Abstract
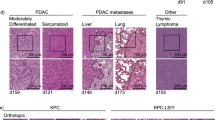
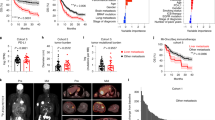
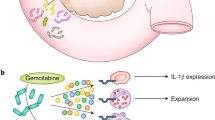
Response to cancer immunotherapy in primary versus metastatic disease has not been well-studied. We found primary pancreatic ductal adenocarcinoma (PDA) is responsive to diverse immunotherapies whereas liver metastases are resistant. We discovered divergent immune landscapes in each compartment. Compared to primary tumor, liver metastases in both mice and humans are infiltrated by highly anergic T cells and MHCIIloIL10+ macrophages that are unable to present tumor-antigen. Moreover, a distinctive population of CD24+CD44−CD40− B cells dominate liver metastases. These B cells are recruited to the metastatic milieu by Muc1hiIL18hi tumor cells, which are enriched >10-fold in liver metastases. Recruited B cells drive macrophage-mediated adaptive immune-tolerance via CD200 and BTLA. Depleting B cells or targeting CD200/BTLA enhanced macrophage and T-cell immunogenicity and enabled immunotherapeutic efficacy of liver metastases. Our data detail the mechanistic underpinnings for compartment-specific immunotherapy-responsiveness and suggest that primary PDA models are poor surrogates for evaluating immunity in advanced disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Change history
27 January 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41388-023-02606-9
References
Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–21.
Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300.
Chen J, Xiao-Zhong G, Qi XS. Clinical outcomes of specific immunotherapy in advanced pancreatic cancer: a systematic review and meta-analysis. J Immunol Res. 2017;2017:8282391.
Daley D, Mani VR, Mohan N, Akkad N, Ochi A, Heindel DW, et al. Dectin 1 activation on macrophages by galectin 9 promotes pancreatic carcinoma and peritumoral immune tolerance. Nat Med. 2017;23:556–67.
Seifert L, Werba G, Tiwari S, Giao Ly NN, Alothman S, Alqunaibit D, et al. The necrosome promotes pancreatic oncogenesis via CXCL1 and Mincle-induced immune suppression. Nature 2016;532:245–9.
Wang W, Marinis JM, Beal AM, Savadkar S, Wu Y, Khan M, et al. RIP1 kinase drives macrophage-mediated adaptive immune tolerance in pancreatic cancer. Cancer Cell. 2018;34:757–74.e7.
Zhu Y, Herndon JM, Sojka DK, Kim KW, Knolhoff BL, Zuo C, et al. Tissue-resident macrophages in pancreatic ductal adenocarcinoma originate from embryonic hematopoiesis and promote tumor progression. Immunity. 2017;47:597.
Daley D, Zambirinis CP, Seifert L, Akkad N, Mohan N, Werba G, et al. gammadelta T cells support pancreatic oncogenesis by restraining alphabeta T cell activation. Cell. 2016;166:1485–99.e15.
Gunderson AJ, Kaneda MM, Tsujikawa T, Nguyen AV, Affara NI, Ruffell B, et al. Bruton tyrosine kinase-dependent immune cell cross-talk drives pancreas cancer. Cancer Discov. 2016;6:270–85.
Pylayeva-Gupta Y, Das S, Handler JS, Hajdu CH, Coffre M, Koralov SB, et al. IL35-producing B cells promote the development of pancreatic neoplasia. Cancer Discov. 2016;6:247–55.
Zhao Y, Shen M, Feng Y, He R, Xu X, Xie Y, et al. Regulatory B cells induced by pancreatic cancer cell-derived interleukin-18 promote immune tolerance via the PD-1/PD-L1 pathway. Oncotarget. 2018;9:14803–14.
Enoksson SL, Grasset EK, Hagglof T, Mattsson N, Kaiser Y, Gabrielsson S, et al. The inflammatory cytokine IL-18 induces self-reactive innate antibody responses regulated by natural killer T cells. Proc Natl Acad Sci USA 2011;108:E1399–407.
Canning C, O’Brien M, Hegarty J, O’Farrelly C. Liver immunity and tumour surveillance. Immunol Lett. 2006;107:83–8.
Moini M, Schilsky ML, Tichy EM. Review on immunosuppression in liver transplantation. World J Hepatol. 2015;7:1355–68.
Wrenshall LE, Ansite JD, Eckman PM, Heilman MJ, Stevens RB, Sutherland DE. Modulation of immune responses after portal venous injection of antigen. Transplantation. 2001;71:841–50.
Mieli-Vergani G, Vergani D, Czaja AJ, Manns MP, Krawitt EL, Vierling JM, et al. Autoimmune hepatitis. Nat Rev Dis Prim. 2018;4:18017.
Disibio G, French SW. Metastatic patterns of cancers: results from a large autopsy study. Arch Pathol Lab Med. 2008;132:931–9.
Yachida S, Iacobuzio-Donahue CA. The pathology and genetics of metastatic pancreatic cancer. Arch Pathol Lab Med. 2009;133:413–22.
Liu Y, Cao X. Immunosuppressive cells in tumor immune escape and metastasis. J Mol Med. 2016;94:509–22.
Liu Y, Cao X. Characteristics and significance of the pre-metastatic niche. Cancer Cell. 2016;30:668–81.
Grunwald B, Harant V, Schaten S, Fruhschutz M, Spallek R, Hochst B, et al. Pancreatic premalignant lesions secrete tissue inhibitor of metalloproteinases-1, which activates hepatic stellate cells via CD63 signaling to create a premetastatic niche in the liver. Gastroenterology. 2016;151:1011–24.e7.
Bodogai M, Moritoh K, Lee-Chang C, Hollander CM, Sherman-Baust CA, Wersto RP, et al. Immunosuppressive and prometastatic functions of myeloid-derived suppressive cells rely upon education from tumor-associated B cells. Cancer Res. 2015;75:3456–65.
Costa-Silva B, Aiello NM, Ocean AJ, Singh S, Zhang H, Thakur BK, et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol. 2015;17:816–26.
Nielsen SR, Quaranta V, Linford A, Emeagi P, Rainer C, Santos A, et al. Macrophage-secreted granulin supports pancreatic cancer metastasis by inducing liver fibrosis. Nat Cell Biol. 2016;18:549–60.
Lee JW, Stone ML, Porrett PM, Thomas SK, Komar CA, Li JH, et al. Hepatocytes direct the formation of a pro-metastatic niche in the liver. Nature. 2019;567:249–52.
Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–71.
Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14:1014–22.
Daley D, Mani VR, Mohan N, Akkad N, Pandian G, Savadkar S, et al. NLRP3 signaling drives macrophage-induced adaptive immune suppression in pancreatic carcinoma. J Exp Med. 2017;214:1711–24.
Funding
This work was supported by the American College of Surgeons Resident Research Fellowship (BD), Deutsche Forschungsgemeinschaft grant AY 126/1-1 (BA), and NIH grants CA168611 (DC, GM), CA203105 (GM), CA215471 (GM), CA19311 (GM), and DK106025 (GM).
Author information
Authors and Affiliations
Contributions
BD and SA prepared the paper, performed in vivo and in vitro experiments and data analysis, and designed, supervised and interpreted the study; GSS, ML, BS, EL, MSS, AF, FY, CH, JG, AP, and YW performed in vitro experiments; JL, RC, RDS, MFC, CB, WW, SAAS, and GW performed in vivo experiments in addition to paper and figure preparation; BA and MK performed data analyis; DC contributed to critical review and paper writing; GM conceived, designed, supervised, analyzed and interpreted the study and provided critical review, and is senior author and corresponding author.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Diskin, B., Adam, S., Soto, G.S. et al. BTLA+CD200+ B cells dictate the divergent immune landscape and immunotherapeutic resistance in metastatic vs. primary pancreatic cancer. Oncogene 41, 4349–4360 (2022). https://doi.org/10.1038/s41388-022-02425-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-022-02425-4
This article is cited by
-
CD200 is overexpressed in the pancreatic tumor microenvironment and predictive of overall survival
Cancer Immunology, Immunotherapy (2024)
-
Molecular and metabolic regulation of immunosuppression in metastatic pancreatic ductal adenocarcinoma
Molecular Cancer (2023)