Abstract
Exploring the relationship between various neurotransmitters and breast cancer cell growth has revealed their likely centrality to improving breast cancer treatment. Neurotransmitters play a key role in breast cancer biology through their effects on the cell cycle, epithelial mesenchymal transition, angiogenesis, inflammation, the tumor microenvironment and other pathways. Neurotransmitters and their receptors are vital to the initiation, progression and drug resistance of cancer and progress in our biological understanding may point the way to lower-cost and lower-risk antitumor therapeutic strategies. This review discusses multiple neurotransmitters in the context of breast cancer. It also discusses risk factors, repurposing of pharmaceuticals impacting neurotransmitter pathways, and the opportunity for better integrated models that encompass exercise, the intestinal microbiome, and other non-pharmacologic considerations. Neurotransmitters’ role in breast cancer should no longer be ignored; it may appear to complicate the molecular picture but the ubiquity of neurotransmitters and their wide-ranging impacts provide an organizing framework upon which further understanding and progress against breast cancer can be based.
Similar content being viewed by others
Introduction
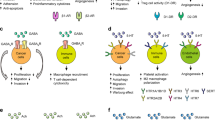
Breast cancer is the most common cancer among women. Despite research progress, it remains the leading cause of cancer death among women [1] and much about its molecular biology is still not understood. Neurotransmitters are nerve-secreted substances that modulate neuronal functions by binding to their respective receptors. They play regulatory roles in the physiological functions of tissues and organs and disruptions are associated with various pathologic states (Fig. 1).
It has been established in recent decades that neurotransmitters are involved in signaling pathways which influence the course of various malignancies, including breast cancer. Emerging data suggest that cancer cells take advantage of neurotransmitter-initiated signaling pathways, including VEGF, p53, AKT, and MAPK, to activate uncontrolled proliferation and metastasis (Fig. 2). In addition, neurotransmitters can affect immune cells and endothelial cells in the tumor microenvironment via monoamine oxidase modulation of tumor-associated macrophages to promote tumor growth (Table 1).
An appreciation of the role played by neurotransmitters is critical given both the numerous ways that neurotransmitters influence cancer and the number of factors that greatly influence neurotransmitter signaling, ranging from common medications (such as beta blockers and antidepressants) to stress (adrenergic activation) [2] (Table 2). Breast cancer tumors are commonly innervated and greater infiltration of nerve fibers into the tumor microenvironment may predict a worse outcome [3, 4]. The significance of neurotransmitters to breast cancer growth could point the way to lower-cost and lower-risk interventions.
An exhaustive detailing of the entire landscape of neurotransmitter interactions with cancer is beyond the scope of a single paper. This review emphasizes the following critical topics, selected as likely closest to affecting standards of breast cancer care and representative of the myriad range of neurotransmitter impacts on breast cancer: epinephrine, norepinephrine, and adrenergic activation; acetylcholine; serotonin; dopamine; histamines; gamma-aminobutyric acid (GABA); and neurotensin; and drugs, such as beta blockers and monoamine oxidase inhibitors, impacting pathways mediated by these neurotransmitters (Table 3). Key questions remain open in most of these areas, and further work on these topics holds the potential of great impact to improve our understanding of breast cancer and our therapeutic arsenal against it.
The wide-ranging impacts of neurotransmitters also provide consistent support for seemingly disparate areas of cancer research, ranging from the microbiome to fasting to exercise.
Adrenergic activation
Epinephrine (adrenaline) and norepinephrine (noradrenaline) are endogenous catecholamines or monoamine neurotransmitters. Release of these hormones mediates the fight-or-flight response as they bind to adrenergic receptors. Breast cancer tissue has been found to overexpress β-adrenergic receptors [5].
Stress induced neuroendocrine activation or pharmacologic activation of adrenergic receptors has been found in mouse models to result in substantial increases to proliferation and distant metastasis [6]. This phenomenon of adrenergic receptors activated by stress neurotransmitters worsening cancer state is not fully understood, but studies are proceeding rapidly [7]. It has also been shown in mice that local mammary tumor sympathetic innervation responds to stress with increased norepinephrine turnover [8] and this is of greater significance to tumor growth than circulating norepinephrine [9]. There are several proposed mechanisms underlying the pro-tumor roles of epinephrine and norepinephrine. One is that activation of β2-adrenoceptor (AR) promotes tumor growth and angiogenesis through increased expression of vascular endothelial growth factor (VEGF), metalloproteases 2 (MMP2), and MMP9. This further potentiates the angiogenic and metastatic evolution of breast cancer [8]. ß2AR signaling may also stimulate tumor growth by promoting DNA damage and p53-associated apoptosis suppression [10].
Chances of recurrence appear to be increased by stress. Perego et al. proposed, and supported with murine evidence for lung and ovarian cancer, a mechanism for the awakening of dormant cancer cells [11]. They proposed that pro-inflammatory S100A8/A9 complexes are released by polymorphonuclear neutrophils via β2-adrenergic receptors, in response to stress hormones, and the complexes cause oxidized lipids to accumulate. The release of S100A8/A9 proteins and modified lipids upregulates a fibroblast growth factor receptor pathway, activating dormant cancer cells.
In metastatic breast cancer, the skeleton is a frequent site of spread. Sympathetic nerves densely network across bones. It has been shown that activation of β2-adrenergic receptors, as in sympathetic nerve activation, in osteoblasts leads to increased bone vascular density and a more favorable bone microenvironment for breast cancer cells [12,13,14].
Beta blockade
If adrenergic activation stimulates breast cancer progression, then a natural question is whether inhibiting this activation can provide clinical anticancer benefits. This question has been aggressively pursued, particularly given the ready availability of beta blocker drugs [15,16,17,18].
Beta blockers are primarily used for cardiac purposes—to treat abnormal heart rhythms and as a second- or later-line treatment for hypertension. Beta blockers are competitive antagonists that block receptor sites for epinephrine and norepinephrine on adrenergic beta receptors. Several of the preclinical studies examining the impact of adrenergic activation on breast cancer growth and migration also tested the impact of beta blockers and found them to have an inhibitory effect [18].
On the clinical side, results have charted a more winding path. A 2017 meta-analysis of six studies, with a cumulative breast cancer patient population over 18,000, found no benefit of beta blockers on overall deaths, cancer-specific deaths, or recurrence [19]. However, this study did not draw conclusions on potential benefits for specific subtypes of breast cancer. Other meta-analyses published over a range of cancers had mixed findings [20,21,22]. A more recent meta-analysis of 17 studies found no significant association between beta blocker use and breast cancer recurrence [23]. Further, an analysis by Modi et al. found existing beta blocker use at the time of anti-HER2 therapy to actually be associated with worse overall survival among patients with advanced HER2-positive breast cancer [24].
Other studies have reported more promising conclusions, particularly in regards to metastasis and recurrence; this evidence is stronger, though not definitive, for certain cancer subtypes [25]. An examination of 800 women with triple negative breast cancer who took beta blockers found significantly reduced risk of recurrence, metastasis, and death [26]. An additional retrospective breast cancer population study found that beta blockers improved relapse-free survival, though not overall survival, after correcting for differences in cancer severity, hypertension, and other factors [27]. Powe et al., Parada-Huerta et al., and Choy et al. all found beta blockers to be correlated with reduced breast cancer metastasis [28,29,30]. Spera et al.’s retrospective analysis found an improvement in progression-free survival, particularly for triple negative phenotype and for patients who were not taking a beta blocker prior to cancer treatment [31].
Furthermore, a meta-analysis of studies published between 2010 and 2013 found that women with breast cancer who took beta blockers had a significantly decreased risk of breast cancer death than women with breast cancer who did not take beta blockers [20]. A recent meta-analysis of nearly 15,000 New Zealand breast cancer patients saw a short term—over 3 months—increased risk of death among patients who took beta blockers post-diagnosis, and a protective effect with long-term use [32].
Propranolol is a beta blocker that was approved for use in the US in 1964. It is still in widespread use and it nonselectively blocks β-adrenergic receptors. In a triple-blind placebo-controlled trial reported in 2020, 30 patients with early-stage breast cancer were given propranolol for 7 days prior to resection and 30 were given placebo [33]. Those given propranolol showed reduced intratumoral mesenchymal polarization and increased immune cell infiltration.
Montoya et al.’s study based on a single stage III breast cancer patient found that neoadjuvant propranolol reduces pro-proliferative Ki-67 and pro-survival Bcl-2 markers, and significantly increases p53 expression and induces apoptosis [34]. Montoya et al. had earlier reported that non-selective beta blockers significantly reduced tumor proliferation in early stage breast cancer, based on a retrospective analysis [35]. They also found that a three-week course of propranolol in one patient with early-stage breast cancer was associated with a reduction in Ki67 positive tumor cells, and selective beta blockers were not as effective.
In a randomized placebo-controlled biomarker trial, perioperative inhibition of COX-2 and β-adrenergic signaling was found to inhibit multiple cellular and molecular pathways related to metastasis and disease recurrence in early-stage breast cancer [36]. Combination with neoadjuvant chemotherapy is also being investigated: A phase II trial tested combining propranolol with neoadjuvant taxane/anthracycline-based chemotherapy demonstrated feasibility in the hopes of regulating angiogenesis and reducing distant metastases [37].
The combination of propranolol with non-chemotherapy medications is also showing promise. For example, Murugan et al. investigated the combination of propranolol with naltrexone (an opiate antagonist used to counteract drug and alcohol dependence) and found it to substantially inhibit tumor growth and improve survival in rat xenografts. The effects were attributed to decreasing tumor cell proliferation, inducing cellular apoptosis, and preventing the epithelial–mesenchymal transition in the tumor [38]. In follow-up work, the glycogen synthase kinase 3 pathway was identified as possibly involved in cross talk between β-adrenergic receptors and mu-opioid receptors, with the conclusion that targeting the receptors or glycogen synthase kinase 3 system could prove fruitful in treating triple negative breast cancer [39]. β1-blockers are also starting to gain interest. Nebivolol, which blocks β1-adrenergic signaling, has been found to halt colon and breast tumor growth in xenograft mice [40].
Combating drug resistance is also an area of promise. β2-andrenergic receptors form a positive feedback loop with Her2 in breast cancer cells [41]. Liu et al. found that β2-adrenergic receptors are predictive marker for response to trastuzumab-based therapy in breast cancer, and that propranolol improves the effectiveness of trastuzumab [42]. They also found that it can re-sensitize resistant cells to trastuzumab and, based on a study of medical records, found that treatment with both trastuzumab and beta blockers significantly improved progression-free survival and overall survival in the patients with Her2-positive metastatic breast cancer. Re-sensitization to existing therapies is a much sought after goal in oncology [43].
α2-Adrenergic receptor agonists
While the interplay with β-adrenergic receptors has received more attention, other adrenergic receptors also appear to be relevant. Szpunar et al. showed that, in mice, treatment with a highly selective α2-adrenergic receptor agonist, dexmedetomidine, increased tumor growth and metastasis [44]. Dexmedetomidine is a common surgical anesthetic, including for breast cancer surgery. Based on in vitro and xenotransplant in vivo assays, Xia et al. reported that dexmedetomidine could increase the proliferation, migration, and invasion of breast cancer cells via activation of α2B-adrenoceptor / ERK signaling [45]. Subsequent studies have corroborated this finding and proposed explanatory mechanisms [46,47,48,49], while none have shown a finding of sufficient significance to discourage use of the anesthetic.
On the other hand, the opioid analgesic tramadol could inhibit proliferation, migration, and invasion of breast cancers by inactivating the α2-adrenoceptor signaling pathway [50]. Based on cellular experiments, Huang et al. concluded that tramadol inhibits the progression of breast cancer cells and should be investigated further for use in combination therapy, especially for triple-negative breast cancer [51]. Like tramadol, the α2-adrenoceptor antagonist rauwolscine also suppressed tumor growth, in mice with human breast cancer cells [52]. Found in multiple botanical sources and marketed as a fat-burning nutritional supplement, it has also been shown to function as a 5-HT1 receptor partial agonist and 5-HT2 receptor antagonist.
Acetylcholine
Acetylcholine (ACh) functions in the nervous system as a neurotransmitter at the autonomic ganglia, the parasympathetic innervated organs, and the neuromuscular junction between motor nerves and skeletal muscle. Acetylcholine receptors (AChRs) fall into one of two categories; the relatively slow activating G protein-coupled metabotropic muscarinic receptors (mAChRs) and the faster activating ionotropic nicotinic receptors (nAChRs). ACh helps regulate cellular proliferation, differentiation, and apoptosis.
Providing a partial explanation for the role of smoking in breast cancer, Nishioka et al. showed a decade ago with MCF10A (benign) and MDA-MB-231 (malignant) breast cells that when nicotine is ligated with nAChR, it promotes EGFR and Src signals [53]. Huang et al. showed in a murine model that advanced stage triple negative breast tumors are associated with higher levels of α9-nAChR gene expression [54]. α7-nAChR also appears to be up and it has been suggested that subtype-specific AChR antagonists could present an attractive pharmaceutical direction to prevent breast cancer progression [55].
Like nAChRs, the mAChRs also appear to influence breast cancer, specifically in that they are upregulated in breast tumors and absent in normal breast cells and tissues. Sales et al. has reported that mAChR agonists can act against breast tumors in a dose-dependent manner and be effective even at low doses [56].
Serotonin
Serotonin (5-hydroxytryptamine, 5-HT) is synthesized from the essential amino acid tryptophan and mediates motility in the gastrointestinal tract and is a vasoactive agent in the blood. As a monoamine neurotransmitter, it also acts in the central nervous system. It regulates epithelial homeostasis in the breast. Serotonin is believed to impact immune signaling and stimulate growth of breast cancer cells [57,58,59,60,61]. Olfati et al. showed that in samples from breast cancer patients, 5HTR2A and 5HTR3A genes are more expressed in tumoral tissues than marginal tissues [62].
Serotonin also initiates angiogenesis by the proliferation, invasion, and migration of endothelial cells [63]. Sonier et al. found that serotonin promotes the growth and division of breast cancer cells, specifically MCF-7 cells, in part through the 5-HT2A receptor. Proliferation and invasion is also facilitated by the 5-HT7 receptor in MDA-MB-231 cells. In these cells, 5-HT is essential to enhance the expression of TPH1 (tryptophan hydroxylase 1) and VEGF, supporting the mitogenic and oncogenic impact of 5-HT on breast cancer.
Tramadol, mentioned earlier in the context of α2-adrenoceptor, also interacts with serotonin receptors. Kim et al. reported that patients who received tramadol after breast cancer surgery had a decreased risk of postoperative recurrence and mortality, with the anti-tumor effect of tramadol appearing to involve inhibition of proliferation, induction of apoptosis, and effects on the serotonin 2B receptor and transient receptor potential vanilloid-1 expression [63]. More generally, antagonists of serotonin biosynthesis, transport, and activity appear to diminish breast cancer stem cell viability [64]. Serotonin production in a tumor sample may be a predictor of poor prognosis [65].
If lowering serotonin activity reduces breast cancer recurrence, then a logical question is the impact of medications that raise serotonin levels. Evidence has been conflicting on this long-standing question. Two decades ago, researchers reported that use of tricyclic medications was associated with significantly increased breast cancer risk [66, 67] and that use of selective serotonin reuptake inhibitor (SSRI) drugs may also pose a breast cancer risk [68, 69]. The SSRI fluoxetine may increase the number of breast cancer brain metastases at least in part due to inflammatory changes in the brain [70]. Supporting these contentions, this year researchers in Israel reported based on an analysis of 7000 patients that use of SSRIs in the years prior to breast cancer diagnosis, or in the years following diagnosis, was associated with substantially increased mortality [71].
However these studies were far from definitive and other analyses have reached the opposite conclusion. For example, a 2012 meta-analysis by Eom et al. on the relationship between SSRI use and breast cancer risk observed only marginal association attenuated over time, with no clinical significance [72]. Similarly, a recent meta-analysis by Li et al. reviewed 19 studies, finding no causal relationship [73]. And breast cancer cell line research has shown no significant effect of SSRIs on cell glucose uptake [74]. Indeed, other work has even suggested that such medications may even be used to positive effect. Murine research has indicated that inhibition of serotonin reuptake can inhibit breast tumor formation and that an SSRI given with docetaxel shrinks breast tumors [75, 76].
While the effectiveness of SSRIs for their primary indication may be in debate, there is little argument that they increase serotonin levels [77]. Further research on their impact on breast cancer is warranted, both to determine the direction of clinical impact of SSRIs in humans and to pursue serotonin receptor agonists as potential therapeutics [78].
Dopamine
Breast cancer overexpresses the hormone prolactin, and prolactin is implicated in breast cancer growth, metastasis, and chemoresistance [79,80,81]. The hormone is under the inhibitory control of the neurotransmitter dopamine, raising the question of whether dopaminergic drugs can improve breast cancer outcomes. Dopamine is a catecholamine, like epinephrine and norepinephrine, and a precursor to their synthesis. It is important for regulation of behavior, movement control, endocrine, and cardiovascular function.
Dopamine or its receptor agonists seem to exhibit inhibitory effect on tumor growth in breast and several other cancer types. However, dopamine fails to diminish the proliferation and invasion of breast and colon cancer cells, indicating that factors such as tumor type, receptors expressed, and doses used play a role [82]. An important mechanism in dopamine’s tumor-suppressive effect is decreased angiogenesis. Activation of the DRD1/cGMP/PKG pathway induces growth arrest in vitro and causes tumor shrinkage and reduced bone metastasis in breast cancer [83].
A small study of giving patients with metastatic breast cancer cabergoline, an agonist of D2 dopamine receptors that has an inhibitory effect on pituitary prolactin secretion, had an inconclusive outcome [84]. Later, Goyette et al. found that phenothiazines, anti-psychotic drugs, reduced invasion and proliferation and increased apoptosis of triple negative cancer cells in vitro, by reducing PI3K/AKT/mTOR and ERK signaling [85]. Furthermore, they observed that administering phenothiazines to mice with triple negative breast cancer xenografts reduced tumor growth and metastasis. Other medications are being actively researched, with side effects being a significant consideration [86].
Histamine
Best known for its role in allergies, histamine is an important monoamine neurotransmitter. Four G-protein-coupled histamine receptor subtypes mediate neuronal histamine’s effects [87]. The histamine H4 receptor, which is primarily expressed in immune cells and has also been found in breast cancer tissue, has been implicated in breast cancer growth [88,89,90]. Histamine decarboxylase expression level is correlated with relapse free and overall survival and histamine administered to mice with 4T1 triple negative tumor cells reduced tumor growth and increased apoptosis [91].
Speisky et al. reported that the H4 receptor may be a useful biomarker for predicting triple negative breast cancer prognosis [92]. Based on data from the Cancer Genome Atlas, they observed that the H4 receptor is downregulated in basal-like/triple negative breast cancer compared with luminal A and normal breast-like tumors. Furthermore, among basal-like/triple negative breast cancer patients, higher expression of the H4 receptor was associated with improved progression-free and overall survival outcomes. Further analysis of 30 triple negative breast cancer tumor samples showed that high H4 receptor expression in peritumoral tissue correlated with lesser lymph node involvement, unifocal triple negative breast cancer, and increased patient survival.
GABA
The primary inhibitory neurotransmitter in the human brain is GABA. GABA may promote cancer cell proliferation and migration and is amplified in multiple cancers including breast. Gumireddy et al. observed that the GABAA receptor alpha3, which is normally exclusively expressed in adult brain, is expressed in breast cancer, with higher tumor expression being associated with poorer survival [93]. As shown in mouse models, the receptor activates the AKT pathway, increasing breast cancer cell invasion and metastasis. Both Neman et al. and Dahn et al. have found that activation of GABAA receptors increase brain metastases in breast cancer patients. The receptor could therefore represent a promising therapeutic target [94, 95].
Propofol is a drug with agonist activity for the GABAA receptor and causes actin reformation and migration of breast cancer cells by collagen matrices. Observational epidemiological studies also show that benzodiazepine use increased the risk of breast cancer and many other cancers in a dose-dependent manner. Mimics of GABA, an inhibitory neurotransmitter, are frequently used to reduce peripheral nerve pain caused by chemotherapy. However, there is concern that GABA treatment could increase breast cancer metastasis [95]. Improved understanding will help ascertain if there are any adverse impacts from existing medication use.
Neurotensin and neuropeptide Y
From 1419 primary breast tumors in a French institute, neurotensin receptor-1 overexpression was found in about one-third of breast tumors from patients undergoing primary surgery [96]. Neurotensin, activating neurotensin receptor, increases tumor cell proliferation, invasion, migration, and antiapoptotic effects [97]. Neurotensin receptor antagonists appear to ameliorate the situation and could provide a method for treating tumors overexpressing neurotensin receptors. Neurotensin is upregulated by estrogen in normal epithelial breast cells. In breast cancer cells, upregulation of the NTS-1 receptor leads to increased cellular migration and invasion. High expression of NTS-1 receptor has been associated with tumor grade, size, and number of metastatic lymph nodes [98].
Neuropeptide Y is a 36 amino-acid neuropeptide that plays a role in various physiological and homeostatic processes in the nervous systems including the osteogenic response. Its major receptors are overexpressed in multiple tumors including breast cancer metastasis [99]. Neuropeptide Y helps breast cancer cells proliferate and metastasize in part due to its role in angiogenesis via its effects on vascular smooth muscle and VEGF [100]. Li et al. elaborated on prior links between breast cancer and osteoporosis noting that Neuropeptide Y and its receptors are also involved in the regulation of bone metabolism [101]. They propose the Y1 receptor as a potential target for stem cell therapy to treat breast cancer and osteoporosis.
MAOI synergy with PD-1/PD-L1 blockade therapy
Immune checkpoint blockade therapy has become a key tool in the treatment of several types of cancer, including triple negative breast cancer, in recent years. There is an active line of research towards improving and broadening the efficacy of such immunotherapies by modifying the tumor microenvironment. Neurotransmitters appear to play an important role here. Targeting tumor-associated macrophages to reduce their inhibition of antitumor T-cell reactivity may improve an immunotherapy’s activity against breast cancer [102].
Monoamine oxidase A is an enzyme bound to the outer mitochondrial membrane. In the brain, it is involved in degrading serotonin, dopamine, epinephrine, and norepinephrine [103]. Given its role in regulating the availability of serotonin and dopamine, there are small molecule monoamine oxidase A inhibitors (MAOIs) that are approved by the FDA for treatment of depression and Parkinson’s disease. Wu et al. demonstrated that MAOA induces the epithelial-to-mesenchymal transition (EMT) and stabilizes the transcription factor HIF1α, which promotes invasiveness and metastasis in prostate cancer [103]. High MAOA expression correlated with worse clinical outcomes. MAO A inhibitors reduced proliferation, microvessel density and invasion, and increased macrophage infiltration in drug-resistant tumors [104]. LaPierre et al. showed that MAO knockout mice had elevated markers of immune stimulation and decreased expression of markers of immune suppression compared MAO A wildtype [105]. They suggested that the deletion of MAO A reduces immune suppression in tumors to enhance antitumor immunity. Thus, MAO A inhibitors may alleviate immune suppression, increase the antitumor immune response and be used for cancer immunotherapy.
An excellent demonstration of this direction was provided by Wang et al. [106]. They reported last year on their investigation of the potential of monoamine oxidase A for reprogramming tumor-associated macrophages. Wang et al. found that monoamine oxidase A promotes tumor-associated macrophage immunosuppressive polarization and subsequent inhibition of antitumor immunity in mice. They showed that MAOI treatment suppressed tumor progression in preclinical mouse syngeneic and human xenograft tumor models, and furthermore that combining MAOI and anti-PD-1 treatments resulted in synergistic tumor suppression. The authors also conducted clinical data correlation studies and found that intratumoral monoamine oxidase A expression level was negatively correlated with patient survival in several cancers, including breast cancer in the GSE9893 cohort [106]. The combination of observations is suggestive that off label use of MAOIs could potentially suppress tumor metastasis and increase antitumor immunity.
Discussion
Piecing together the puzzle of neurotransmitters’ influence on breast cancer is vital to better understand breast cancer biology and discover novel treatment approaches. It is also urgent to clarify the effects—positive, negative, or neutral —on breast cancer of commonly used medications, including beta blockers, SSRIs, MAOIs, and many more. Even the anesthesia used for breast cancer surgery may need thoughtful examination [107].
While all this potentially complicates the picture of breast cancer prevention and treatment, one reason that neurotransmitters provide a compelling framework for new approaches to breast cancer is the manner in which they link seemingly disparate observations and research directions. This is a source of intricacy, even outside the domain of pharmaceuticals. For example, exercise is advised as preventive against breast cancer, and cancers in general but is also a stressor that releases adrenaline and, as discussed, β2-adrenergic receptor signaling appears to be detrimental in regards to breast cancer. Jensen et al. propose combining exercise and beta blockers for breast cancer patients, such that the positive adrenergic signaling advantages of exercise can be obtained while avoiding chronic adrenergic signaling in the tumor microenvironment [108]. Wackerhage et al. suggest explanations for the seeming paradox of catecholamines’ varying effects [109].
Psychological stress has been observed to be a negative prognostic factor for survival among breast cancer patients [110] and beta adrenergic signaling provides a molecular mechanism to explain this [2, 111]. Addressing this factor could provide benefits at low cost. For example, a meta-analysis of studies of the effect of meditation on the psychological stress level of breast cancer patients found significant benefits in self-reported stress and molecular markers [112] and a randomized trial teaching techniques from cognitive behavioral therapy or relaxation training to women being treated for breast cancer showed reductions in stress and serum inflammatory markers [113].
Neurotransmitters may also provide an underpinning for observations related to the influence of the intestinal microbiome on breast cancer. The gut microbiota has been found to influence treatment side effects and prognosis in breast cancer patients [114]. This is consistent with the finding that intestinal microbiota impact regulation of various neurotransmitters in the body [115]. Indeed, dopamine, GABA, and the vast majority of serotonin in the body are all produced in the gut.
As indicated in prior sections and Table 4, there have been numerous clinical trials that relate to the role of neurotransmitters and breast cancer. These are essential, but further progress in our understanding would also be sped up by large-scale epidemiological studies. Prospective studies have their role, but given the commonality of factors such as the various medications discussed in this review, there is also a significant opportunity for further retrospective studies, both exploratory and to examine specific hypotheses. Access to larger patient corpora than are normally obtained should be sought to provide greater statistical significance.
At the other end of the spectrum, insights can also be expected from advances in sophisticated molecular experimental techniques. McCallum et al.’s recording of neural activity in a triple-negative mammary cancer mouse model while the tumor grew and metastasized is a recent example [116]. Another example is the finding that in mice triple negative breast cancer tumors have more sensory neurons and the axon guidance molecule Plexin B3 mediates cancer cells’ adhesion and migration on sensory nerves [117].
Lastly, it is worth considering why neurotransmitters would have a substantial impact on breast cancer and whether it is reasonable for this to be the case. Indeed, a reason why this relationship was not meaningfully considered for decades is that it was not self-evident that there should be one. However, findings over recent years reveal the ubiquity of neurotransmitters in the body’s various signaling pathways. Evidence even suggests that certain neurotransmitter precursors evolutionarily predate the appearance of neurons and animals [118]. Neurotransmitters have come to play a number of roles as messengers, and there is little surprise that these signaling molecules would also be critical in the growth and spread of cancer.
Neurotransmitters appear to complicate the clinical and mechanistic picture of breast cancer, but denying this complexity is unlikely to pay dividends. Taking a signaling oriented view of breast cancer, particularly incorporating neurotransmitters, unifies various observations and provides clear direction for obtaining clinically significant short-term and long-term results in improving therapeutic strategies.
References
Lei S, Zheng R, Zhang S, Wang S, Chen R, Sun K, et al. Global patterns of breast cancer incidence and mortality: A population-based cancer registry data analysis from 2000 to 2020. Cancer Commun. 2021;41:1183–94.
Liu H, Li C, Cao B, Jiang Y, Han L, Xu R, et al. The molecular mechanism of chronic stress affecting the occurrence and development of breast cancer and potential drug therapy. Transl Oncol. 2022;15:101281.
Huang D, Su S, Cui X, Shen X, Zeng Y, Wu W, et al. Nerve fibers in breast cancer tissues indicate aggressive tumor progression. Medicine. 2014;93:e172.
Zahalka AH, Frenette PS. Nerves in cancer. Nat Rev Cancer. 2020;20:143–57.
Rains SL, Amaya CN, Bryan BA. Beta-adrenergic receptors are expressed across diverse cancers. Oncoscience. 2017;4:95.
Sloan EK, Priceman SJ, Cox BF, Yu S, Pimentel MA, Tangkanangnukul V, et al. The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res. 2010;70:7042–52.
Eckerling A, Ricon-Becker I, Sorski L, Sandbank E, Ben-Eliyahu S. Stress and cancer: mechanisms, significance and future directions. Nat Rev Cancer. 2021;21:767–85.
Szpunar MJ, Belcher EK, Dawes RP, Madden KS. Sympathetic innervation, norepinephrine content, and norepinephrine turnover in orthotopic and spontaneous models of breast cancer. Brain, Behav, Immun. 2016;53:223–33.
Walker AK, Martelli D, Ziegler AI, Lambert GW, Phillips SE, Hill SJ, et al. Circulating epinephrine is not required for chronic stress to enhance metastasis. Psychoneuroendocrinology. 2019;99:191–5.
Hara MR, Kovacs JJ, Whalen EJ, Rajagopal S, Strachan RT, Grant W, et al. A stress response pathway regulates DNA damage through β2-adrenoreceptors and β-arrestin-1. Nature. 2011;477:349–53.
Perego M, Tyurin VA, Tyurina YY, Yellets J, Nacarelli T, Lin C, et al. Reactivation of dormant tumor cells by modified lipids derived from stress-activated neutrophils. Sci Transl Med. 2020;12:eabb5817.
Mulcrone PL, Campbell JP, Cl’ement-Demange L, Anbinder AL, Merkel AR, Brekken RA, et al. Skeletal colonization by breast cancer cells is stimulated by an osteoblast and β2AR-dependent neo-angiogenic switch. J Bone Miner Res. 2017;32:1442–54.
Madel M-B, Elefteriou F. Mechanisms Supporting the Use of Beta-Blockers for the Management of Breast Cancer Bone Metastasis. Cancers. 2021;13:2887.
Lourenco C, Conceicão F, Jeronimo C, Lamghari M, Sousa DM. Stress in Metastatic Breast Cancer: To the Bone and Beyond. Cancers. 2022;14:1881.
Peixoto R, Pereira M, de L, Oliveira M. Beta-blockers and cancer: where are we? Pharmaceuticals. 2020;13:105.
Phadke S, Clamon G. Beta blockade as adjunctive breast cancer therapy: A review. Crit Rev Oncol/Hematol. 2019;138:173–7.
Spini A, Roberto G, Gini R, Bartolini C, Bazzani L, Donnini S, et al. Evidence of β-blockers drug repurposing for the treatment of triple negative breast cancer: a systematic review. Neoplasma. 2019;66:963–70.
Caparica R, Bruzzone M, Agostinetto E, De Angelis C, Fede A, Ceppi M, et al. Beta-blockers in early-stage breast cancer: A systematic review and meta-analysis. ESMO Open. 2021;6:100066.
Kim HY, Jung YJ, Lee SH, Jung HJ, Pak K. Is Beta-Blocker Use Beneficial in Breast Cancer? A Meta-Analysis. Oncology. 2017;92:264–8.
Childers WK, Hollenbeak CS, Cheriyath P. β-blockers reduce breast cancer recurrence and breast cancer death: a meta-analysis. Clin Breast Cancer. 2015;15:426–31.
Zhong S, Yu D, Zhang X, Chen X, Yang S, Tang J, et al. β-Blocker use and mortality in cancer patients: systematic review and meta-analysis of observational studies. Eur J Cancer Prev. 2016;25:440–8.
Na Z, Qiao X, Hao X, Fan L, Xiao Y, Shao Y, et al. The effects of beta-blocker use on cancer prognosis: a meta-analysis based on 319,006 patients. OncoTargets Ther. 2018;11:4913.
Li C, Li T, Tang R, Yuan S, Zhang W. β-Blocker use is not associated with improved clinical outcomes in women with breast cancer: a meta-analysis. Biosci Reports. 2020;40:BSR20200721.
Modi ND, Tan JQE, Rowland A, Koczwara B, Kichenadasse G, McKinnon RA, et al. The Influence of Pre-existing Beta-Blockers Use on Survival Outcomes in HER2 Positive Advanced Breast Cancer: Pooled Analysis of Clinical Trial Data. Front Oncol. 2020;10:1130.
Barron TI, Connolly RM, Sharp L, Bennett K, Visvanathan K. Beta blockers and breast cancer mortality: a population-based study. J Clin Oncol. 2011;29:2635–44.
Botteri E, Munzone E, Rotmensz N, Cipolla C, De Giorgi V, Santillo B, et al. Therapeutic effect of β-blockers in triple-negative breast cancer postmenopausal women. Breast cancer Res Treat. 2013;140:567–75.
Melhem-Bertrandt A, Chavez-MacGregor M, Lei X, Brown EN, Lee RT, Meric-Bernstam F, et al. Beta-blocker use is associated with improved relapse-free survival in patients with triple-negative breast cancer. J Clin Oncol. 2011;29:2645.
Powe DG, Voss MJ, Zanker KS, Habashy HO, Green AR, Ellis IO, et al. Beta-blocker drug therapy reduces secondary cancer formation in breast cancer and improves cancer specific survival. Oncotarget. 2010;1:628.
Parada-Huerta E, Ponce-Medrano J, Alvarez-Dominguez T, Uribe-Escamilla R, Rodriguez-Joya J, Padron-Lucio S, et al. Metastasis risk reduction related with beta-blocker treatment in Mexican women with breast cancer. Asian Pac J Cancer Prev. 2016;17:2953–7.
Choy C, Raytis JL, Smith DD, Duenas M, Neman J, Jandial R, et al. Inhibition of β2-adrenergic receptor reduces triple-negative breast cancer brain metastases: The potential benefit of perioperative β-blockade. Oncol Rep. 2016;35:3135–42.
Spera G, Fresco R, Fung H, Dyck J, Pituskin E, Paterson I, et al. Beta blockers and improved progression-free survival in patients with advanced HER2 negative breast cancer: a retrospective analysis of the ROSE/TRIO-012 study. Ann Oncol. 2017;28:1836–41.
Scott OW, Tin Tin S, Elwood JM, Cavadino A, Habel LA, Kuper-Hommel M, et al. Post-diagnostic beta blocker use and breast cancer-specific mortality: a population-based cohort study. Breast Cancer Res Treat. 2022;193:225–35.
Hiller JG, Cole SW, Crone EM, Byrne DJ, Shackleford DM, Pang J-MB, et al. Preoperative β-blockade with propranolol reduces biomarkers of metastasis in breast cancer: a phase II randomized trial. Clin Cancer Res. 2020;26:1803–11.
Montoya A, Varela-Ramirez A, Dickerson E, Pasquier E, Torabi A, Aguilera R, et al. The beta adrenergic receptor antagonist propranolol alters mitogenic and apoptotic signaling in late stage breast cancer. Biomed J. 2019;42:155–65.
Montoya A, Amaya CN, Belmont A, Diab N, Trevino R, Villanueva G, et al. Use of non-selective β-blockers is associated with decreased tumor proliferative indices in early stage breast cancer. Oncotarget. 2017;8:6446.
Shaashua L, Shabat-Simon M, Haldar R, Matzner P, Zmora O, Shabtai M, et al. Perioperative COX-2 and β-Adrenergic Blockade Improves Metastatic Biomarkers in Breast Cancer Patients in a Phase-II Randomized TrialPerioperative COX-2 and β-Adrenergic Blockade in Breast Cancer. Clin Cancer Res. 2017;23:4651–61.
Hopson MB, Lee S, Accordino M, Trivedi M, Maurer M, Crew KD, et al. Phase II study of propranolol feasibility with neoadjuvant chemotherapy in patients with newly diagnosed breast cancer. Breast Cancer Res Treat. 2021;188:427–32.
Murugan S, Rousseau B, Sarkar DK. Beta 2 Adrenergic receptor antagonist propranolol and opioidergic receptor antagonist naltrexone produce synergistic effects on breast cancer growth prevention by acting on cancer cells and immune environment in a preclinical model of breast cancer. Cancers. 2021;13:4858.
Rousseau B, Murugan S, Palagani A, Sarkar DK. Beta 2 adrenergic receptor and mu opioid receptor interact to potentiate the aggressiveness of human breast cancer cell by activating the glycogen synthase kinase 3 signaling. Breast Cancer Res. 2022;24:1–17.
Nuevo-Tapioles C, Santacatterina F, Stamatakis K, Nuñez de Arenas C, Gomez de Cedron M, Formentini L, et al. Coordinate β-adrenergic inhibition of mitochondrial activity and angiogenesis arrest tumor growth. Nat Commun. 2020;11:1–18.
Shi M, Liu D, Duan H, Qian L, Wang L, Niu L, et al. The β2-adrenergic receptor and Her2 comprise a positive feedback loop in human breast cancer cells. Breast cancer Res Treat. 2011;125:351–62.
Liu D, Yang Z, Wang T, Chen H, Hu Y, Hu C, et al. β2-AR signaling controls trastuzumab resistance-dependent pathway. Oncogene. 2016;35:47–58.
Derakhshani A, Rezaei Z, Safarpour H, Sabri M, Mir A, Sanati MA, et al. Overcoming trastuzumab resistance in HER2-positive breast cancer using combination therapy. J Cell Physiol. 2020;235:3142–56.
Szpunar MJ, Burke KA, Dawes RP, Brown EB, Madden KS. The antidepressant desipramine and α2-adrenergic receptor activation promote breast tumor progression in association with altered collagen structure. Cancer Prevention. Research. 2013;6:1262–72.
Xia M, Ji N, Duan M, Tong J, Xu J, Zhang Y, et al. Dexmedetomidine regulate the malignancy of breast cancer cells by activating α2-adrenoceptor/ERK signaling pathway. Eur Rev Med Pharm Sci. 2016;20:3500–6.
Liu Y, Sun J, Wu T, Lu X, Du Y, Duan H, et al. Effects of serum from breast cancer surgery patients receiving perioperative dexmedetomidine on breast cancer cell malignancy: A prospective randomized controlled trial. Cancer Med. 2019;8:7603–12.
Chi M, Shi X, Huo X, Wu X, Zhang P, Wang G. Dexmedetomidine promotes breast cancer cell migration through Rab11-mediated secretion of exosomal TMPRSS2. Ann Transl Med. 2020;8:8.
Nair AS, Saifuddin MS, Naik V, Rayani BK, et al. Dexmedetomidine in cancer surgeries: Present status and consequences with its use. Indian J Cancer. 2020;57:234.
Wen G, Xin N. Dexmetomidine promotes the activity of breast cancer cells through miR-199a/HIF-1α axis. Transl Cancer Res. 2021;10:4817.
Xia M, Tong J, Zhou Z, Duan M, Xu J, Zeng H, et al. Tramadol inhibits proliferation, migration and invasion via α2-adrenoceptor signaling in breast cancer cells. Eur Rev Med Pharm Sci. 2016;20:157–65.
Huang Y-H, Sue S-H, Wu Z-S, Huang S-M, Lee S-Y, Wu Z-F. Antitumorigenic Effect of Tramadol and Synergistic Effect With Doxorubicin in Human Breast Cancer Cells. Frontiers in Oncology. 2022;12:811716.
Avalos-Moreno M, Lopez-Tejada A, Blaya-Canovas JL, Cara-Lupiañez FE, Gonzalez-Gonzalez A, Lorente JA, et al. Drug repurposing for triple-negative breast cancer. J Personalized Med. 2020;10:200.
Nishioka T, Kim H-S, Luo L-Y, Huang Y, Guo J, Yan Chen C. Sensitization of epithelial growth factor receptors by nicotine exposure to promote breast cancer cell growth. Breast Cancer Res. 2011;13:1–11.
Huang L-C, Lin C-L, Qiu J-Z, Lin C-Y, Hsu K-W, Tam K-W, et al. Nicotinic Acetylcholine Receptor Subtype Alpha-9 Mediates Triple-Negative Breast Cancers Based on a Spontaneous Pulmonary Metastasis Mouse Model. Front Cell Neurosci. 2017;11:336.
Chen J, Cheuk IW, Shin VY, Kwong A. Acetylcholine receptors: Key players in cancer development. Surgical Oncol. 2019;31:46–53.
Sales ME, Español AJ, Salem AR, Pulido PM, Sanchez Y, Sanchez F. Role of Muscarinic Acetylcholine Receptors in Breast Cancer: Design of Metronomic Chemotherapy. Curr Clin Pharmacol. 2019;14:91–100.
Sonier B, Arseneault M, Lavigne C, Ouellette RJ, Vaillancourt C. The 5-HT2A serotoninergic receptor is expressed in the MCF-7 human breast cancer cell line and reveals a mitogenic effect of serotonin. Biochemical biophysical Res Commun. 2006;343:1053–9.
Kopparapu PK, Tinzl M, Anagnostaki L, Persson JL, Dizeyi N. Expression and localization of serotonin receptors in human breast cancer. Anticancer Res. 2013;33:363–70.
Sola-Penna M, Paixão LP, Branco JR, Ochioni AC, Albanese JM, Mundim DM, et al. Serotonin activates glycolysis and mitochondria biogenesis in human breast cancer cells through activation of the Jak1/STAT3/ERK1/2 and adenylate cyclase/PKA, respectively. Br J Cancer. 2020;122:194–208.
Karmakar S, Lal G. Role of serotonin receptor signaling in cancer cells and anti-tumor immunity. Theranostics. 2021;11:5296.
Balakrishna P, George S, Hatoum H, Mukherjee S. Serotonin pathway in cancer. Int J Mol Sci. 2021;22:1268.
Olfati Z, Rigi G, Vaseghi H, Zamanzadeh Z, Sohrabi M, Hejazi SH. Evaluation of serotonin receptors (5HTR2A and 5HTR3A) mRNA expression changes in tumor of breast cancer patients. Medical J Islamic Republic of Iran. 2020;34:99.
Kim MH, Oh JE, Park S, Kim JH, Lee KY, Bai SJ, et al. Tramadol use is associated with enhanced postoperative outcomes in breast cancer patients: A retrospective clinical study with in vitro confirmation. Br J Anaesth. 2019;123:865–76.
Gwynne WD, Shakeel MS, Girgis-Gabardo A, Hassell JA. The role of serotonin in breast cancer stem cells. Molecules. 2021;26:3171.
Leoncikas V, Wu H, Ward LT, Kierzek AM, Plant NJ. Generation of 2,000 breast cancer metabolic landscapes reveals a poor prognosis group with active serotonin production. Sci Rep. 2016;6:1–13.
Cotterchio M, Kreiger N, Darlington G, Steingart A. Antidepressant medication use and breast cancer risk. Am J Epidemiol. 2000;151:951–7.
Sharpe C, Collet J, Belzile E, Hanley J, Boivin J. The effects of tricyclic antidepressants on breast cancer risk. Br J Cancer. 2002;86:92–7.
Moorman PG, Grubber JM, Millikan RC, Newman B. Antidepressant medications and their association with invasive breast cancer and carcinoma in situ of the breast. Epidemiology 2003;14:307–14.
Steingart A, Cotterchio M, Kreiger N, Sloan M. Antidepressant medication use and breast cancer risk: a case-control study. Int J Epidemiol. 2003;32:961–6.
Shapovalov Y, Zettel M, Spielman SC, Amico-Ruvio SA, Kelly EA, Sipe GO, et al. Fluoxetine modulates breast cancer metastasis to the brain in a murine model. BMC Cancer. 2014;14:598.
Fischer A, Rennert HS, Rennert G. Selective serotonin reuptake inhibitors associated with increased mortality risk in breast cancer patients in Northern Israel. Int J Epidemiology. 2022;51:807–16.
Eom C-S, Park SM, Cho K-H. Use of antidepressants and the risk of breast cancer: a meta-analysis. Breast cancer Res Treat. 2012;136:635–45.
Li R, Li X, Yan P, Bing Z, Cao L, Hui X, et al. Relationship between antidepressive agents and incidence risk of breast cancer: systematic review and meta-analysis. Future Oncol. 2020;17:1105–24.
Stapel B, Melzer C, von der Ohe J, Hillemanns P, Bleich S, Kahl KG, et al. Effect of SSRI exposure on the proliferation rate and glucose uptake in breast and ovary cancer cell lines. Sci Rep. 2021;11:1–14.
Hallett RM, Girgis-Gabardo A, Gwynne WD, Giacomelli AO, Bisson JN, Jensen JE, et al. Serotonin transporter antagonists target tumor-initiating cells in a transgenic mouse model of breast cancer. Oncotarget. 2016;7:53137.
Gwynne WD, Hallett RM, Girgis-Gabardo A, Bojovic B, Dvorkin-Gheva A, Aarts C, et al. Serotonergic system antagonists target breast tumor initiating cells and synergize with chemotherapy to shrink human breast tumor xenografts. Oncotarget. 2017;8:32101.
Kirsch I, Deacon BJ, Huedo-Medina TB, Scoboria A, Moore TJ, Johnson BT. Initial severity and antidepressant benefits: a meta-analysis of data submitted to the Food and Drug Administration. PLoS Med. 2008;5:e45.
Jose J, Tavares CD, Ebelt ND, Lodi A, Edupuganti R, Xie X, et al. Serotonin analogues as inhibitors of breast cancer cell growth. ACS medicinal Chem Lett. 2017;8:1072–6.
Borcherding DC, Hugo ER, Fox SR, Jacobson EM, Hunt BG, Merino EJ, et al. Suppression of breast cancer by small molecules that block the prolactin receptor. Cancers. 2021;13:2662.
Aranha AF, Dos Anjos LG, Turri JA, Simões RS, Maciel GA, Baracat EC, et al. Impact of the prolactin levels in breast cancer: a systematic review and meta-analysis. Gynecol Endocrinol. 2022;38:385–90.
Aticı Odul K, Govindrajan N, Lopetegui-Gonzalez I, et al. CS. Prolactin: a hormone with diverse functions from mammary gland development to cancer metastasis. In: Seminars in Cell & Developmental Biology. 2021;114:159–70.
Sarkar C, Chakroborty D, Chowdhury UR, Dasgupta PS, Basu S. Dopamine increases the efficacy of anticancer drugs in breast and colon cancer preclinical models. Clinical cancer research: an official journal of the American Association for. Cancer Res. 2008;14:2502–10.
Minami K, Liu S, Liu Y, Chen A, Wan Q, Na S, et al. Inhibitory Effects of Dopamine Receptor D(1) Agonist on Mammary Tumor and Bone Metastasis. Sci Rep. 2017;7:45686.
Costa R, Santa-Maria C, Scholtens D, Jain S, Flaum L, Gradishar W, et al. A pilot study of cabergoline for the treatment of metastatic breast cancer. Breast Cancer Res Treat. 2017;165:585–92.
Goyette M-A, Cusseddu R, Elkholi I, Abu-Thuraia A, El-Hachem N, Haibe-Kains B, et al. AXL knockdown gene signature reveals a drug repurposing opportunity for a class of antipsychotics to reduce growth and metastasis of triple-negative breast cancer. Oncotarget. 2019;10:2055.
Grant CE, Flis A, Ryan BM. Understanding the Role of Dopamine in Cancer: Past, Present, and Future. Carcinogenesis. 2022;43:517–27.
Nuutinen S, Panula P. Histamine in neurotransmission and brain diseases. Histamine in Inflammation. Adv Exp Med Biol. 2010;95–107.
Martinel Lamas DJ, Croci M, Carabajal E, Crescenti EJ, Sambuco L, Massari NA, et al. Therapeutic potential of histamine H4 receptor agonists in triple-negative human breast cancer experimental model. Br J Pharmacol. 2013;170:188–99.
Sterle HA, Nicoud MB, Massari NA, T’aquez Delgado MA, Herrero Ducloux MV, Cremaschi GA, et al. Immunomodulatory role of histamine H4 receptor in breast cancer. Br J Cancer. 2019;120:128–38.
Galarza TE, Delgado MAT, Mohamad NA, Mart’∈ GA, Cricco GP. Histamine H4 receptor agonists induce epithelial-mesenchymal transition events and enhance mammosphere formation via Src and TGF-β signaling in breast cancer cells. Biochemical Pharmacol. 2020;180:114177.
Nicoud MB, Sterle HA, Massari NA, T’aquez Delgado MA, Formoso K, Herrero Ducloux MV, et al. Study of the antitumour effects and the modulation of immune response by histamine in breast cancer. Br J Cancer. 2020;122:348–60.
Speisky D, T’aquez Delgado MA, Iotti A, Nicoud MB, Ospital IA, Vigovich F, et al. Histamine H4 Receptor Expression in Triple-negative Breast Cancer: An Exploratory Study. J Histochemistry Cytochemistry. 2022;70:311–22.
Gumireddy K, Li A, Kossenkov AV, Sakurai M, Yan J, Li Y, et al. The mRNA-edited form of GABRA3 suppresses GABRA3-mediated Akt activation and breast cancer metastasis. Nat Commun. 2016;7:1–9.
Neman J, Termini J, Wilczynski S, Vaidehi N, Choy C, Kowolik CM, et al. Human breast cancer metastases to the brain display GABAergic properties in the neural niche. Proc Natl Acad Sci. 2014;111:984–9.
Dahn ML, Walsh HR, Dean CA, Giacomantonio MA, Fernando W, Murphy JP, et al. Metabolite profiling reveals a connection between aldehyde dehydrogenase 1A3 and GABA metabolism in breast cancer metastasis. Metabolomics. 2022;18:1–14.
Morgat C, Brouste V, Chastel A, V’elasco V, Macgrogan G, Hindi’e E. Expression of neurotensin receptor-1 (NTS1) in primary breast tumors, cellular distribution, and association with clinical and biological factors. Breast Cancer Res Treat. 2021;190:403–13.
Sanchez ML, Coveñas R. The Neurotensinergic System: A Target for Cancer Treatment. Curr Medicinal Chem. 2022;29:3231–60.
Souaze F, Dupouy S, Viardot-Foucault V, Bruyneel E, Attoub S, Gespach C, et al. Expression of neurotensin and NT1 receptor in human breast cancer: a potential role in tumor progression. Cancer Res. 2006;66:6243–9.
Reubi JC, Gugger M, Waser B, Schaer JCY. Y(1)-mediated effect of neuropeptide Y in cancer:breast carcinomas as targets. C. Cancer Res.2001;61:4636–41.
Medeiros PJ, Jackson DN, Neuropeptide Y. Y5-receptor activation on breast cancer cells acts as a paracrine system that stimulates VEGF expression and secretion to promote angiogenesis. Peptides 2013;48:106–13.
Lin ST, Li YZ, Sun XQ, et al. Update on the Role of Neuropeptide Y and Other Related Factors in Breast Cancer and Osteoporosis. Front Endocrinol (Lausanne). 2021;12:705499.
Qiu S-Q, Waaijer SJ, Zwager MC, de Vries EG, van der Vegt B, Schroder CP. Tumor-associated macrophages in breast cancer: Innocent bystander or important player? Cancer Treat Rev. 2018;70:178–89.
Wu JB, Shao C, Li X, Li Q, Hu P, Shi C, et al. Monoamine oxidase A mediates prostate tumorigenesis and cancer metastasis. J Clin Invest. 2014;124:2891–908.
Kushal S, Wang W, Vaikari VP, Kota R, Chen K, Yeh TS, et al. Monoamine oxidase A (MAO A) inhibitors decrease glioma progression. Oncotarget. 2016;7:13842–53.
Lapierre JA, Geary LA, Jang JK, Epstein AL, Hong F, Shih JC. Deletion of monoamine oxidase A in a prostate cancer model enhances anti-tumor immunity through reduced immune suppression. Biochem Biophys Res Commun. 2022;634:100–7.
Wang Y-C, Wang X, Yu J, Ma F, Li Z, Zhou Y, et al. Targeting monoamine oxidase A-regulated tumor-associated macrophage polarization for cancer immunotherapy. Nat Comm. 2021;12:3530.
Kim R, Kawai A, Wakisaka M, Kin T. Current Status and Prospects of Anesthesia and Breast Cancer: Does Anesthetic Technique Affect Recurrence and Survival Rates in Breast Cancer Surgery? Front Oncol. 2022;12:795864.
Jensen AWP, Carnaz, Simões AM. thor Straten P, Holmen Olofsson G. Adrenergic signaling in immunotherapy of cancer: friend or foe? Cancers. 2021;13:394.
Wackerhage H, Christensen J, Ilmer M, von Luettichau I, Renz B, Schonfelder M. Cancer catecholamine conundrum. Trends Cancer. 2022;8:110–22.
Adeyemi OJ, Gill TL, Paul R, Huber LB. Evaluating the association of self-reported psychological distress and self-rated health on survival times among women with breast cancer in the US. PloS one. 2021;16:e0260481.
Gosain R, Gage-Bouchard E, Ambrosone C, Repasky E, Gandhi S. Stress reduction strategies in breast cancer: Review of pharmacologic and non-pharmacologic based strategies. In Seminars in immunopathology. 2020;42:719–34.
Araujo RV, Fernandes AFC, Nery IS, Andrade EMLR, Nogueira LT, Azevedo FHC. Meditation effect on psychological stress level in women with breast cancer: a systematic review. Revista da Escola de Enfermagem da USP. 2019;53:e03529.
Diaz A, Taub CJ, Lippman ME, Antoni MH, Blomberg BB. Effects of brief stress management interventions on distress and leukocyte nuclear factor kappa B expression during primary treatment for breast cancer: a randomized trial. Psychoneuroendocrinology. 2021;126:105163.
Terrisse S, Derosa L, Iebba V, Ghiringhelli I, Vaz-Luis F, Kroemer G, et al. Intestinal microbiota influences clinical outcome and side effects of early breast cancer treatment. Cell Death Differ. 2021;28:2778–96.
Barandouzi ZA, Lee J, del Carmen Rosas M, Chen J, Henderson WA, Starkweather AR, et al. Associations of neurotransmitters and the gut microbiome with emotional distress in mixed type of irritable bowel syndrome. Sci Rep. 2022;12:1–11.
McCallum GA, Shiralkar J, Suciu D, Covarrubias G, Yu JS, Karathanasis E, et al. Chronic neural activity recorded within breast tumors. Sci Rep. 2020;10:1–13.
Le TT, Payne SL, Buckwald MN, Hayes LA, Parker SR, Burge CB, et al. Sensory nerves enhance triple-negative breast cancer invasion and metastasis via the axon guidance molecule PlexinB3. NPJ Breast Cancer. 2022;8:116.
Yañez-Guerra LA, Thiel D, J’ekely G. Premetazoan Origin of Neuropeptide Signaling. Mol Biol evolution. 2022;39:msac051.
Pouya FD, Rasmi Y, Asl ER. Role of neurotransmitters and neuropeptides in breast cancer metastasis. Biochem (Mosc), Suppl Ser A: Membr Cell Biol. 2020;14:107–16.
Jiang S-H, Hu L-P, Wang X, Li J, Zhang Z-G. Neurotransmitters: emerging targets in cancer. Oncogene 2020;39:503–15.
Funding
This work was partly supported by National Cancer Institute (grant number P30CA014089), Gloria Borges WunderGlo Foundation, Dhont Family Foundation, Gene Gregg Pancreas Research Fund, San Pedro Peninsula Cancer Guild, Daniel Butler Research Fund, V Foundation for Cancer Research, Victoria and Philip Wilson Research Fund, Fong research project and Ming Hsieh research fund. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute of the National Institutes of Health. Open access funding provided by SCELC, Statewide California Electronic Library Consortium.
Author information
Authors and Affiliations
Contributions
HJL conceived of the presented idea. PJ conducted the primary review of the literature, analysis, and manuscript preparation. All authors, including FB, CS, AL, SA, SS, JHL, YY, JM, WZ, JCS, JL, SMM, DS, JN, ETRT, PJ, and HJL, reviewed the results, provided critical feedback and comments, and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jayachandran, P., Battaglin, F., Strelez, C. et al. Breast cancer and neurotransmitters: emerging insights on mechanisms and therapeutic directions. Oncogene 42, 627–637 (2023). https://doi.org/10.1038/s41388-022-02584-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-022-02584-4
This article is cited by
-
Development, Optimization, and Evaluation of Nano-platforms for Delivery of siRNA and BPTES in c-Myc Induced Breast Cancer
Journal of Pharmaceutical Innovation (2023)