Abstract
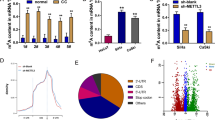
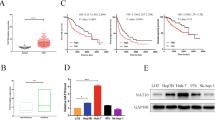
N6-methyladenosine (m6A) is the most abundant chemical modification on mRNA and plays significant roles in many bioprocesses. However, the functions of m6A on cervical cancer (CC) tumorigenesis remain unclear. Here we found methyltransferase-like 3 (METTL3), a core member of the m6A methyltransferase family, was greatly upregulated as an independent prognostic factor in CC. Mechanistically, the transcription factor ETS1 recruited P300 and WDR5 which separately mediated H3K27ac and H3K4me3 histone modification in the promoter of METTL3 and induced METTL3 transcription activation. Furthermore, we identified TXNDC5 as a target of METTL3-mediated m6A modification through MeRIP-seq, and revealed that METTL3-mediated TXNDC5 expression relied on the m6A reader-dependent manner. Functionally, we verified that METTL3 promoted proliferation and metastasis of CC cells by regulating of TXNDC5 expression through in vitro and in vivo experiments. In addition, our study verified the effect of METTL3/TXNDC5 axis on ER stress. Taken together, METTL3 facilitates the malignant progression of CC, suggesting that METTL3 might be a potential prognostic biomarker and therapeutic target for CC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Zou D, Dong L, Li C, Yin Z, Rao S, Zhou Q. The m(6)A eraser FTO facilitates proliferation and migration of human cervical cancer cells. Cancer Cell Int. 2019;19:321.
Wang T, Kong S, Tao M, Ju S. The potential role of RNA N6-methyladenosine in Cancer progression. Mol Cancer. 2020;19:88.
Liu K, Ding Y, Ye W, Liu Y, Yang J, Liu J, et al. Structural and Functional Characterization of the Proteins Responsible for N(6)-Methyladenosine Modification and Recognition. Curr Protein Pept Sci. 2016;17:306–18.
Sun T, Wu R, Ming L. The role of m6A RNA methylation in cancer. Biomed Pharmacother. 2019;112:108613.
Tong J, Flavell RA, Li HB. RNA m(6)A modification and its function in diseases. Front Med. 2018;12:481–9.
Horna-Terrón E, Pradilla-Dieste A, Sánchez-de-Diego C, Osada J. TXNDC5, a newly discovered disulfide isomerase with a key role in cell physiology and pathology. Int J Mol Sci. 2014;15:23501–18.
Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334:1081–6.
Sullivan DC, Huminiecki L, Moore JW, Boyle JJ, Poulsom R, Creamer D, et al. EndoPDI, a novel protein-disulfide isomerase-like protein that is preferentially expressed in endothelial cells acts as a stress survival factor. J Biol Chem. 2003;278:47079–88.
Funkner A, Parthier C, Schutkowski M, Zerweck J, Lilie H, Gyrych N, et al. Peptide binding by catalytic domains of the protein disulfide isomerase-related protein ERp46. J Mol Biol. 2013;425:1340–62.
Chen X, Li C, Liu J, He Y, Wei Y, Chen J. Inhibition of ER stress by targeting the IRE1alpha-TXNDC5 pathway alleviates crystalline silica-induced pulmonary fibrosis. Int Immunopharmacol. 2021;95:107519.
Chawsheen HA, Jiang H, Ying Q, Ding N, Thapa P, Wei Q. The redox regulator sulfiredoxin forms a complex with thioredoxin domain-containing 5 protein in response to ER stress in lung cancer cells. J Biol Chem. 2019;294:8991–9006.
Berridge MJ. The endoplasmic reticulum: a multifunctional signaling organelle. Cell Calcium. 2002;32:235–49.
Jørgensen MM, Bross P, Gregersen N. Protein quality control in the endoplasmic reticulum. APMIS Suppl. 2003;109:86–91.
Xu Y, Li D, Zeng L, Wang C, Zhang L, Wang Y, et al. Proteasome inhibitor lactacystin enhances cisplatin cytotoxicity by increasing endoplasmic reticulum stress-associated apoptosis in HeLa cells. Mol Med Rep. 2015;11:189–95.
Senft D, Ronai ZA. Ronai, UPR, autophagy, and mitochondria crosstalk underlies the ER stress response. Trends Biochem Sci. 2015;40:141–8.
Peng F, Zhang H, Du Y, Tan P. Cetuximab enhances cisplatin-induced endoplasmic reticulum stress-associated apoptosis in laryngeal squamous cell carcinoma cells by inhibiting expression of TXNDC5. Mol Med Rep. 2018;17:4767–76.
Zeng C, Huang W, Li Y, Weng H. Roles of METTL3 in cancer: mechanisms and therapeutic targeting. J Hematol Oncol. 2020;13:117.
Beacon TH, Delcuve GP, López C, Nardocci G, Kovalchuk I, van Wijnen AJ, et al. The dynamic broad epigenetic (H3K4me3, H3K27ac) domain as a mark of essential genes. Clin Epigenetics. 2021;13:138.
Froimchuk E, Jang Y, Ge K. Histone H3 lysine 4 methyltransferase KMT2D. Gene. 2017;627:337–42.
Biancotto C, Frigè G, Minucci S. Histone modification therapy of cancer. Adv Genet. 2010;70:341–86.
Luo G, Xu W, Zhao Y, Jin S, Wang S, Liu Q, et al. RNA m(6) A methylation regulates uveal melanoma cell proliferation, migration, and invasion by targeting c-Met. J Cell Physiol. 2020;235:7107–19.
Yue B, Cui R, Zheng R, Jin W, Song C, Bao T, et al. Essential role of ALKBH5-mediated RNA demethylation modification in bile acid-induced gastric intestinal metaplasia. Mol Ther Nucl Acids. 2021;26:458–72.
Du QY, Zhu ZM, Pei DS. The biological function of IGF2BPs and their role in tumorigenesis. Invest N. Drugs. 2021;39:1682–93.
Kojima R, Okumura M, Masui S, Kanemura S, Inoue M, Saiki M, et al. Radically different thioredoxin domain arrangement of ERp46, an efficient disulfide bond introducer of the mammalian PDI family. Structure. 2014;22:431–43.
Datan E, Roy SG, Germain G, Zali N, McLean JE, Golshan G, et al. Dengue-induced autophagy, virus replication and protection from cell death require ER stress (PERK) pathway activation. Cell Death Dis. 2016;7:e2127.
Hasanain M, Bhattacharjee A, Pandey P, Ashraf R, Singh N, Sharma S, et al. alpha-Solanine induces ROS-mediated autophagy through activation of endoplasmic reticulum stress and inhibition of Akt/mTOR pathway. Cell Death Dis. 2015;6:e1860.
Maldonado López A, Capell BC. The METTL3-m(6)A Epitranscriptome: Dynamic Regulator of Epithelial Development, Differentiation, and Cancer. Genes (Basel). 2021;12:1019.
Liu S, Zhuo L, Wang J, Zhang Q, Li Q, Li G. METTL3 plays multiple functions in biological processes. Am J Cancer Res. 2020;10:1631–46.
Li X, Tang J, Huang W, Wang F, Li P, Qin C, et al. The M6A methyltransferase METTL3: acting as a tumor suppressor in renal cell carcinoma. Oncotarget. 2017;8:96103–16.
Wang Q, Guo X, Li L, Gao Z, Su X, Ji M, et al. N(6)-methyladenosine METTL3 promotes cervical cancer tumorigenesis and Warburg effect through YTHDF1/HK2 modification. Cell Death Dis. 2020;11:911.
Wang X, Li Z, Kong B, Song C, Cong J, Hou J, et al. Reduced m(6)A mRNA methylation is correlated with the progression of human cervical cancer. Oncotarget. 2017;8:98918–30.
Shen C, Xuan B, Yan T, Ma Y, Xu P, Tian X, et al. m(6)A-dependent glycolysis enhances colorectal cancer progression. Mol Cancer. 2020;19:72.
Lin Y, Wei X, Jian Z, Zhang X. METTL3 expression is associated with glycolysis metabolism and sensitivity to glycolytic stress in hepatocellular carcinoma. Cancer Med. 2020;9:2859–67.
Lu M, Zhang Z, Xue M, Zhao BS, Harder O, Li A, et al. N(6)-methyladenosine modification enables viral RNA to escape recognition by RNA sensor RIG-I. Nat Microbiol. 2020;5:584–98.
Xu J, Cai Y, Ma Z, Jiang B, Liu W, Cheng J, et al. The RNA helicase DDX5 promotes viral infection via regulating N6-methyladenosine levels on the DHX58 and NFkappaB transcripts to dampen antiviral innate immunity. PLoS Pathog. 2021;17:e1009530.
Yang Y, Song S, Meng Q, Wang L, Li X, Xie S, et al. miR24-2 accelerates progression of liver cancer cells by activating Pim1 through tri-methylation of Histone H3 on the ninth lysine. J Cell Mol Med. 2020;24:2772–90.
Yan J, Huang X, Zhang X, Chen Z, Ye C, Xiang W, et al. LncRNA LINC00470 promotes the degradation of PTEN mRNA to facilitate malignant behavior in gastric cancer cells. Biochem Biophys Res Commun. 2020;521:887–93.
Zhu L, Zhu Y, Han S, Chen M, Song P, Dai D, et al. Impaired autophagic degradation of lncRNA ARHGAP5-AS1 promotes chemoresistance in gastric cancer. Cell Death Dis. 2019;10:383.
Wei W, Huo B, Shi X. miR-600 inhibits lung cancer via downregulating the expression of METTL3. Cancer Manag Res. 2019;11:1177–87.
Dittmer J. The biology of the Ets1 proto-oncogene. Mol Cancer. 2003;2:29.
Teruyama K, Abe M, Nakano T, Iwasaka-Yagi C, Takahashi S, Yamada S, et al. Role of transcription factor Ets-1 in the apoptosis of human vascular endothelial cells. J Cell Physiol. 2001;188:243–52.
Kumar P, Pandey KN. Cooperative activation of Npr1 gene transcription and expression by interaction of Ets-1 and p300. Hypertension. 2009;54:172–8.
Lee CG, Kwon HK, Sahoo A, Hwang W, So JS, Hwang JS, et al. Interaction of Ets-1 with HDAC1 represses IL-10 expression in Th1 cells. J Immunol. 2012;188:2244–53.
Liu J, Li D, Zhang X, Li Y, Ou J. Histone Demethylase KDM3A Promotes Cervical Cancer Malignancy Through the ETS1/KIF14/Hedgehog Axis. Onco Targets Ther. 2020;13:11957–73.
Ren TW, Zhou YN, Wu J, Zhang ZY, Hao TJ, Wang JX, et al. Relationship between Ets-1 expression and angiogenesis, clinicopathological features and survival of patients with gastric carcinoma. Zhonghua Zhong Liu Za Zhi. 2009;31:674–8.
Wang Q, Chen C, Ding Q, Zhao Y, Wang Z, Chen J, et al. METTL3-mediated m(6)A modification of HDGF mRNA promotes gastric cancer progression and has prognostic significance. Gut. 2020;69:1193–205.
Li T, Hu PS, Zuo Z, Lin JF, Li X, Wu QN, et al. METTL3 facilitates tumor progression via an m(6)A-IGF2BP2-dependent mechanism in colorectal carcinoma. Mol Cancer. 2019;18:112.
Zhao W, Cui Y, Liu L, Ma X, Qi X, Wang Y, et al. METTL3 Facilitates Oral Squamous Cell Carcinoma Tumorigenesis by Enhancing c-Myc Stability via YTHDF1-Mediated m(6)A Modification. Mol Ther Nucl Acids. 2020;20:1–12.
Li J, Chang X. [Progress of research on TXNDC5]. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2017;34:448–50.
Shi H, Wang X, Lu Z, Zhao BS, Ma H, Hsu PJ, et al. YTHDF3 facilitates translation and decay of N(6)-methyladenosine-modified RNA. Cell Res. 2017;27:315–28.
Liu S, Li G, Li Q, Zhang Q, Zhuo L, Chen X, et al. The roles and mechanisms of YTH domain-containing proteins in cancer development and progression. Am J Cancer Res. 2020;10:1068–84.
Matsusaki M, Kanemura S, Kinoshita M, Lee YH, Inaba K, Okumura M. The Protein Disulfide Isomerase Family: from proteostasis to pathogenesis. Biochim Biophys Acta Gen Subj. 2020;1864:129338.
Xu B, Li J, Liu X, Li C, Chang X. TXNDC5 is a cervical tumor susceptibility gene that stimulates cell migration, vasculogenic mimicry and angiogenesis by down-regulating SERPINF1 and TRAF1 expression. Oncotarget. 2017;8:91009–24.
Wang L, Song G, Chang X, Tan W, Pan J, Zhu X, et al. The role of TXNDC5 in castration-resistant prostate cancer-involvement of androgen receptor signaling pathway. Oncogene. 2015;34:4735–45.
Park MS, Kim SK, Shin HP, Lee SM, Chung JH. TXNDC5 gene polymorphism contributes to increased risk of hepatocellular carcinoma in the Korean male population. Anticancer Res. 2013;33:3983–7.
Tan F, Zhu H, He X, Yu N, Zhang X, Xu H, et al. Role of TXNDC5 in tumorigenesis of colorectal cancer cells: In vivo and in vitro evidence. Int J Mol Med. 2018;42:935–45.
Song S, Tan J, Miao Y, Li M, Zhang Q. Crosstalk of autophagy and apoptosis: Involvement of the dual role of autophagy under ER stress. J Cell Physiol. 2017;232:2977–84.
Pecoraro A, Pagano M, Russo G, Russo A. Role of Autophagy in Cancer Cell Response to Nucleolar and Endoplasmic Reticulum Stress. Int J Mol Sci. 2020;21:7334.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No.81872080), Jiangsu Provincial Medical Talent (ZDRCA2016055).
Author information
Authors and Affiliations
Contributions
QYD and FCH performed the experiments and wrote the manuscript. WQD partially participated in vivo experiments. XLS and XJ partially participated in vitro experiments. All authors contributed to data analysis. LSZ reviewed and supervised the manuscript. DSP obtained funding and designed the research. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Du, QY., Huo, FC., Du, WQ. et al. METTL3 potentiates progression of cervical cancer by suppressing ER stress via regulating m6A modification of TXNDC5 mRNA. Oncogene 41, 4420–4432 (2022). https://doi.org/10.1038/s41388-022-02435-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-022-02435-2
This article is cited by
-
Nucleic acid and protein methylation modification in renal diseases
Acta Pharmacologica Sinica (2024)
-
New insights into the regulation of METTL3 and its role in tumors
Cell Communication and Signaling (2023)
-
Methyltransferase-like proteins in cancer biology and potential therapeutic targeting
Journal of Hematology & Oncology (2023)
-
The roles of m6A methylation in cervical cancer: functions, molecular mechanisms, and clinical applications
Cell Death & Disease (2023)
-
RNA N6-methyladenosine modification mediates downregulation of NR4A1 to facilitate malignancy of cervical cancer
Cell & Bioscience (2022)