Abstract
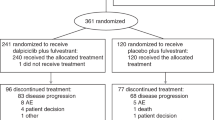
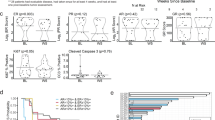
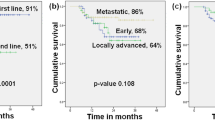
SOLAR-1 and BYLieve trials documented the efficacy of the PI3K-inhibitor alpelisib in pre-treated PIK3CA-mutant, hormone receptor-positive, HER2-negative (HR+/HER2-) advanced breast cancer (ABC) patients. We report here real-life data of patients prospectively registered in the French alpelisib early access program (EAP) opened to PIK3CA-mutant HR+/HER2- ABC patients treated with alpelisib and fulvestrant. Primary endpoint was PFS by local investigators using RECIST1.1. Eleven centers provided individual data on 233 consecutive patients. Patients had received a median number of 4 (range: 1–16) prior systemic treatments for ABC, including CDK4/6 inhibitor, chemotherapy, fulvestrant and everolimus in 227 (97.4%), 180 (77.3%), 175 (75.1%) and 131 (56.2%) patients, respectively. After a median follow-up of 7.1 months and 168 events, median PFS was 5.3 months (95% CI: 4.7–6.0). Among 186 evaluable patients, CBR at 6 months was 45.3% (95% CI: 37.8–52.8). In multivariable analysis, characteristics significantly associated with a shorter PFS were age < 60 years (HR = 1.5, 95% CI = 1.1–2.1), >5 lines of prior treatments (HR = 1.4, 95% CI = 1.0–2.0) and the C420R PI3KCA mutation (HR = 4.1, 95% CI = 1.3–13.6). N = 91 (39.1%) patients discontinued alpelisib due to adverse events. To our knowledge, this is the largest real-life assessment of alpelisib efficacy. Despite heavy pre-treatments, patients derived a clinically relevant benefit from alpelisib and fulvestrant.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
Change history
15 March 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41388-023-02615-8
References
Miller TW, Balko JM, Arteaga CL. Phosphatidylinositol 3-kinase and antiestrogen resistance in breast cancer. J Clin Oncol J Am Soc Clin Oncol. 2011;29:4452–61.
Bosch A, Li Z, Bergamaschi A, Ellis H, Toska E, Prat A, et al. PI3K inhibition results in enhanced estrogen receptor function and dependence in hormone receptor-positive breast cancer. Sci Transl Med. 2015;7:283ra51.
Mosele F, Stefanovska B, Lusque A, Tran Dien A, Garberis I, Droin N, et al. Outcome and molecular landscape of patients with PIK3CA-mutated metastatic breast cancer. Ann Oncol. 2020;31:377–86.
Sobhani N, Roviello G, Corona SP, Scaltriti M, Ianza A, Bortul M, et al. The prognostic value of PI3K mutational status in breast cancer: A meta‐analysis. J Cell Biochem. 2018;119:4287–92.
Fritsch C, Huang A, Chatenay-Rivauday C, Schnell C, Reddy A, Liu M, et al. Characterization of the novel and specific PI3Kα inhibitor NVP-BYL719 and development of the patient stratification strategy for clinical trials. Mol Cancer Ther. 2014;13:1117–29.
André F, Ciruelos E, Rubovszky G, Campone M, Loibl S, Rugo HS, et al. Alpelisib for PIK3CA-Mutated, Hormone Receptor-Positive Advanced Breast Cancer. N Engl J Med. 2019;380:1929–40.
Cardoso F, Paluch-Shimon S, Senkus E, Curigliano G, Aapro MS, André F, et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann Oncol J Eur Soc Med Oncol. 2020;31:1623–49.
Rugo HS, Lerebours F, Ciruelos E, Drullinsky P, Ruiz-Borrego M, Neven P, et al. Alpelisib plus fulvestrant in PIK3CA-mutated, hormone receptor-positive advanced breast cancer after a CDK4/6 inhibitor (BYLieve): one cohort of a phase 2, multicentre, open-label, non-comparative study. Lancet Oncol. 2021;22:489–98.
Patil S. Early access programs: Benefits, challenges, and key considerations for successful implementation. Perspect Clin Res. 2016;7:4.
André F, Ciruelos EM, Juric D, Loibl S, Campone M, Mayer IA, et al. Alpelisib plus fulvestrant for PIK3CA-mutated, hormone receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: final overall survival results from SOLAR-1. Ann Oncol J Eur Soc Med Oncol. 2021;32:208–17.
Bidard F-C, Kaklamani VG, Neven P, Streich G, Montero AJ, Forget F et al. Elacestrant (oral selective estrogen receptor degrader) versus standard endocrine therapy for estrogen receptor–positive, human epidermal growth factor receptor 2–negative advanced breast cancer: results from the randomized phase III EMERALD trial. J Clin Oncol. 2022;40:JCO.22.00338.
Barrios MSDM, Wang DG, Blinder VS, Bromberg J, Drullinsky P, Funt SA, et al. Prevalence and characterization of dermatologic adverse events related to alpelisib (BYL719) in breast cancer patients. J Clin Oncol. 2020;38:1063–1063.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Acknowledgements
This work was supported by Novartis Pharma Sas. Novartis grants have been dedicated to the research purposes detailed below: Principal investigator & local sub-investigator time. Abstract & publication fees. Clinical Research Assistant time Database/Datamanager time. Statistical analysis. Preliminary data have been published as an abstract and a poster at the 2021 ASCO Annual Meeting (abstract number: 1064).
Author information
Authors and Affiliations
Contributions
Conceptualization: DBR, AG, FCB, FL. Methodology: DBR, AG, FCB, FL. Software: DBR, BR. Validation: DBR, AB, TDLMR, CB, SA, CB, MAB, ID, ZT, ER, JG, MS, BR, JG, HS, SD, MB, PC, AG, DL, JYP, FCB, FL. Formal analysis: BR. Resources: DBR, AB, TDLMR, CB, SA, CB, MAB, ID, ZT, ER, JG, MS, BR, JG, HS, SD, MB, PC, AG, DL, JYP, FCB, FL. Data Curation: DBR, BR, FCB, FL. Investigation: DBR, BR, FCB, FL. Writing - Original Draft: DBR, AB, TDLMR, CB, SA, CB, MAB, ID, ZT, ER, JG, MS, BR, JG, HS, SD, MB, PC, AG, DL, JYP, FCB, FL. Writing - Review & Editing: DBR, AB, TDLMR, CB, SA, CB, MAB, ID, ZT, ER, JG, MS, BR, JG,HS, SD, MB, PC, AG, DL, JYP, FCB, FL. Visualization: DBR, BR, FCB, FL. Supervision: DBR, AG, FCB, FL. Project administration: DBR, FCB, FL. Funding acquisition: FL.
Corresponding author
Ethics declarations
Competing interests
Institut Curie, represented in this work by Dr Florence Lerebours, has been funded by Novartis. DBR: Travel accommodations expenses: Daiichi Sankyo; Lilly; GSK. AB, TDLMR, CB, SA, CB, MAB, ID, ZT, ER, JG, MS: no relationships to disclose. JG: Travel accommodations expenses: AstraZeneca; Esai. HS: Honoraria: Lilly; Pfizer; Tesaro. Consulting or advisory role: Lilly; Mundipharla; Mylan; Novartis; Pierre Fabre; Roche; Sandoz; Vifor Pharma. Travel accommodations expenses: AstraZeneca; Lilly; Mundipharma; Novartis; Pfizer; Roche. SD: Consulting or advisory role: AstraZeneca; Pierre Fabre. Research funding: AstraZeneca; Exact Sciences; Lilly; Novartis; Pfiizer; Puma Biotechnolohy; Roche/Genentech; Sanofi. Travel accommodations expenses: AstraZeneca; Pfizer; Roche. MB: Travel accommodations expenses: Pfizer. Paul Cottu: Honoraria: Lilly; NanoString Technologies; Novartis; Pfizer; Pierre Fabre; Roche. Consulting or Advisory role: Lilly; Pfizer; Roche/Genentech. Research funding: Novartis; Pfizer. Travel accommodations expenses: Novartis; Pfizer. AG: Research Funding: Abbvie; AstaZeneca; Boehringer Ingelheim; Bristol-Myers Squibb; Cascadian Therapeutics; Lilly; Merus; MSD; Nektar; Novartis; Roche; Roche/Genentech; Sanofi/Aventis; Seattle Genetics. Travel accommodations expenses: Novartis. DL: Consulting or Advisory role: MSD Travel accommodations expenses: Bristol-Myers Squibb. JYP: Consulting or Advisory role or travel accommodations expenses: Pfizer, Lilly MSD, Roche, Novartis, AstraZeneca, Pierre Fabre, Servier, Daiichi et Ipsen. FCB: Consulting or advisory role: Archer; Lilly; Novartis; Pfizer; Radius Health; Sanofi. Speakers’ bureau: AstraZeneca; Novartis; Pfizer; Roche. Research Funding: Menarini Silicon Biosystems; Novartis; Pfizer; ProLynx; Roche; Servier. Patents, royalties, other intellectual property: ctDNA detection techniques. Travel accommodations expenses: Chugai Pharma; Novartis; Pfizer. FL: Travel accommodations expenses: Lilly; Novartis; Pfizer; Pierre Fabre; Roche.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bello Roufai, D., Gonçalves, A., De La Motte Rouge, T. et al. Alpelisib and fulvestrant in PIK3CA-mutated hormone receptor-positive HER2-negative advanced breast cancer included in the French early access program. Oncogene 42, 1951–1956 (2023). https://doi.org/10.1038/s41388-022-02585-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-022-02585-3
This article is cited by
-
Fulvestrant and everolimus efficacy after CDK4/6 inhibitor: a prospective study with circulating tumor DNA analysis
Oncogene (2024)
-
Sacituzumab govitecan in metastatic triple-negative breast cancer patients treated at Institut Curie Hospitals: efficacy, safety, and impact of brain metastases
Breast Cancer (2024)