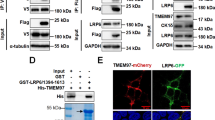
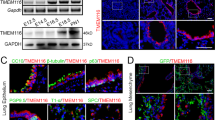
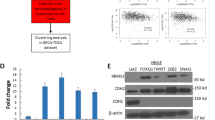
Abstract
Emerging evidence shows the association between nuclear envelope and tumor progression, however, the functional contributions of specific constituents of the nuclear envelope remain largely unclear. We found that the expression level of transmembrane protein 201 (TMEM201), an integral inner nuclear membrane protein of unknown function, was significantly elevated in invasive breast cancer and predicted poor breast cancer prognosis. We showed that TMEM201, as a positive modulator, was both necessary and sufficient to regulate the migration and invasion of breast cancer cells in vitro and in vivo. Mechanistically, RNA-sequencing analysis and validation showed that TMEM201 deficiency inhibited epithelial-to-mesenchymal transition and transforming growth factor-β signaling. Finally, we showed that TMEM201 physically interacted with SMAD2/3 and was required for the phosphorylation of SMAD2/3, nuclear translocation and transcriptional activation of the TGFβ. Thus, we demonstrated that specific inner nuclear membrane component mediated signal-dependent transcriptional effects to control breast cancer metastasis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Code availability
R language package of survival can be accessed in Supplementary Table 6.
References
Hetzer MW. The nuclear envelope. Cold Spring Harb Perspect Biol. 2010;2:a000539.
Gruenbaum Y, Margalit A, Goldman RD, Shumaker DK, Wilson KL. The nuclear lamina comes of age. Nat Rev Mol Cell Biol. 2005;6:21–31.
Goldberg M, Lu H, Stuurman N, Ashery-Padan R, Weiss AM, Yu J, et al. Interactions among Drosophila nuclear envelope proteins lamin, otefin, and YA. Mol Cell Biol. 1998;18:4315–23.
Schirmer EC, Foisner R. Proteins that associate with lamins: many faces, many functions. Exp Cell Res. 2007;313:2167–79.
Dreger M, Bengtsson L, Schöneberg T, Otto H, Hucho F. Nuclear envelope proteomics: novel integral membrane proteins of the inner nuclear membrane. Proc Natl Acad Sci USA. 2001;98:11943–8.
Korfali N, Wilkie GS, Swanson SK, Srsen V, Batrakou DG, Fairley EA, et al. The leukocyte nuclear envelope proteome varies with cell activation and contains novel transmembrane proteins that affect genome architecture. Mol Cell Proteom. 2010;9:2571–85.
Schirmer EC, Florens L, Guan T, Yates JR 3rd, Gerace L. Nuclear membrane proteins with potential disease links found by subtractive proteomics. Science.2003;301:1380–2.
Wilkie GS, Korfali N, Swanson SK, Malik P, Srsen V, Batrakou DG, et al. Several novel nuclear envelope transmembrane proteins identified in skeletal muscle have cytoskeletal associations. Mol Cell Proteom. 2011;10:M110.003129.
Korfali N, Wilkie GS, Swanson SK, Srsen V, de Las Heras J, Batrakou DG, et al. The nuclear envelope proteome differs notably between tissues. Nucleus.2012;3:552–64.
Schreiber KH, Kennedy BK. When lamins go bad: nuclear structure and disease. Cell.2013;152:1365–75.
Shi J, Li C, Wang H, Xiao B, Qiu W. NUP58 facilitates metastasis and epithelial-mesenchymal transition of lung adenocarcinoma via the GSK-3β/Snail signaling pathway. Am J Transl Res. 2019;11:393–405.
Lu T, Bao Z, Wang Y, Yang L, Lu B, Yan K, et al. Karyopherinβ1 regulates proliferation of human glioma cells via Wnt/β-catenin pathway. Biochem Biophys Res Commun. 2016;478:1189–97.
Rodriguez-Bravo V, Pippa R, Song WM, Carceles-Cordon M, Dominguez-Andres A, Fujiwara N, et al. Nuclear pores promote lethal prostate cancer by increasing POM121-Driven E2F1, MYC, and AR nuclear import. Cell.2018;174:1200–15.e20.
Bell ES, Lammerding J. Causes and consequences of nuclear envelope alterations in tumour progression. Eur J Cell Biol. 2016;95:449–64.
de Las Heras JI, Batrakou DG, Schirmer EC. Cancer biology and the nuclear envelope: a convoluted relationship. Semin Cancer Biol. 2013;23:125–37.
Chow KH, Factor RE, Ullman KS. The nuclear envelope environment and its cancer connections. Nat Rev Cancer. 2012;12:196–209.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
Weigelt B, Peterse JL, van ‘t Veer LJ. Breast cancer metastasis: markers and models. Nat Rev Cancer. 2005;5:591–602.
Wolff AC, Hammond MEH, Allison KH, Harvey BE, Mangu PB, Bartlett JMS, et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J Clin Oncol. 2018;36:2105–22.
Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N. Engl J Med. 2010;363:1938–48.
Liedtke C, Mazouni C, Hess KR, André F, Tordai A, Mejia JA, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008;26:1275–81..
Kao KJ, Chang KM, Hsu HC, Huang AT. Correlation of microarray-based breast cancer molecular subtypes and clinical outcomes: implications for treatment optimization. BMC Cancer. 2011;11:143.
Curtis C, Shah SP, Chin SF, Turashvili G, Rueda OM, Dunning MJ. et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature. 2012;486:346–52.
Pereira B, Chin SF, Rueda OM, Vollan HK, Provenzano E, Bardwell HA, et al. The somatic mutation profiles of 2,433 breast cancers refines their genomic and transcriptomic landscapes. Nat Commun. 2016;7:11479.
Buch C, Lindberg R, Figueroa R, Gudise S, Onischenko E, Hallberg E. An integral protein of the inner nuclear membrane localizes to the mitotic spindle in mammalian cells. J Cell Sci. 2009;122:2100–7.
Dephoure N, Zhou C, Villén J, Beausoleil SA, Bakalarski CE, Elledge SJ, et al. A quantitative atlas of mitotic phosphorylation. Proc Natl Acad Sci USA. 2008;105:10762–7.
Olsen JV, Vermeulen M, Santamaria A, Kumar C, Miller ML, Jensen LJ, et al. Quantitative phosphoproteomics reveals widespread full phosphorylation site occupancy during mitosis. Sci Signal. 2010;3:ra3.
Zhou H, Di Palma S, Preisinger C, Peng M, Polat AN, Heck AJ, et al. Toward a comprehensive characterization of a human cancer cell phosphoproteome. J Proteome Res. 2013;12:260–71.
Schaffer BE, Levin RS, Hertz NT, Maures TJ, Schoof ML, Hollstein PE, et al. Identification of AMPK phosphorylation sites reveals a network of proteins involved in cell invasion and facilitates large-scale substrate prediction. Cell Metab. 2015;22:907–21.
Siegel PM, Shu W, Cardiff RD, Muller WJ, Massagué J. Transforming growth factor beta signaling impairs Neu-induced mammary tumorigenesis while promoting pulmonary metastasis. Proc Natl Acad Sci USA. 2003;100:8430–5.
Moustakas A, Heldin CH. Mechanisms of TGFβ-induced epithelial-mesenchymal transition. J Clin Med. 2016;5:63.
Reis-Sobreiro M, Chen JF, Novitskaya T, You S, Morley S, Steadman K, et al. Emerin deregulation links nuclear shape instability to metastatic potential. Cancer Res. 2018;78:6086–97.
Liu C, Yu H, Shen X, Qiao J, Wu X, Chang J, et al. Prognostic significance and biological function of Lamina-associated polypeptide 2 in non-small-cell lung cancer. Onco Targets Ther. 2019;12:3817–27.
Borrego-Pinto J, Jegou T, Osorio DS, Auradé F, Gorjánácz M, Koch B. et al. Samp1 is a component of TAN lines and is required for nuclear movement. J Cell Sci. 2012;125:1099–105.
Derynck R, Zhang YE, Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature.2003;425:577–84.
Massagué J, Wotton D. Transcriptional control by the TGF-beta/Smad signaling system. EMBO J. 2000;19:1745–54.
Lin F, Morrison JM, Wu W, Worman HJ. MAN1, an integral protein of the inner nuclear membrane, binds Smad2 and Smad3 and antagonizes transforming growth factor-beta signaling. Hum Mol Genet. 2005;14:437–45.
Pan D, Estévez-Salmerón LD, Stroschein SL, Zhu X, He J, Zhou S, et al. The integral inner nuclear membrane protein MAN1 physically interacts with the R-Smad proteins to repress signaling by the transforming growth factor-{beta} superfamily of cytokines. J Biol Chem. 2005;280:15992–6001.
Osada S, Ohmori SY, Taira M. XMAN1, an inner nuclear membrane protein, antagonizes BMP signaling by interacting with Smad1 in Xenopus embryos. Development.2003;130:1783–94.
Raju GP, Dimova N, Klein PS, Huang HC. SANE, a novel LEM domain protein, regulates bone morphogenetic protein signaling through interaction with Smad1. J Biol Chem. 2003;278:428–37.
Mansharamani M, Wilson KL. Direct binding of nuclear membrane protein MAN1 to emerin in vitro and two modes of binding to barrier-to-autointegration factor. J Biol Chem. 2005;280:13863–70.
Vijayaraghavan B, Figueroa RA, Bergqvist C, Gupta AJ, Sousa P, Hallberg E. RanGTPase regulates the interaction between the inner nuclear membrane proteins, Samp1 and Emerin. Biochim Biophys Acta Biomembr. 2018;1860:1326–34.
Karlsson G, Liu Y, Larsson J, Goumans MJ, Lee JS, Thorgeirsson SS, et al. Gene expression profiling demonstrates that TGF-beta1 signals exclusively through receptor complexes involving Alk5 and identifies targets of TGF-beta signaling. Physiol Genomics. 2005;21:396–403.
Yang YC, Piek E, Zavadil J, Liang D, Xie D, Heyer J, et al. Hierarchical model of gene regulation by transforming growth factor beta. Proc Natl Acad Sci USA. 2003;100:10269–74.
Bengtsson L. What MAN1 does to the Smads. TGFbeta/BMP signaling and the nuclear envelope. FEBS J. 2007;274:1374–82.
Acknowledgements
This work is supported by the National Natural Science Foundation of China (Grant 31871414, 81971265), Science and Technology Commission of Shanghai Municipality (19JC1416300), Shanghai Institute of Materia Medica (SIMM2004KF-01), Shanghai Municipal Human Resources and Social Security Bureau (Y91204R).
Author information
Authors and Affiliations
Contributions
YK designed and performed most experiments and data analysis. YTZ helped with the knockout cell line construction and relevant analysis. HLW helped with the TCGA and other databases analysis and RNA sequencing analysis. WJK contributed to the in vivo mouse model construction. HRG assisted the immunoblots. YL helped with the plasmids construction and lentivirus package. JL and YZ participated in experimental design, data interpretation and supervised the project. YK wrote the article. All authors contributed to the final editing and approval of the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Kong, Y., Zhang, Y., Wang, H. et al. Inner nuclear membrane protein TMEM201 promotes breast cancer metastasis by positive regulating TGFβ signaling. Oncogene 41, 647–656 (2022). https://doi.org/10.1038/s41388-021-02098-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-021-02098-5
This article is cited by
-
The role of inner nuclear membrane proteins in tumourigenesis and as potential targets for cancer therapy
Cancer and Metastasis Reviews (2022)