Abstract
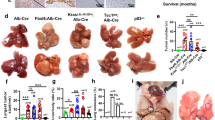
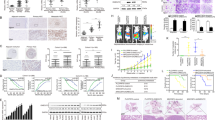
The cellular origin of hepatocellular carcinomas (HCC) and the role of Notch1 signalling in HCC initiation are controversial. Herein, we establish Notch1 as a regulator of HCC development and progression. Clinically, high Notch1 expression correlates with enhanced cancer progression, elevated lung metastasis, increased cancer stem cell (CSC)-like cells’ gene signature expression, and poor overall survival in HCC patients. Notch1 intracellular domain (N1ICD) overexpression spontaneously transforms rat liver progenitor cells (LPC) into CSC-like cells (WBN1ICD C5) under a selective growth environment, while orthotopic injection of these cells generates liver tumors and spontaneous pulmonary metastasis in an isogenic rat model. Mechanistically, the elevated Notch1 activity increases c-myc expression, which then transcriptionally upregulates VCAM1 expression to activate macrophage dependent HCC transendothelial migration. In vivo, silencing c-myc prohibits the tumorigenicity of WBN1ICD C5 cells, while depletion of VCAM1 reduces spontaneous lung metastasis without affecting primary WBN1ICD C5 orthotopic liver tumor growth. Importantly, depletion of macrophage or blockade of macrophage VCAM1 binding receptor α4β1-integrin reduces the number of WBN1ICD C5 lung nodules in an experimental metastasis model. Overall, our work discovers that the Notch1-c-myc-VCAM1 signaling axis initiates LPC-driven hepatocarcinogenesis and metastasis, providing a preclinical model for HCC study and therapeutic targets for an improved HCC treatment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
All the data supporting the findings of this study are available within the article and its supplementary information files and from the corresponding author upon reasonable request.
References
Hooth MJ, Coleman WB, Presnell SC, Borchert KM, Grisham JW, Smith GJ. Spontaneous neoplastic transformation of WB-F344 rat liver epithelial cells. Am J Pathol. 1998;153:1913–21.
Chan LH, Luk ST, Ma S. Turning hepatic cancer stem cells inside out-a deeper understanding through multiple perspectives. Mol Cells. 2015;38:202–9.
Sell S, Leffert HL. Liver cancer stem cells. J Clin Oncol. 2008;26:2800–5.
Font-Burgada J, Shalapour S, Ramaswamy S, Hsueh B, Rossell D, Umemura A, et al. Hybrid Periportal Hepatocytes Regenerate the Injured Liver without Giving Rise to Cancer. Cell. 2015;162:766–79.
Benhamouche S, Curto M, Saotome I, Gladden AB, Liu CH, Giovannini M, et al. Nf2/Merlin controls progenitor homeostasis and tumorigenesis in the liver. Genes Dev. 2010;24:1718–30.
Ntziachristos P, Lim JS, Sage J, Aifantis I. From fly wings to targeted cancer therapies: a centennial for notch signaling. Cancer cell. 2014;25:318–34.
Zhang R, Han P, Yang H, Ouyang K, Lee D, Lin YF, et al. In vivo cardiac reprogramming contributes to zebrafish heart regeneration. Nature. 2013;498:497–501.
Rand MD, Grimm LM, Artavanis-Tsakonas S, Patriub V, Blacklow SC, Sklar J, et al. Calcium depletion dissociates and activates heterodimeric notch receptors. Mol Cell Biol. 2000;20:1825–35.
Zhu W, Niu J, He M, Zhang L, Lv X, Liu F, et al. SNORD89 promotes stemness phenotype of ovarian cancer cells by regulating Notch1-c-Myc pathway. J Transl Med. 2019;17:259.
Verginelli F, Adesso L, Limon I, Alisi A, Gueguen M, Panera N, et al. Activation of an endothelial Notch1-Jagged1 circuit induces VCAM1 expression, an effect amplified by interleukin-1beta. Oncotarget. 2015;6:43216–29.
Sun Q, Wang R, Wang Y, Luo J, Wang P, Cheng B. Notch1 is a potential therapeutic target for the treatment of human hepatitis B virus X protein-associated hepatocellular carcinoma. Oncol Rep. 2014;31:933–9.
Qi R, An H, Yu Y, Zhang M, Liu S, Xu H, et al. Notch1 signaling inhibits growth of human hepatocellular carcinoma through induction of cell cycle arrest and apoptosis. Cancer Res. 2003;63:8323–9.
Viatour P, Ehmer U, Saddic LA, Dorrell C, Andersen JB, Lin C, et al. Notch signaling inhibits hepatocellular carcinoma following inactivation of the RB pathway. J Exp Med. 2011;208:1963–76.
Ahn S, Hyeon J, Park CKNotch1. and Notch4 are markers for poor prognosis of hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2013;12:286–94.
Zhang Y, Li D, Feng F, An L, Hui F, Dang D, et al. Progressive and prognosis value of notch receptors and ligands in hepatocellular carcinoma: a systematic review and meta-analysis. Sci Rep. 2017;7:14809.
Strazzabosco M, Fabris L. Notch signaling in hepatocellular carcinoma: guilty in association! Gastroenterology. 2012;143:1430–4.
Kim MJ, Xia B, Suh HN, Lee SH, Jun S, Lien EM, et al. PAF-Myc-controlled cell stemness is required for intestinal regeneration and tumorigenesis. Dev Cell. 2018;44:582–96 e584.
Zhang HL, Wang P, Lu MZ, Zhang SD, Zheng L. c-Myc maintains the self-renewal and chemoresistance properties of colon cancer stem cells. Oncol Lett. 2019;17:4487–93.
Wieland E, Rodriguez-Vita J, Liebler SS, Mogler C, Moll I, Herberich SE, et al. Endothelial notch1 activity facilitates metastasis. Cancer Cell. 2017;31:355–67.
Chen Q, Massague J. Molecular pathways: VCAM-1 as a potential therapeutic target in metastasis. Clin Cancer Res. 2012;18:5520–5.
Lu X, Mu E, Wei Y, Riethdorf S, Yang Q, Yuan M, et al. VCAM-1 promotes osteolytic expansion of indolent bone micrometastasis of breast cancer by engaging alpha4beta1-positive osteoclast progenitors. Cancer Cell. 2011;20:701–14.
Luo Z, Mu L, Zheng Y, Shen W, Li J, Xu L, et al. NUMB enhances Notch signaling by repressing ubiquitination of NOTCH1 intracellular domain. J Mol Cell Biol. 2020;12:345–58.
Hussain SP, Schwank J, Staib F, Wang XW, Harris CC. TP53 mutations and hepatocellular carcinoma: insights into the etiology and pathogenesis of liver cancer. Oncogene. 2007;26:2166–76.
Chen Q, Zhang XH, Massague J. Macrophage binding to receptor VCAM-1 transmits survival signals in breast cancer cells that invade the lungs. Cancer Cell. 2011;20:538–49.
Mishra A, Guo Y, Zhang L, More S, Weng T, Chintagari NR, et al. A critical role for P2X7 receptor-induced VCAM-1 shedding and neutrophil infiltration during acute lung injury. J Immunol. 2016;197:2828–37.
Jin H, Su J, Garmy-Susini B, Kleeman J, Varner J. Integrin alpha4beta1 promotes monocyte trafficking and angiogenesis in tumors. Cancer Res. 2006;66:2146–52.
Lai YS, Wahyuningtyas R, Aui SP, Chang KT. Autocrine VEGF signalling on M2 macrophages regulates PD-L1 expression for immunomodulation of T cells. J Cell Mol Med. 2019;23:1257–67.
Harney AS, Arwert EN, Entenberg D, Wang Y, Guo P, Qian BZ, et al. Real-time imaging reveals local, transient vascular permeability, and tumor cell intravasation stimulated by TIE2hi macrophage-derived VEGFA. Cancer Disco. 2015;5:932–43.
Solinas G, Schiarea S, Liguori M, Fabbri M, Pesce S, Zammataro L, et al. Tumor-conditioned macrophages secrete migration-stimulating factor: a new marker for M2-polarization, influencing tumor cell motility. J Immunol. 2010;185:642–52.
Wei C, Yang C, Wang S, Shi D, Zhang C, Lin X, et al. Crosstalk between cancer cells and tumor associated macrophages is required for mesenchymal circulating tumor cell-mediated colorectal cancer metastasis. Mol Cancer. 2019;18:64.
Mishra L, Banker T, Murray J, Byers S, Thenappan A, He AR, et al. Liver stem cells and hepatocellular carcinoma. Hepatology. 2009;49:318–29.
Lohuizen van M, Verbeek S, Scheijen B, Wientjens E, Gulden H, Berns A. Identification of cooperating oncogenes in E mu-myc transgenic mice by provirus tagging. Cell. 1991;65:737–52.
Villanueva A, Alsinet C, Yanger K, Hoshida Y, Zong Y, Toffanin S, et al. Notch signaling is activated in human hepatocellular carcinoma and induces tumor formation in mice. Gastroenterology. 2012;143:1660–9.e1667.
Gurzu S, Kobori L, Fodor D, Jung I. Epithelial mesenchymal and endothelial mesenchymal transitions in hepatocellular carcinoma: a review. BioMed Res Int. 2019;2019:2962580.
Wurmbach E, Chen YB, Khitrov G, Zhang W, Roayaie S, Schwartz M, et al. Genome-wide molecular profiles of HCV-induced dysplasia and hepatocellular carcinoma. Hepatology. 2007;45:938–47.
Ding L, Ding YN, Lin Y, Wen JM, Xue L. Proteins related to early changes in carcinogenesis of hepatic oval cells after treatment with methylnitronitrosoguanidine. Exp Toxicol Pathol. 2014;66:139–46.
Li X, Li Y, Kang X, Guo K, Li H, Gao D, et al. Dynamic alteration of protein expression profiles during neoplastic transformation of rat hepatic oval-like cells. Cancer Sci. 2010;101:1099–107.
Wu K, Ding J, Chen C, Sun W, Ning BF, Wen W, et al. Hepatic transforming growth factor beta gives rise to tumor-initiating cells and promotes liver cancer development. Hepatology. 2012;56:2255–67.
Lai GH, Zhang Z, Shen XN, Ward DJ, Dewitt JL, Holt SE, et al. erbB-2/neu transformed rat cholangiocytes recapitulate key cellular and molecular features of human bile duct cancer. Gastroenterology. 2005;129:2047–57.
Padilla-Nash HM, Hathcock K, McNeil NE, Mack D, Hoeppner D, Ravin R, et al. Spontaneous transformation of murine epithelial cells requires the early acquisition of specific chromosomal aneuploidies and genomic imbalances. Genes Chromosomes Cancer. 2012;51:353–74.
Llovet JM, Zucman-Rossi J, Pikarsky E, Sangro B, Schwartz M, Sherman M, et al. Hepatocellular carcinoma. Nat Rev Dis Prim. 2016;2:16018.
Fan B, Malato Y, Calvisi DF, Naqvi S, Razumilava N, Ribback S, et al. Cholangiocarcinomas can originate from hepatocytes in mice. J Clin Invest. 2012;122:2911–5.
Cook J, Hagemann T. Tumour-associated macrophages and cancer. Curr Opin Pharm. 2013;13:595–601.
Cardoso AP, Pinto ML, Pinto AT, Oliveira MI, Pinto MT, Goncalves R, et al. Macrophages stimulate gastric and colorectal cancer invasion through EGFR Y(1086), c-Src, Erk1/2 and Akt phosphorylation and smallGTPase activity. Oncogene. 2014;33:2123–33.
Byrne GJ, Ghellal A, Iddon J, Blann AD, Venizelos V, Kumar S, et al. Serum soluble vascular cell adhesion molecule-1: role as a surrogate marker of angiogenesis. J Natl Cancer Inst. 2000;92:1329–36.
Nagy A, Lanczky A, Menyhart O, Gyorffy B. Validation of miRNA prognostic power in hepatocellular carcinoma using expression data of independent datasets. Sci Rep. 2018;8:9227.
Zhang R, Wu WR, Shi XD, Xu LB, Zhu MS, Zeng H, et al. Dysregulation of Bmi1 promotes malignant transformation of hepatic progenitor cells. Oncogenesis. 2016;5:e203.
Vandereyken M, Jacques S, Van Overmeire E, Amand M, Rocks N, Delierneux C, et al. Dusp3 deletion in mice promotes experimental lung tumour metastasis in a macrophage dependent manner. PLoS One. 2017;12:e0185786.
Acknowledgements
Some of the illustrations were created using Biorender.com. This work was supported by grants from the National Natural Science Foundation of China (81972255, 82173195, 81920108028, 81872142, 81772597, 82173229, 81702904, 81972262), Guangdong Natural Science Foundation (2021A1515010095, 712055485047), Guangzhou Science and Technology Program Key Project (201904020008), Sun Yat-sen University Clinical Research 5010 Program (2018008), Science and Technology Program of Guangzhou (202102010326), Sun Yat-sen University Fundamental Research Funds (19ykpy113), the Key Training Program for Young Scholars of Sun Yat-sen University (18ykzd07), grant from the Key Laboratory of Malignant Tumor Molecular Mechanism and Translational Medicine of Guangzhou Bureau of Science and Information Technology ([2013]163), the Key Laboratory of Malignant Tumor Gene Regulation and Target Therapy of Guangdong Higher Education Institutes (KLB09001), Guangdong Science and Technology Department (2020A0505100029, 2020B1212060018, 2020B1212030004), the Guangdong Translational Medicine Public Platform of Guangdong Province (4202037).
Author information
Authors and Affiliations
Contributions
WRW, XDS, and FPZ carried out the majority of the experiments and contributed equally to the paper; KZ, YFQ, SPH, HWF, LZ, and MSZ assisted the animal experiments; FPZ and HZ carried out the RNA-seq database and immunohistochemistry analysis; RZ and XHY provided technical advice. LBX, PPW, and CL conceived the study, co-supervised the research, and co-wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
All animal procedures were approved by the animal care and use committee of Sun Yat-sen University.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Wu, WR., Shi, XD., Zhang, FP. et al. Activation of the Notch1-c-myc-VCAM1 signalling axis initiates liver progenitor cell-driven hepatocarcinogenesis and pulmonary metastasis. Oncogene 41, 2340–2356 (2022). https://doi.org/10.1038/s41388-022-02246-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-022-02246-5
This article is cited by
-
PARD3 drives tumorigenesis through activating Sonic Hedgehog signalling in tumour-initiating cells in liver cancer
Journal of Experimental & Clinical Cancer Research (2024)
-
Cathepsin-facilitated invasion of BMI1-high hepatocellular carcinoma cells drives bile duct tumor thrombi formation
Nature Communications (2023)
-
MYC in liver cancer: mechanisms and targeted therapy opportunities
Oncogene (2023)