGG [59]. FGFR aberrations are incredibly rare in HGG, and include a handful of cases of FGFR1 N546K [12, 56], FGFR1 K656E [11], FGFR1 N546D [14], and FGFR1 D577N [14]. Analysis of a cohort of 94 paediatric RMS patients identified 14 FGFR4 missense mutations, including six mutations in the tyrosine kinase domain (TKD) (N353D/K, V550E/L, A554V, and G576D), which were more commonly identified in PAX3-FOXO1 fusion negative RMS [49]. FGFR SNVs are not commonly associated with other paediatric sarcoma subtypes and are only rarely identified in haematological malignancies, including in B-ALL where FGFR activating mutations are identified in <1% of cases [10, 60].
Gene fusions and other structural variants
Gene fusions involving FGFRs have been identified in a range of paediatric and adult cancers. There are two classes of fusion genes which involve either an N-terminal partner (termed type I) or a C-terminal partner (termed type II). In either case, the fusion partners commonly contribute protein-protein interaction domains, facilitating the dimerisation and activation of FGFR kinase signalling [45] (Fig. 3C). Type I fusions generally involve the loss of the N-terminal ligand binding (regulatory) and transmembrane (membrane localisation) domains of FGFRs and are commonly identified in haematological malignancies. Single cases of FGFR1-activating fusions have been reported in B-ALL (HOOK3-FGFR1) and RMS (FOXO1-FGFR1), although the exact molecular structure of these fusions is unknown [61, 62]. Type I FGFR1 fusions are associated with “myeloid/lymphoid neoplasms with FGFR1 rearrangement”, an extremely rare myeloproliferative disorder characterised by the presence of FGFR1 rearrangement. This disease primarily affects adults but has been described in 16 childhood or adolescent (≤21 years of age) cases in the literature [17, 40]. In this age group, the disease primarily presents as eosinophilia and T-cell lymphoblastic lymphoma with a short chronic phase that can transform to acute myeloid leukaemia [17]. While at least 11 FGFR1 partner genes have been reported in adult cases, only three have been reported in paediatric cases, CNTRL (formerly CEP110), ZMYM2 (formerly ZNF198) and BCR [17, 40].
Functional analysis of the BCR-FGFR1, CNTRL-FGFR1, and ZNF198-FGFR1 fusion proteins in Ba/F3 cells has shown that each of these fusions can transform cells to cytokine independence and are sensitive to inhibition with FGFR-targeting inhibitors, including ponatinib and dovitinib [39, 40, 63, 64]. Further analysis of BCR-FGFR1 and ZNF198-FGFR1 has demonstrated the mechanism of activation mediated by BCR or ZNF198 and downstream signalling activation differs between these fusions. ZNF198-FGFR1 is localised to the cytoplasm, forms a dimeric structure, activates STAT proteins in a similar manner to WT FGFR1, and induces relatively increased phosphorylation and activation of STAT1, STAT3 and ERK1/2 compared to BCR-FGFR1. [65]. Importantly, in addition to autophosphorylation of key tyrosine residues in the activation loop of FGFR1, the same as ligand stimulated FGFR1, the ZNF198 region of the fusion protein itself has been shown to be phosphorylated at seven tyrosine residues that could act as docking sites for signalling proteins [66]. The development of a kinase-dead BCR-FGFR1 construct, by introducing the K514A mutation in the FGFR1 region, demonstrated that FGFR1 kinase activity is essential for STAT and MAPK activation, and cellular transformation [67]. Further, mutation of phosphorylation sites within the BCR region of the fusion, excluding the GRB2 binding site Y177, had little effect on the transforming capacity, but mutation of key residues within the coiled-coil domain, responsible for salt-bridge formation, abolished the transforming capacity. Taken together, these data demonstrate that although FGFR1 kinase activation is the primary transforming event for FGFR1 fusions, fusion partners likely mediate different mechanisms of activation and intermolecular interactions.
There is currently no consensus for the clinical management of paediatric myeloid/lymphoid neoplasms with an FGFR rearrangement and treatments vary based on symptoms and haematological condition at diagnosis [17]. Of available data, 42% patients (5/12) succumb to their disease (follow-up time varies from 1.4-13 years) suggesting that new therapies are urgently needed [17, 40]. Data supporting the potential efficacy of FGFR-targeted TKIs in this disease is mostly limited to pre-clinical studies [40, 63, 64]. The potential clinical efficacy of ponatinib has been demonstrated in an adult patient with BCR-FGFR1 + trilineage mixed-phenotype acute leukaemia [68], but there is very limited data available in paediatric patients [40]. Whether FGFR1-targeting TKIs could improve outcomes for patients with this disease is yet to be established.
Type II fusions generally retain most functional domains of FGFRs including the ligand binding, transmembrane domain and TKD, and are more commonly identified in solid tumours. Functionally, these fusions are membrane bound and constitutively activated by the addition of C-terminal dimerisation domains. In paediatrics, Type II fusions are most frequently associated with various subtypes of LGG [42, 55, 59], most notably the FGFR1-TACC1 and FGFR3-TACC3 fusions, but have also been identified in rare cases of other tumour types including FGFR1-ERC1 in HGG [12], FGFR1-HOOK3 in a young adult gastrointestinal stromal tumour (GIST) [69], and FGFR1-EBF1 in spindle cell sarcoma [42] (Fig. 4). FGFR2 or FGFR3 gene fusions are also a characteristic molecular feature of a recently described subtype of low grade neuroepithelial tumour—Polymorphous Low-grade Neuroepithelial Tumour of the Young (PLNTY) [70]. Interestingly, gene rearrangements including FGFR1 ITD, FGFR1 fusions, and FGFR2 fusions, have been classified as low risk in LGG, compared to FGFR1 mutations which are associated with intermediate risk and inferior overall survival [34].
The FGFR1-TACC1 and FGFR3-TACC3 fusions transform rat fibroblasts and induce glial-like tumour formation when ectopically expressed in immunodeficient mice [71]. When expressed in astrocytes, the FGFR3-TACC3 fusion is localised to spindle microtubules and may not activate canonical FGFR downstream signalling pathways, PI3K/AKT or MAPK. Interestingly, FGFR3-TACC3 and FGFR1-TACC1 expression induces a range of mitotic errors, suggesting in this context it is largely the TACC region of the protein that is contributing to the function of the fusion. Despite this, FGFR inhibitors are effective at killing FGFR-TACC-expressing cells in vitro and prolong survival of mice bearing FGFR3-TACC3-induced tumours in vivo, suggesting the TK activity is important. Interestingly, mutation of tyrosine residues that are normally phosphorylated in the TACC3 region of the fusion increased colony formation in NIH3T3 cells and IL-3 independent proliferation in 32D cells, suggesting that phosphorylation of these residues may have a negative regulatory effect on signal transduction [47]. These data suggest that FGFR-TACC fusions may be weaker oncogenic drivers and may provide a mechanistic explanation for the favourable outcomes observed in LGG patients with FGFR fusions, compared to FGFR mutations [59].
In addition to gene fusions, other SVs, including FGFR1 ITD which results in a duplication of the TKD at a protein level (Fig. 3D), also promote FGFR1 kinase activation [54]. FGFR1 ITD is the most frequently observed FGFR aberration in paediatric LGG [54, 59] but there are few studies that have investigated the exact mechanism of kinase activation. FGFR1 ITD results in an FGFR1 protein with two TKDs (TKD-1 and TKD-2) separated by a linker region of variable length (74-104 amino acids) [54]. Transplantation of Tp-53 null astrocytes transfected with FGFR1 ITD into the brains of nude mice rapidly induces astrocytic tumour formation, characterised by PI3K/AKT and MAPK pathway activation, demonstrating that this alteration is sufficient for oncogenesis. Further, the FGFR inhibitor, PD173074 was shown to effectively block autophosphorylation of FGFR1 ITD, suggesting that these inhibitors may be clinically effective in patients with FGFR1 ITD. Much like FGFR gene fusions, FGFR1 ITD positive tumours are thought to be less aggressive than FGFR mutated tumours [59]. Of note, a single case of FGFR1 ITD has also been reported in neuroblastoma where the duplicated region spans part of exon 17 encoding the kinase domain [57]. Whether this would functionally result in FGFR1 activation was not investigated, which highlights the value of model systems to functionally validate the novel molecular findings that will inevitably come with sequencing more patients.
Other mechanisms: transcriptional upregulation of FGFRs and FGF ligand overexpression
The application of transcriptome sequencing in pan-cancer studies has highlighted the frequency and potential importance of increased RNA expression of RTKs, including FGFRs, and their ligands. Here, we distinguish increased RNA expression arising in the absence of an associated gene amplification or mutational event. In such instances, increased FGFR RNA expression may arise as a result of mutations in non-coding or regulatory regions, epigenetic dysregulation, or as a secondary consequence of other oncogenic drivers [45]. The principal question is under what circumstances is overexpression of a WT FGFR indicative of FGFR signalling activation and, more pertinently from a therapeutic standpoint, when is it indicative of tumour dependence on FGFR signalling? While this question is still open, it is likely that a combination of specific factors, including FGF ligand overexpression, are associated with receptor activation (Fig. 3E). Some of this evidence comes from studies of FGFR gene amplification, the consequence of what is essentially WT FGFR expression. Overexpression of FGF ligands has been identified in multiple cancer types and is recurrently associated with aggressive tumour types and inferior clinical outcomes. Studies exploring the role of FGF ligands in cancer are mostly limited to adult cancer subtypes and include FGF2, the most commonly implicated FGF in cancer, FGF1 in ovarian, FGF4, FGF9 and FGF22 in lung, FGF5 in breast, FGF6, FGF19, and FGF21 in liver, FGF7 and FGF18 in gastric, and FGF8, FGF17, FGF19, and FGF23 in prostate cancer (reviewed in [72]). Most of these studies have focussed on the prognostic implications of increased FGF expression but have not explored the functional implications on FGFR signalling activation or corresponding therapeutic sensitivity.
There are few studies that have established clear obligate relationships between specific FGFRs and FGFs in the context of cancer development and progression, and these again are mainly limited to adult cancers. For example, FGF19, which is commonly amplified in hepatocellular carcinoma, promotes hepatocyte proliferation through activation of FGFR4 [73]. In addition, FGF7 and FGF18 are thought to cooperate with FGFR2 amplification to promote gastric cancer [74, 75]. In non-small cell lung cancer (NSCLC), FGF/FGFR pathway activation, mediated by FGF2 and FGF9 signalling through FGFR1 or FGFR2, has been suggested to promote EGFR inhibitor resistance [76].
Recently, paediatric precision medicine trials have started to include the analysis and reporting of RNA overexpression of targetable genes and have highlighted FGFR overexpression as a feature of multiple subtypes of paediatric cancer [12, 15]. The ZERO program was the first paediatric precision medicine program to publish reportable findings of RNA overexpression, using a z-score and fold change cut-off of greater than 2 to determine outlier overexpression, when compared to the entire cohort (all tumour types). In the first 252 tumours published from ZERO, overexpression of FGFR genes was reported in 16 tumours (6.3%), dominated by FGFR4 overexpression in fusion positive RMS (9 tumours), in which FGFR4 overexpression is a known molecular feature and is directly regulated by PAX3-FOXO1 [77]. The INFORM study reported RNA outlier expression, in the absence of genomic aberration, of either FGFR (most commonly FGFR3 or FGFR4) or FGF genes in 32 and 8 cases, respectively, from a total of 781 patients with RNAseq data [15]. Of note, combined overexpression of both FGFR and FGF genes was not identified in any of the tumours analysed. In both ZERO and INFORM, aberrant expression of FGFR pathway genes was restricted to sarcomas, brain and CNS tumours, and solid tumours. This observation, together with the low reported frequencies of genomic aberration of FGFR pathway genes, suggests that the FGFR pathway may be less important in haematological malignancies.
FGFR1, FGFR4 and FGF8 expression is recurrently altered in RMS. The prevalence of FGFR1 overexpression in the two histological subtypes of RMS, alveolar RMS (aRMS) and embryonal RMS (eRMS) has varied in different patient cohorts, with some studies reporting higher levels of expression in eRMS [78], while others have identified higher levels in aRMS but only in PAX3-FOXO1 negative cases [79]. One study identified FGFR1 overexpression in the absence of genomic alteration in both RMS primary samples and cell lines and showed that it was associated with hypomethylation of a 5‘ CPG island and abnormal expression of AKT1, NOG and BMP4 genes in primary samples [80]. FGFR4 overexpression in RMS is associated with the aRMS histological subtype, which is molecularly characterised by the PAX3/7-FOXO1 fusion, advanced-stage disease, and poor survival [49]. While FGFR4 is an established transcriptional target of PAX3-FOXO1 in fusion positive RMS, and increased FGFR4 RNA and protein expression is frequently identified in fusion positive RMS patients, the dependency on and as such therapeutic targetability of FGFR4 in this disease is debated. The oncogenic potential of WT FGFR4 is supported by the findings that FGFR4 knockdown reduced tumour growth in a human RMS cell line and lung metastases in a RMS mouse model [49], and WT FGFR4 overexpressing mouse-myoblasts induced tumour formation in vivo [81]. However, the transforming potential of WT FGFR4 was not as aggressive as FGFR4-activating mutations, and most WT FGFR4 tumours lacked RMS-specific immunohistochemistry markers [81]. Importantly, cells expressing WT FGFR4 are substantially less sensitive to FGFR inhibitors, such as ponatinib, compared to cells expressing FGFR4-activating mutants, N535K and V550E [49, 53]. Based on these preclinical studies, targeting overexpressed WT FGFR4 is currently considered to be only minimally effective in RMS. Recently, FGF8 was identified as a direct transcriptional target of PAX3-FOXO1, where FGF8 upregulation represents a novel mechanism of PAX3-FOXO1 independent growth in aRMS [82]. More research is needed to determine whether specific targeting of FGF8 in PAX-FOXO1-positive RMS is an effective treatment strategy.
In addition to RMS, aberrant expression of FGFR pathway genes has also been identified in OS, Ewing sarcoma (ES), Malignant Peripheral Nerve Sheath Tumour (MPNST), and synovial sarcoma (SS). There are a multiple studies in OS cell lines that have used either phosphoproteomics or immunohistochemistry methods to investigate the expression and activation of FGFRs in small numbers of OS cell lines, where conclusions have been mixed but FGFR1, FGFR2, FGFR3, and FGF2 have all been implicated [83,84,85]. Notably, one study suggested that APE1 promotes angiogenesis in OS through upregulation of FGFR3 and FGF2 [85]. A preclinical study using in vivo transgenic models of c-Fos oncogene-induced OS identified FGFR1 as a novel c-Fos/AP-1 regulated gene. Expression of c-Fos induced an increase in FGFR1 RNA and protein, promoted anchorage-independent growth, and MAPK activation in a FGF2-dependent manner. Pharmacological inhibition of FGFR1 blocked colony forming of OS cells in vitro and reduced OS metastasis in vivo [86]. In ES, one study showed that FGFR1 was expressed and activated in 78% of primary ES cells and FGF2 stimulation induced motility and invasion of these cells [87]. In addition, another study identified FGFR4 as a potential gene dependency in ES using an siRNA screen targeting 287 cancer associated genes in four ES cell lines. Further, subsequent treatment with the selective FGFR4 inhibitor BLU9931 inhibited ES cell growth [88]. In MPNST, combined expression of FGFR1 and FGFR2 is associated with superior overall survival, while FGFR4 expression is associated with poor disease-free survival [89]. FGFR3 has also been postulated to be a positive prognostic marker in SS, with high FGFR3 being associated with improved progression free survival in untreated SS patients [90]. Expression of the SS18-SSX2 fusion, which molecularly characterises SS, results in transcriptional upregulation of FGFR2 and increased expression of FGF ligands, FGF3, FGF7, FGF9 and FGF18, suggesting possible autocrine feedback loops function to upregulate FGFR signalling [91]. Another study showed that either Fgfr1, Fgfr2, or Fgfr3 gene knockout impeded SS18-SSX2-induced tumour formation in vivo and FGFR inhibitor (BGJ398) treatment reduced SS tumour cell growth in vitro and in vivo [92]. Analysis of FGFR and FGF mRNA expression in SS primary tumours and cell lines demonstrated that these lines express most FGFRs and some FGF ligands, including FGF2, FGF8, FGF9, FGF11 and FGF18. Further, FGF8 stimulation of SS cell lines promoted proliferation and FGFR inhibitor treatment demonstrated efficacy in in vitro and in vivo, via downregulation of ERK [93]. These data together demonstrate that the FGFR pathway may play a role in multiple sarcoma subtypes.
In paediatric brain tumours, elevated expression of FGFRs have been reported in medulloblastoma and ependymoma, two common paediatric brain tumours in which genomic aberration of FGFRs is not a characteristic [11, 94]. Increased FGFR3 expression has been associated with treatment failure in medulloblastoma [95], and FGFR inhibitor treatment has demonstrated some efficacy, albeit at high doses, in some medulloblastoma cell lines [96]. In ependymoma, high FGFR1 and FGFR3 expression has been associated with high tumour grade and poor prognosis, in the absence of FGFR1 or FGFR3 mutation [97]. Co-expression of FGFR1/3 with FGFs including FGF1, FGF2 and FGF9 has been identified in specific molecular subtypes and cell sub-populations of ependymoma. FGF2 has also been implicated in glioblastoma multiform (GBM), a grade IV HGG, which also frequently overexpresses FGFR1 [98].
Other mechanisms: alternative splicing of FGFR RNAs
As described earlier, alternative splicing of the FGFR Ig domains occurs normally and mediates ligand binding specificity. This aspect of normal FGFR regulation has also been implicated in cancer. Alternative splicing of the distal portion of the IgIII domain in FGFR1-3 results in the inclusion of either IIIb or IIIc exon and expression of these isoforms for FGFR1 and FGFR2 is generally restricted to epithelial cells for FGFR IIIb and mesenchymal cells for FGFR IIIc (reviewed in [25]). Further, these isoforms generally bind FGF ligands that are expressed in the reciprocal tissue, and this plays a key role in organ development. This pattern of expression and signalling by FGFR3 isoforms is not strictly observed. The majority of FGF ligands preferentially signal through FGFR IIIc isoforms, except for the FGF7 subfamily (FGF3, FGF7, FGF10, and FGF22) that signals through FGFR IIIb isoforms [25, 99]. In the context of cancer, aberrant signalling of FGF10 through FGFR2 IIIb, and to a lesser extent FGFR1 IIIb, has been implicated in a range of cancers [100]. For example, in breast cancer, FGF10 promotes cell migration through activation of FGFR1 IIIb, promoting translocation to the nucleus and transcription upregulation of cell migration genes, and activation of FGFR2 IIIb, promoting PI3K/SH3BP4 recruitment and receptor recycling [101, 102]. In paediatric cancer subtypes, there are few studies that have investigated the role of alternate splicing of FGFR IgIII domains. However, one study has shown that FGFR1 and FGFR3 IIIc splice variants are the dominant expressed isoforms in ependymoma [103]. In addition, expression of the FGFR1 IIIc isoform and FGF5 is elevated in astrocytoma and GBM patient samples compared to non-malignant controls, and FGF5 promotes oncogenic cell growth and survival through autocrine and paracrine signalling through the FGF/FGFR signalling axis [104].
Splice isoforms lacking the IgI domain and/or the IgI-IgII linker region of FGFRs also occur naturally (Fig. 3F), the most important of which is FGFR1β, which lacks the IgI domain and is upregulated in a range of cancers, including astrocytoma, pancreatic, breast, and bladder cancer [105,106,107,108]. Further, an increase in the ratio of FGFR1β to the full-length isoform (FGFR1α), is associated with more aggressive tumours and inferior clinical outcomes in the tumours in which it has been described. Recently, FGFR1 was shown to be preferentially spliced, resulting in the FGFR1β isoform, in paediatric HGG compared to normal brain, which was also accompanied by upregulation of total FGFR1 expression [109]. Functionally, FGFR1 isoform switching alters affinity for particular FGF ligands in bladder cancer cell lines [108]. In addition to FGFR1β, other splice isoforms, including FGFR3ΔΑΒ, an FGFR3 isoform that lacks the IgI-IgII linker region, containing the acid box (AB), have been shown to alter heparin binding and FGF affinity and represent another mechanism of FGFR activation that may be exploited in cancer [110].
Co-occurring mutations in FGFR-driven paediatric cancers
The presence of co-occurring mutations, and potential co-operation, with FGFR alterations in paediatric cancer is not completely understood, largely due to the rarity of these mutations across the spectrum of paediatric cancer. Recently, specific analysis of FGFR aberrations in paediatric cancer by Lazo De La Vega and colleagues has characterised the breadth of co-occurring mutations in FGFR-driven paediatric maliganancies. In the 41 cases in which an activating FGFR alteration was identified, 66% of cases (27/41) had at least one additional oncogenic or likely oncogenic mutation in another gene [42]. Amplification of MYC/MYCN (7 cases) and mutation of TP53 (6 cases) were the most common co-occurring mutations observed, as well as smaller numbers of cases of MDM2/4 amplification and CDKN2A/2B deletion. Importantly, mutations in therapeutically targetable genes were also observed including PIK3CA mutations (3 cases), CDK4 amplification (4 cases), and mutations in the RAS pathway (NF1/2, NRAS, KRAS, HRAS; 7 cases), highlighting potential opportunities for combination therapy approaches. Indeed, initial analysis of the molecular profiles of the first 1000 patients enroled in the NCI-Paediatric MATCH trial also identified co-occurrence of PIK3CA mutation (1 case), CDK4/6 amplification (3 cases), and RAS pathway activating mutations (NRAS, PTPN11; 4 cases) in 40 patients identified with FGFR alterations [41]. Notably, in both studies, co-occurring mutations did not appear to be associated with specific FGFR alterations or tumour types, although larger cohorts would be needed to determine any association. In LGG, FGFR alterations have been observed in patients with germline NF1 mutations, and in combination with BRAF V600E, CDKN2A deletion, and mutations in PIK3CA, NRAS, MAPK2, SET2D, and JAK2 [59]. In addition, dual mutations in FGFR1 are also observed. Whether these mutations co-operate to drive tumorigenesis, or they represent potential combination therapy options, is yet to be explored.
Recent developments in FGFR targeted therapies
In recent years, a range of FGFR-inhibitors have been developed and tested in pre-clinical and, in some instances, clinical studies. First generation multi-target TKIs (eg. ponatinib, dovitinib and lenvatinib) inhibit a range of TKs, including but not limited to VEGF1/3, KIT, and RET, in addition to FGFRs. The broad-spectrum activity of these inhibitors has resulted in a lack of anti-FGFR activity and the occurrence of adverse events, demonstrated in a Phase II clinical trial of dovitinib for FGFR2-mutated metastatic endometrial cancer [111]. A number of these broad-spectrum multi-kinase inhibitors have been trialled clinically, without molecular indication, in a range of sarcoma subtypes where efficacy was mainly observed in OS. Regorafenib and sorafenib have been shown to modestly prolong progression-free survival (PFS) of OS patients [112,113,114], and regorafenib has demonstrated comparable efficacy in ES patients [115, 116] (Table 2). Lenvatinib, which has a similar target profile, demonstrated similar clinical efficacy to regorafenib when used as a monotherapy and tested specifically in a young patient cohort (2-25 years) with relapsed/refractory OS, where disease control and objective responses of 48% and 6.7%, respectively, were achieved [117]. However, when combined with etoposide and ifosfamide, disease control and objective response rates of 71% and 9%, respectively, were achieved [118]. A partial response to ponatinib has also been reported in a paediatric oligodendroglioma patient with an FGFR3-PHGDH fusion [119]. As with any multi-kinase inhibitor, it is however very difficult to assess how much of this anti-tumour effect, if any, can be attributed to inhibition of the FGFR-pathway. Although with these inhibitors the overall toxicity was manageable, for some patients these can be severe. It should be noted that the efficacy of Regorafenib will also be tested in combination with immunotherapy in OS (NCT04803877), and with chemotherapy in relapsed RMS (FaR-RMS, NCT04625907), without molecular indication, in clinical trials. In addition, surufatinib will be tested in paediatric patients with relapsed or refractory solid tumours with FGFR1 aberrations (NCT05093322) (Table 2).
Multiple kinase inhibitors are also in clinical trial for different subtypes of paediatric brain tumours, but these are based on molecular targets, and the agents themselves are more selective [120]. The potentially efficacy of the MEK inhibitor, trametinib in paediatric LGG patients with MAPK pathway activation (BRAF or NF1 aberrations) has been demonstrated in multiple retrospective studies [121,122,123], and are now in clinical trial. Given RAS pathway activating mutations co-occur with FGFR alternations in multiple paediatric tumour types, including LGG, trametinib and FGFR inhibitor represents a potential effective therapeutic combination for these patients.
The refinement in both molecular selection of patients, aided by personalised medicine, with FGFR-driven disease has been accompanied with the development of selective FGFR inhibitors. A number of these more selective FGFR inhibitors are in clinical development, including but not limited to erdafitinib, AZD4557, and pemigatinib. Some of these are currently being trialled in paediatric cancer, although little clinical efficacy data is available, and is limited to case reports or small cohort studies (Table 2). A single-centre study of Debio1347 (FGFR1/2/3 inhibitor) in 5 paediatric patients with recurrent or refractory FGFR-altered gliomas demonstrated promising results, inducing partial responses (PR) in two (1 x LGG and 1 x HGG) and stable disease (SD) in two (2 x LGG) of the four evaluable patients [124]. In addition, a partial response was observed in a young adult FGFR1 N546K-mutant pilocytic astrocytoma patient treated with pemigatinib, an FGFR1/2/3/4 ATP-competitive inhibitor [125]. This was an interesting result as the FGFR1 N546K mutation has been shown to mediate resistance to other ATP-competitive inhibitors, as previously described [52]. Erdafitinib is currently being tested in a Phase II clinical trial for treatment of paediatric patients (1–21 years of age) with relapsed or refractory solid tumours, non-Hodgkin lymphoma, or histiocytic disorders with identified FGFR mutations, as part of the NCI-COG Paediatric MATCH trial [41]. In addition, paediatric patients are currently being recruited for the RAGNAR study (NCT04083976) of erdafitinib in advanced solid tumours with FGFR alterations [126]. Outside of these clinical trials, erdafitinib demonstrated some clinical efficacy in a case of FGFR1-EBF2 positive spindle and round cell neoplasm [42] Notably, infigratinib, an FGFR12/3 selective inhibitor, has also been trialled in adults with FGFR-altered recurrent gliomas, where an 6-month PFS rate of 16% and sustained responses of more than a year were observed [127]. Interestingly, 3 of the 4 patients with durable clinical responses harboured FGFR1 K656E (n = 2) or the equivalent FGFR3 K650E (n = 1) mutations, the only patients with these specific variants in the cohort, while most patients harboured FGFR3 amplifications or FGFR3-TACC3 fusions. At present, erdafitinib and pemigatinib are the only FDA approved FGFR inhibitors, approved for adult advanced urothelial carcinoma and cholangiocarcinoma, respectively.
Conclusions
FGFR genomic alteration, alternate transcript expression, and aberrant FGF ligand expression have individually been shown to contribute to FGFR pathway activation in multiple cancer types. While mechanistic studies of these variants in paediatric cancer subtypes is limited, there are numerous studies in adult cancers from which the potential functional impact of these variants can be speculated. The impact of overexpression of FGFR RNA in the absence of genomic alteration and the possible cooperation of multiple FGF/FGFR events to drive FGFR pathway activation has not been fully explored or established. The rapid development and advancement of personalised medicine pipelines, to not only include genomic, but now transcriptome analysis, allows for the comprehensive analysis of the vast array of aberrations that can lead to FGFR pathway activation in paediatric cancer. As personalised medicine becomes standard, there is a greater capacity to identify individual patients that are likely to benefit from FGFR-targeted therapies.
References
Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2000;103:211–25.
Fleuren EDG, Zhang LX, Wu JM, Daly RJ. The kinome ‘at large’ in cancer. Nat Rev Cancer. 2016;16:83–98.
Li SG, Ilaria RL, Million RP, Daley GQ, Van Etten RA. The P190, P210, and P230 forms of the BCR/ABL oncogene induce a similar chronic myeloid leukemia-like syndrome in mice but have different lymphoid leukemogenic activity. J Exp Med. 1999;189:1399–412.
Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–405.
Schultz KR, Bowman WP, Aledo A, Slayton WB, Sather H, Devidas M, et al. Improved early event-free survival with imatinib in Philadelphia chromosome-positive acute lymphoblastic leukemia: a children’s oncology group study. J Clin Oncol. 2009;27:5175–81.
Slayton WB, Schultz KR, Kairalla JA, Devidas M, Mi XL, Pulsipher MA, et al. Dasatinib plus intensive chemotherapy in children, adolescents, and young adults with philadelphia chromosome-positive acute lymphoblastic leukemia: results of children’s oncology group trial AALL0622. J Clin Oncol. 2018;36:2306.
Drilon A, Laetsch TW, Kummar S, DuBois SG, Lassen UN, Demetri GD, et al. Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. N Engl J Med. 2018;378:731–9.
Katoh M, Nakagama H. FGF receptors: cancer biology and therapeutics. Med Res Rev. 2014;34:280–300.
Schuler M, Cho BC, Sayehli CM, Navarro A, Soo RA, Richly H, et al. Rogaratinib in patients with advanced cancers selected by FGFR mRNA expression: a phase 1 dose-escalation and dose-expansion study. Lancet Oncol. 2019;20:1454–66.
Ma X, Liu Y, Liu Y, Alexandrov LB, Edmonson MN, Gawad C, et al. Pan-cancer genome and transcriptome analyses of 1,699 paediatric leukaemias and solid tumours. Nature. 2018;555:371–6.
Gröbner SN, Worst BC, Weischenfeldt J, Buchhalter I, Kleinheinz K, Rudneva VA, et al. The landscape of genomic alterations across childhood cancers. Nature. 2018;555:321–7.
Wong M, Mayoh C, Lau LMS, Khuong-Quang D-A, Pinese M, Kumar A, et al. Whole genome, transcriptome and methylome profiling enhances actionable target discovery in high-risk pediatric cancer. Nat Med. 2020;26:1742–53.
Newman S, Nakitandwe J, Kesserwan CA, Azzato EM, Wheeler DA, Rusch M, et al. Genomes for Kids: the scope of pathogenic mutations in pediatric cancer revealed by comprehensive DNA and RNA sequencing. Cancer Discov. 2021;11:3008–27.
Harttrampf AC, Lacroix L, Deloger M, Deschamps F, Puget S, Auger N, et al. Molecular screening for cancer treatment optimization (MOSCATO-01) in pediatric patients: a single-institutional prospective molecular stratification trial. Clin Cancer Res. 2017;23:6101.
van Tilburg CM, Pfaff E, Pajtler KW, Langenberg KPS, Fiesel P, Jones BC, et al. The pediatric precision oncology INFORM registry: clinical outcome and benefit for patients with very high-evidence targets. Cancer Discov. 2021;11:2764–79. candisc.0094.2021
Berlanga P, Pierron G, Lacroix L, Chicard M, de Beaumais TA, Marchais A, et al. The European MAPPYACTS Trial: Precision Medicine Program in Pediatric and Adolescent Patients with Recurrent Malignancies. Cancer Discov. 2022;12:1266–81.
Sarthy JF, Reddivalla N, Radhi M, Chastain K. Pediatric 8p11 eosinophilic myeloproliferative syndrome (EMS): a case report and review of the literature. Pediatr Blood Cancer. 2017;64:e26310.
Loriot Y, Necchi A, Park SH, Garcia-Donas J, Huddart R, Burgess E, et al. Erdafitinib in locally advanced or metastatic urothelial carcinoma. N. Engl J Med. 2019;381:338–48.
Abou-Alfa GK, Sahai V, Hollebecque A, Vaccaro G, Melisi D, Al-Rajabi R, et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, phase 2 study. Lancet Oncol. 2020;21:671–84.
Deng CX, Wynshawboris A, Shen MM, Daugherty C, Ornitz DM, Leder P. Murine Fgfr-1 is required for early postimplantation growth and axial organization. Genes Dev. 1994;8:3045–57.
Arman E, Haffner-Krausz R, Chen Y, Heath JK, Lonai P. Targeted disruption of fibroblast growth factor (FGF) receptor 2 suggests a role for FGF signaling in pregastrulation mammalian development. Proc Natl Acad Sci USA. 1998;95:5082–7.
Colvin JS, Bohne BA, Harding GW, McEwen DG, Ornitz DM. Skeletal overgrowth and deafness in mice lacking fibroblast growth factor receptor 3. Nat Genet. 1996;12:390–7.
Weinstein M, Xu XL, Ohyama K, Deng CX. FGFR-3 and FGFR-4 function cooperatively to direct alveogenesis in the murine lung. Development. 1998;125:3615–23.
Johnson DE, Williams LT. Structural and functional diversity in the FGF receptor multigene family. Adv Cancer Res. 1993;60:1–41.
Ornitz DM, Itoh N. The fibroblast growth factor signaling pathway. Wiley Interdiscip Rev Dev Biol. 2015;4:215–66.
Goetz R, Beenken A, Ibrahimi OA, Kalinina J, Olsen SK, Eliseenkova AV, et al. Molecular insights into the klotho-dependent, endocrine mode of action of fibroblast growth factor 19 subfamily members. Mol Cell Biol. 2007;27:3417–28.
Plotnikov AN, Hubbard SR, Schlessinger J, Mohammadi M. Crystal structures of two FGF-FGFR complexes reveal the determinants of ligand-receptor specificity. Cell 2000;101:413–24.
Tiong KH, Mah LY, Leong C-O. Functional roles of fibroblast growth factor receptors (FGFRs) signaling in human cancers. Apoptosis. 2013;18:1447–68.
Partanen J, Makela TP, Eerola E, Korhonen J, Hirvonen H, Claesson-Welsh L, et al. FGFR-4, a novel acidic fibroblast growth factor receptor with a distinct expression pattern. EMBO J. 1991;10:1347–54.
Kalinina J, Dutta K, Ilghari D, Beenken A, Goetz R, Eliseenkova AV, et al. The alternatively spliced acid box region plays a key role in FGF receptor autoinhibition. Structure. 2012;20:77–88.
Roghani M, Moscatelli D. Prostate cells express two isoforms of fibroblast growth factor receptor 1 with different affinities for fibroblast growth factor-2. Prostate. 2007;67:115–24.
Furdui CM, Lew ED, Schlessinger J, Anderson KS. Autophosphorylation of FGFR1 kinase is mediated by a sequential and precisely ordered reaction. Mol cell. 2006;21:711–7.
Eswarakumar VP, Lax I, Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev. 2005;16:139–49.
Mohammadi M, Honegger AM, Rotin D, Fischer R, Bellot F, Li W, et al. A tyrosine-phosphorylated carboxy-terminal peptide of the fibroblast growth-factor receptor (FLG) is a binding-site for the sh2 domain of phospholipase C-GAMMA-1. Mol Cell Biol. 1991;11:5068–78.
Gillani R, Seong BKA, Crowdis J, Conway JR, Dharia NV, Alimohamed S, et al. Gene fusions create partner and collateral dependencies essential to cancer cell survival. Cancer Res. 2021;81:3971–84.
Weiss J, Sos ML, Seidel D, Peifer M, Zander T, Heuckmann JM, et al. Frequent and focal FGFR1 amplification associates with therapeutically tractable FGFR1 dependency in squamous cell lung cancer. Sci Transl Med. 2010;2:62ra93.
Shiang CY, Qi Y, Wang B, Lazar V, Wang J, Fraser Symmans W, et al. Amplification of fibroblast growth factor receptor-1 in breast cancer and the effects of brivanib alaninate. Breast Cancer Res Treat. 2010;123:747–55.
Ornitz DM, Yayon A, Flanagan JG, Svahn CM, Levi E, Leder P. Heparin is required for cell-free binding of basic fibroblast growth factor to a soluble receptor and for mitogenesis in whole cells. Mol Cell Biol. 1992;12:240–7.
Demiroglu A, Steer EJ, Heath C, Taylor K, Bentley M, Allen SL, et al. The t(8;22) in chronic myeloid leukemia fuses BCR to FGFR1: transforming activity and specific inhibition of FGFR1 fusion proteins. Blood. 2001;98:3778–83.
Brown LM, Bartolo RC, Davidson NM, Schmidt B, Brooks I, Challis J, et al. Targeted therapy and disease monitoring in CNTRL-FGFR1-driven leukaemia. Pediatr Blood Cancer. 2019;66:e27897.
Parsons DW, Janeway KA, Patton DR, Winter CL, Coffey B, Williams PM, et al. Actionable tumor alterations and treatment protocol enrollment of pediatric and young adult patients with refractory cancers in the national cancer institute-children’s oncology group pediatric MATCH trial. J Clin Oncol. 2022;40:2224.
Lazo De La Vega L, Comeau H, Sallan S, Al-Ibraheemi A, Gupta H, Li YY, et al. Rare FGFR oncogenic alterations in sequenced pediatric solid and brain tumors suggest FGFR is a relevant molecular target in childhood cancer. JCO Precis Oncol. 2022;6:e2200390.
Fernanda Amary M, Ye H, Berisha F, Khatri B, Forbes G, Lehovsky K, et al. Fibroblastic growth factor receptor 1 amplification in osteosarcoma is associated with poor response to neo-adjuvant chemotherapy. Cancer Med. 2014;3:980–7.
Guagnano V, Kauffmann A, Wöhrle S, Stamm C, Ito M, Barys L, et al. FGFR genetic alterations predict for sensitivity to NVP-BGJ398, a selective pan-FGFR inhibitor. Cancer Disco. 2012;2:1118–33.
Katoh M. Fibroblast growth factor receptors as treatment targets in clinical oncology. Nat Rev Clin Oncol. 2019;16:105–22.
Lew ED, Furdui CM, Anderson KS, Schlessinger J. The precise sequence of FGF receptor autophosphorylation is kinetically driven and is disrupted by oncogenic mutations. Sci Signal. 2009;2:ra6.
Nelson KN, Meyer AN, Siari A, Campos AR, Motamedchaboki K, Donoghue DJ. Oncogenic gene fusion FGFR3-TACC3 is regulated by tyrosine phosphorylation. Mol Cancer Res. 2016;14:458–69.
Hart KC, Robertson SC, Kanemitsu MY, Meyer AN, Tynan JA, Donoghue DJ. Transformation and Stat activation by derivatives of FGFR1, FGFR3, and FGFR4. Oncogene 2000;19:3309–20.
Taylor JGT, Cheuk AT, Tsang PS, Chung JY, Song YK, Desai K, et al. Identification of FGFR4-activating mutations in human rhabdomyosarcomas that promote metastasis in xenotransplanted models. J Clin Invest. 2009;119:3395–407.
Nakamura IT, Kohsaka S, Ikegami M, Ikeuchi H, Ueno T, Li K, et al. Comprehensive functional evaluation of variants of fibroblast growth factor receptor genes in cancer. npj Precis Oncol. 2021;5:66.
Sohl CD, Ryan MR, Luo B, Frey KM, Anderson KS. Illuminating the molecular mechanisms of tyrosine kinase inhibitor resistance for the FGFR1 gatekeeper mutation: the Achilles’ heel of targeted therapy. ACS Chem Biol. 2015;10:1319–29.
Yoza K, Himeno R, Amano S, Kobashigawa Y, Amemiya S, Fukuda N, et al. Biophysical characterization of drug-resistant mutants of fibroblast growth factor receptor 1. Genes Cells. 2016;21:1049–58.
Li SQ, Cheuk AT, Shern JF, Song YK, Hurd L, Liao H, et al. Targeting wild-type and mutationally activated FGFR4 in rhabdomyosarcoma with the inhibitor ponatinib (AP24534). PloS One. 2013;8:e76551.
Zhang J, Wu G, Miller CP, Tatevossian RG, Dalton JD, Tang B, et al. Whole-genome sequencing identifies genetic alterations in pediatric low-grade gliomas. Nat Genet. 2013;45:602–12.
Surrey LF, MacFarland SP, Chang F, Cao K, Rathi KS, Akgumus GT, et al. Clinical utility of custom-designed NGS panel testing in pediatric tumors. Genome Med. 2019;11:32.
Wu G, Diaz AK, Paugh BS, Rankin SL, Ju BS, Li YJ, et al. The genomic landscape of diffuse intrinsic pontine glioma and pediatric non-brainstem high-grade glioma. Nat Genet. 2014;46:444–50.
Brady SW, Liu Y, Ma X, Gout AM, Hagiwara K, Zhou X, et al. Pan-neuroblastoma analysis reveals age- and signature-associated driver alterations. Nat Commun. 2020;11:5183.
Qaddoumi I, Orisme W, Wen J, Santiago T, Gupta K, Dalton JD, et al. Genetic alterations in uncommon low-grade neuroepithelial tumors: BRAF, FGFR1, and MYB mutations occur at high frequency and align with morphology. Acta Neuropathol. 2016;131:833–45.
Ryall S, Zapotocky M, Fukuoka K, Nobre L, Guerreiro Stucklin A, Bennett J, et al. Integrated molecular and clinical analysis of 1,000 pediatric low-grade gliomas. Cancer Cell. 2020;37:569–83.e5.
Jerchel IS, Hoogkamer AQ, Ariës IM, Boer JM, Besselink NJM, Koudijs MJ, et al. Fibroblast growth factor receptor signaling in pediatric B-cell precursor acute lymphoblastic leukemia. Sci Rep. 2019;9:1875.
Reshmi SC, Harvey RC, Roberts KG, Stonerock E, Smith A, Jenkins H, et al. Targetable kinase gene fusions in high-risk B-ALL: a study from the Children’s Oncology Group. Blood. 2017;129:3352–61.
Liu J, Guzman MA, Pezanowski D, Patel D, Hauptman J, Keisling M, et al. FOXO1-FGFR1 fusion and amplification in a solid variant of alveolar rhabdomyosarcoma. Mod Pathol. 2011;24:1327–35.
Chase A, Grand FH, Cross NC. Activity of TKI258 against primary cells and cell lines with FGFR1 fusion genes associated with the 8p11 myeloproliferative syndrome. Blood. 2007;110:3729–34.
Chase A, Bryant C, Score J, Cross NC. Ponatinib as targeted therapy for FGFR1 fusions associated with the 8p11 myeloproliferative syndrome. Haematologica. 2013;98:103–6.
Baumann H, Kunapuli P, Tracy E, Cowell JK. The oncogenic fusion protein-tyrosine kinase ZNF198/fibroblast growth factor receptor-1 has signaling function comparable with interleukin-6 cytokine receptors. J Biol Chem. 2003;278:16198–208.
Kasyapa C, Gu TL, Natarajan L, Polakiewicz R, Cowell JK. Phosphorylation of the SSBP2 and ABL proteins by the ZNF198-FGFR1 fusion kinase seen in atypical myeloproliferative disorders as revealed by phosphopeptide-specific MS. Proteomics. 2009;9:3979–88.
Peiris MN, Meyer AN, Nelson KN, Bisom-Rapp EW, Donoghue DJ. Oncogenic fusion protein BCR-FGFR1 requires the breakpoint cluster region-mediated oligomerization and chaperonin Hsp90 for activation. Haematologica. 2020;105:1262–73.
Khodadoust MS, Luo B, Medeiros BC, Johnson RC, Ewalt MD, Schalkwyk AS, et al. Clinical activity of ponatinib in a patient with FGFR1-rearranged mixed-phenotype acute leukemia. Leukemia. 2016;30:947–50.
Shi E, Chmielecki J, Tang CM, Wang K, Heinrich MC, Kang G, et al. FGFR1 and NTRK3 actionable alterations in “Wild-Type” gastrointestinal stromal tumors. J Transl Med. 2016;14:339.
Huse JT, Snuderl M, Jones DTW, Brathwaite CD, Altman N, Lavi E, et al. Polymorphous low-grade neuroepithelial tumor of the young (PLNTY): an epileptogenic neoplasm with oligodendroglioma-like components, aberrant CD34 expression, and genetic alterations involving the MAP kinase pathway. Acta Neuropathol. 2017;133:417–29.
Singh D, Chan JM, Zoppoli P, Niola F, Sullivan R, Castano A, et al. Transforming fusions of FGFR and TACC genes in human glioblastoma. Science. 2012;337:1231–5.
Xie Y, Su N, Yang J, Tan Q, Huang S, Jin M, et al. FGF/FGFR signaling in health and disease. Signal Transduct Target Ther. 2020;5:181.
Wu X, Ge H, Lemon B, Vonderfecht S, Weiszmann J, Hecht R, et al. FGF19-induced hepatocyte proliferation is mediated through FGFR4 activation. J Biol Chem. 2010;285:5165–70.
Zhang JL, Zhou YH, Huang TT, Wu F, Pan Y, Dong YJ, et al. FGF18, a prominent player in FGF signaling, promotes gastric tumorigenesis through autocrine manner and is negatively regulated by miR-590-5p. Oncogene. 2019;38:33–46.
Huang T, Wang L, Liu D, Li P, Xiong H, Zhuang L, et al. FGF7/FGFR2 signal promotes invasion and migration in human gastric cancer through upregulation of thrombospondin-1. Int J Oncol. 2017;50:1501–12.
Marek L, Ware KE, Fritzsche A, Hercule P, Helton WR, Smith JE, et al. Fibroblast growth factor (FGF) and FGF receptor-mediated autocrine signaling in non-small-cell lung cancer cells. Mol Pharm. 2009;75:196–207.
Marshall AD, van der Ent MA, Grosveld GC. PAX3-FOXO1 and FGFR4 in alveolar rhabdomyosarcoma. Mol Carcinog. 2012;51:807–15.
Missiaglia E, Selfe J, Hamdi M, Williamson D, Schaaf G, Fang C, et al. Genomic imbalances in rhabdomyosarcoma cell lines affect expression of genes frequently altered in primary tumors: An approach to identify candidate genes involved in tumor development. Genes Chromosomes Cancer. 2009;48:455–67.
Williamson D, Missiaglia E, de Reyniès A, Pierron G, Thuille B, Palenzuela G, et al. Fusion gene-negative alveolar rhabdomyosarcoma is clinically and molecularly indistinguishable from embryonal rhabdomyosarcoma. J Clin Oncol. 2010;28:2151–8.
Goldstein M, Meller I, Orr-Urtreger A. FGFR1 over-expression in primary rhabdomyosarcoma tumors is associated with hypomethylation of a 5’ CpG island and abnormal expression of the AKT1, NOG, and BMP4 genes. Genes Chromosomes Cancer. 2007;46:1028–38.
McKinnon T, Venier R, Yohe M, Sindiri S, Gryder BE, Shern JF, et al. Functional screening of FGFR4-driven tumorigenesis identifies PI3K/mTOR inhibition as a therapeutic strategy in rhabdomyosarcoma. Oncogene. 2018;37:2630–44.
Boudjadi S, Pandey PR, Chatterjee B, Nguyen TH, Sun W, Barr FG. A fusion transcription factor-driven cancer progresses to a fusion-independent relapse via constitutive activation of a downstream transcriptional target. Cancer Res. 2021;81:2930–42.
Fordham AM, Ekert PG, Fleuren EDG. Precision medicine and phosphoproteomics for the identification of novel targeted therapeutic avenues in sarcomas. Biochim Biophys Acta Rev Cancer. 2021;1876:188613.
Hassan SE, Bekarev M, Kim MY, Lin J, Piperdi S, Gorlick R, et al. Cell surface receptor expression patterns in osteosarcoma. Cancer. 2012;118:740–9.
Ren T, Qing Y, Dai N, Li M, Qian C, Yang Y, et al. Apurinic/apyrimidinic endonuclease 1 induced upregulation of fibroblast growth factor 2 and its receptor 3 induces angiogenesis in human osteosarcoma cells. Cancer Sci. 2014;105:186–94.
Weekes D, Kashima TG, Zandueta C, Perurena N, Thomas DP, Sunters A, et al. Regulation of osteosarcoma cell lung metastasis by the c-Fos/AP-1 target FGFR1. Oncogene. 2016;35:2852–61.
Kamura S, Matsumoto Y, Fukushi JI, Fujiwara T, Iida K, Okada Y, et al. Basic fibroblast growth factor in the bone microenvironment enhances cell motility and invasion of Ewing’s sarcoma family of tumours by activating the FGFR1-PI3K-Rac1 pathway. Br J Cancer. 2010;103:370–81.
Montoya JJ, Wai DH, Lee DW, Azorsa PA, Pepper OB, Arceci RJ, et al. Abstract 3338: a role for FGFR4 in growth and survival of Ewing sarcoma cells. Cancer Res. 2017;77:3338.
Zhou W, Du X, Song F, Zheng H, Chen K, Zhang W, et al. Prognostic roles for fibroblast growth factor receptor family members in malignant peripheral nerve sheath tumor. Oncotarget. 2016;7:22234–44.
Charbonneau B, Vogel RI, Manivel JC, Rizzardi A, Schmechel SC, Ognjanovic S, et al. Expression of FGFR3 and FGFR4 and clinical risk factors associated with progression-free survival in synovial sarcoma. Hum Pathol. 2013;44:1918–26.
Garcia CB, Shaffer CM, Alfaro MP, Smith AL, Sun J, Zhao Z, et al. Reprogramming of mesenchymal stem cells by the synovial sarcoma-associated oncogene SYT–SSX2. Oncogene. 2012;31:2323–34.
DeSalvo J, Ban Y, Li L, Sun X, Jiang Z, Kerr DA, et al. ETV4 and ETV5 drive synovial sarcoma through cell cycle and DUX4 embryonic pathway control. J Clin Invest. 2021;131:e141908.
Ishibe T, Nakayama T, Okamoto T, Aoyama T, Nishijo K, Shibata KR, et al. Disruption of fibroblast growth factor signal pathway inhibits the growth of synovial sarcomas: potential application of signal inhibitors to molecular target therapy. Clin Cancer Res. 2005;11:2702–12.
Northcott PA, Buchhalter I, Morrissy AS, Hovestadt V, Weischenfeldt J, Ehrenberger T, et al. The whole-genome landscape of medulloblastoma subtypes. Nature. 2017;547:311.
Pomeroy SL, Tamayo P, Gaasenbeek M, Sturla LM, Angelo M, McLaughlin ME, et al. Prediction of central nervous system embryonal tumour outcome based on gene expression. Nature. 2002;415:436–42.
Holzhauser S, Lukoseviciute M, Andonova T, Ursu RG, Dalianis T, Wickstrom M, et al. Targeting fibroblast growth factor receptor (FGFR) and phosphoinositide 3-kinase (PI3K) signaling pathways in medulloblastoma cell lines. Anticancer Res. 2020;40:53–66.
Lehtinen B, Raita A, Kesseli J, Annala M, Nordfors K, Yli-Harja O, et al. Clinical association analysis of ependymomas and pilocytic astrocytomas reveals elevated FGFR3 and FGFR1 expression in aggressive ependymomas. Bmc Cancer. 2017;17:310.
Jimenez-Pascual A, Mitchell K, Siebzehnrubl FA, Lathia JD. FGF2: a novel druggable target for glioblastoma? Expert Opin Ther Targets. 2020;24:311–8.
Ornitz DM, Xu J, Colvin JS, McEwen DG, MacArthur CA, Coulier F, et al. Receptor specificity of the fibroblast growth factor family. J Biol Chem. 1996;271:15292–7.
Clayton NS, Grose RP. Emerging roles of fibroblast growth factor 10 in cancer. Front Genet. 2018;9:499.
Chioni A-M, Grose R. FGFR1 cleavage and nuclear translocation regulates breast cancer cell behavior. J Cell Biol. 2012;197:801–17.
Francavilla C, Rigbolt KT, Emdal KB, Carraro G, Vernet E, Bekker-Jensen DB, et al. Functional proteomics defines the molecular switch underlying FGF receptor trafficking and cellular outputs. Mol Cell. 2013;51:707–22.
Lotsch D, Kirchhofer D, Englinger B, Jiang L, Okonechnikov K, Senfter D, et al. Targeting fibroblast growth factor receptors to combat aggressive ependymoma. Acta Neuropathol. 2021;142:339–60.
Allerstorfer S, Sonvilla G, Fischer H, Spiegl-Kreinecker S, Gauglhofer C, Setinek U, et al. FGF5 as an oncogenic factor in human glioblastoma multiforme: autocrine and paracrine activities. Oncogene. 2008;27:4180–90.
Yamaguchi F, Saya H, Bruner JM, Morrison RS. Differential expression of two fibroblast growth factor-receptor genes is associated with malignant progression in human astrocytomas. Proc Natl Acad Sci USA 1994;91:484–8.
Kobrin MS, Yamanaka Y, Friess H, Lopez ME, Korc M. Aberrant expression of type I fibroblast growth factor receptor in human pancreatic adenocarcinomas. Cancer Res. 1993;53:4741–4.
Luqmani YA, Mortimer C, Yiangou C, Johnston CL, Bansal GS, Sinnett D, et al. Expression of 2 variant forms of fibroblast growth factor receptor 1 in human breast. Int J Cancer. 1995;64:274–9.
Tomlinson DC, Knowles MA. Altered splicing of FGFR1 is associated with high tumor grade and stage and leads to increased sensitivity to FGF1 in bladder cancer. Am J Pathol. 2010;177:2379–86.
Siddaway R, Milos S, Vadivel AKA, Dobson THW, Swaminathan J, Ryall S, et al. Splicing is an alternate oncogenic pathway activation mechanism in glioma. Nat Commun. 2022;13:588.
Shimizu A, Tada K, Shukunami C, Hiraki Y, Kurokawa T, Magane N, et al. A novel alternatively spliced fibroblast growth factor receptor 3 isoform lacking the acid box domain is expressed during chondrogenic differentiation of ATDC5 cells. J Biol Chem. 2001;276:11031–40.
Konecny GE, Finkler N, Garcia AA, Lorusso D, Lee PS, Rocconi RP, et al. Second-line dovitinib (TKI258) in patients with FGFR2-mutated or FGFR2-non-mutated advanced or metastatic endometrial cancer: a non-randomised, open-label, two-group, two-stage, phase 2 study. Lancet Oncol. 2015;16:686–94.
Davis LE, Bolejack V, Ryan CW, Ganjoo KN, Loggers ET, Chawla S, et al. Randomized double-blind phase II study of regorafenib in patients with metastatic osteosarcoma. J Clin Oncol. 2019;37:1424.
Duffaud F, Mir O, Boudou-Rouquette P, Piperno-Neumann S, Penel N, Bompas E, et al. Efficacy and safety of regorafenib in adult patients with metastatic osteosarcoma: a non-comparative, randomised, double-blind, placebo-controlled, phase 2 study. Lancet Oncol. 2019;20:120–33.
Grignani G, Palmerini E, Dileo P, Asaftei SD, D’Ambrosio L, Pignochino Y, et al. A phase II trial of sorafenib in relapsed and unresectable high-grade osteosarcoma after failure of standard multimodal therapy: an Italian Sarcoma Group study. Ann Oncol. 2012;23:508–16.
Duffaud F, Blay JY, Mir O, Chevreau CM, Rouquette PB, Kalbacher E, et al. LBA68 Results of the randomized, placebo (PL)-controlled phase II study evaluating the efficacy and safety of regorafenib (REG) in patients (pts) with metastatic relapsed Ewing sarcoma (ES), on behalf of the French Sarcoma Group (FSG) and UNICANCER. Ann Oncol. 2020;31:S1199.
Attia S, Bolejack V, Ganjoo KN, George S, Agulnik M, Rushing D, et al. A phase II trial of regorafenib in patients with advanced Ewing sarcoma and related tumors of soft tissue and bone: SARC024 trial results. Cancer Med. 2023;12:1532–9.
Gaspar N, Campbell-Hewson Q, Melcon SG, Locatelli F, Venkatramani R, Hecker-Nolting S, et al. Phase I/II study of single-agent lenvatinib in children and adolescents with refractory or relapsed solid malignancies and young adults with osteosarcoma (ITCC-050). ESMO Open. 2021;6:100250.
Gaspar N, Venkatramani R, Hecker-Nolting S, Melcon SG, Locatelli F, Bautista F, et al. Lenvatinib with etoposide plus ifosfamide in patients with refractory or relapsed osteosarcoma (ITCC-050): a multicentre, open-label, multicohort, phase 1/2 study. Lancet Oncol. 2021;22:1312–21.
Linzey JR, Marini B, McFadden K, Lorenzana A, Mody R, Robertson PL, et al. Identification and targeting of an FGFR fusion in a pediatric thalamic “central oligodendroglioma”. NPJ Precis. Oncol 2017;1:29.
Mueller T, Stucklin ASG, Postlmayr A, Metzger S, Gerber N, Kline C, et al. Advances in targeted therapies for pediatric brain tumors. Curr Treat Options Neurol. 2020;22:43.
Paul MR, Pehlivan KC, Milburn M, Yeh-Nayre L, Elster J, Crawford JR. Trametinib-based treatment of pediatric CNS tumors: a single institutional experience. J Pediatr Hematol/Oncol. 2020;42:e730–7.
Selt F, van Tilburg CM, Bison B, Sievers P, Harting I, Ecker J, et al. Response to trametinib treatment in progressive pediatric low-grade glioma patients. J Neuro-Oncol. 2020;149:499–510.
Manoharan N, Choi J, Chordas C, Zimmerman MA, Scully J, Clymer J, et al. Trametinib for the treatment of recurrent/progressive pediatric low-grade glioma. J Neurooncol. 2020;149:253–62.
Farouk Sait S, Gilheeney SW, Bale TA, Haque S, Dinkin MJ, Vitolano S, et al. Debio1347, an oral FGFR inhibitor: results from a single-center study in pediatric patients with recurrent or refractory FGFR-altered gliomas. JCO Precis Oncol. 2021;5:PO.20.00444.
Capone S, Ketonen L, Weathers SP, Subbiah V. Activity of pemigatinib in pilocytic astrocytoma and FGFR1(N546K) Mutation. JCO Precis Oncol. 2022;6:e2100371.
Witt O, Geoerger B, Dirksen U, Farouk Sait S, Reardon DA, Shih KC, et al. Erdafitinib in pediatric patients with advanced solid tumors with fibroblast growth factor receptor (FGFR) gene alterations: RAGNAR study pediatric cohort. J Clin Oncol. 2022;40:TPS10058-TPS.
Lassman AB, Sepúlveda-Sánchez JM, Cloughesy TF, Gil-Gil MJ, Puduvalli VK, Raizer JJ, et al. Infigratinib in patients with recurrent gliomas and FGFR alterations: a multicenter phase II study. Clin Cancer Res. 2022;28:2270–7.
Zhou X, Edmonson MN, Wilkinson MR, Patel A, Wu G, Liu Y, et al. Exploring genomic alteration in pediatric cancer using ProteinPaint. Nat Genet. 2016;48:4–6.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Contributions
LMB, PGE and EDGF developed the idea of the review and wrote the manuscript. All authors reviewed and edited the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Brown, L.M., Ekert, P.G. & Fleuren, E.D.G. Biological and clinical implications of FGFR aberrations in paediatric and young adult cancers. Oncogene 42, 1875–1888 (2023). https://doi.org/10.1038/s41388-023-02705-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-023-02705-7
This article is cited by
-
PI3K/AKT/mTOR signaling transduction pathway and targeted therapies in cancer
Molecular Cancer (2023)