Abstract
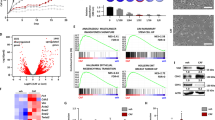
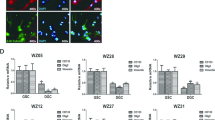
Breast cancer (BC) is the most common cancer in women worldwide, and the exploration of aberrantly expressed genes might clarify tumorigenesis and help uncover new therapeutic strategies for BC. Although RGMA was recently recognized as a tumor suppressor gene, its detailed biological function and regulation in BC remain unclear. Herein, we found that RGMA was downregulated in BC tissues compared with non-tumorous breast tissues, particularly in metastatic BC samples, and that patients with low RGMA expression manifested a poorer prognosis. Furthermore, DNMT1 and DNMT3A were found to be recruited to the RGMA promoter and induced aberrant hypermethylation, resulting in downregulation of RGMA expression in BC. In contrast, RGMA overexpression suppressed BC cell proliferation and colony-formation capabilities and increased BC cell apoptosis. Furthermore, RGMA knockdown accelerated BC cell proliferation and suppressed cellular apoptosis in vitro and in vivo. Reversal of RGMA promoter methylation with 5-Aza-CdR restored RGMA expression and blocked tumor growth. Overall, DNMT1- and DNMT3A-mediated RGMA promoter hypermethylation led to downregulation of RGMA expression, and low RGMA expression contributed to BC growth via activation of the FAK/Src/PI3K/AKT-signaling pathway. Our data thus suggested that RGMA might be a promising therapeutic target in BC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
Schoeps A, Rudolph A, Seibold P, Dunning AM, Milne RL, Bojesen SE, et al. Identification of New Genetic Susceptibility Loci for Breast Cancer Through Consideration of Gene-Environment Interactions. Genet Epidemiol. 2014;38:84–93.
Abdel-Hafiz HA, Horwitz KB. Role of epigenetic modifications in luminal breast cancer. Epigenomics. 2015;7:847–62.
Siebold C, Yamashita T, Monnier PP, Mueller BK, Pasterkamp RJ. RGMs: Structural Insights, Molecular Regulation, and Downstream Signaling. Trends Cell Biol. 2017;27:365–78.
O’Leary C, Cole SJ, Langford M, Hewage J, White A, Cooper HM. RGMa regulates cortical interneuron migration and differentiation. PLoS ONE. 2013;8:e81711.
Korecka JA, Moloney EB, Eggers R, Hobo B, Scheffer S, Ras-Verloop N, et al. Repulsive Guidance Molecule a (RGMa) Induces Neuropathological and Behavioral Changes That Closely Resemble Parkinson’s Disease. J Neurosci. 2017;37:9361–79.
Lu Y, Li Y, Wang Z, Xie S, Wang Q, Lei X, et al. Downregulation of RGMA by HIF-1A/miR-210-3p axis promotes cell proliferation in oral squamous cell carcinoma. Biomed Pharmacother. 2019;112:108608.
Zhao ZW, Lian WJ, Chen GQ, Zhou HY, Wang GM, Cao X, et al. Decreased expression of repulsive guidance molecule member A by DNA methylation in colorectal cancer is related to tumor progression. Oncol Rep. 2012;27:1653–9.
Li J, Ye L, Kynaston HG, Jiang WG. Repulsive guidance molecules, novel bone morphogenetic protein co-receptors, are key regulators of the growth and aggressiveness of prostate cancer cells. Int J Oncol. 2012;40:544–50.
Li VSW, Yuen ST, Chan TL, Yan HHN, Law WL, Yeung BHY, et al. Frequent Inactivation of Axon Guidance Molecule RGMA in Human Colon Cancer Through Genetic and Epigenetic Mechanisms. Gastroenterology. 2009;137:176–87.
Zhang G, Wang R, Cheng K, Li Q, Wang Y, Zhang R, et al. Repulsive Guidance Molecule a Inhibits Angiogenesis by Downregulating VEGF and Phosphorylated Focal Adhesion Kinase In Vitro. Front Neurol. 2017;8:504.
Xiao B, Chen L, Ke Y, Hang J, Cao L, Zhang R, et al. Identification of methylation sites and signature genes with prognostic value for luminal breast cancer. BMC Cancer. 2018;18:405.
Li Y, Wang YW, Chen X, Ma RR, Guo XY, Liu HT, et al. MicroRNA-4472 Promotes Tumor Proliferation and Aggressiveness in Breast Cancer by Targeting RGMA and Inducing EMT. Clin Breast Cancer. 2020;20:e113–26.
Klutstein M, Nejman D, Greenfield R, Cedar H. DNA Methylation in Cancer and Aging. Cancer Res. 2016;76:3446–50.
Endo M, Yamashita T. Inactivation of Ras by p120GAP via focal adhesion kinase dephosphorylation mediates RGMa-induced growth cone collapse. J Neurosci. 2009;29:6649–62.
Li Y, Li X-Y, Li L-X, Zhou R-C, Sikong Y.GuX, et al. S100A10 Accelerates Aerobic Glycolysis and Malignant Growth by Activating mTOR-Signaling Pathway in Gastric Cancer. Front Cell Dev Biol. 2020;8:559486.
Niit M, Hoskin V, Carefoot E, Geletu M, Arulanandam R, Elliott B, et al. Cell-cell and cell-matrix adhesion in survival and metastasis: Stat3 versus Akt. Biomol Concepts. 2015;6:383–99.
Feng FB, Qiu HY. Effects of Artesunate on chondrocyte proliferation, apoptosis and autophagy through the PI3K/AKT/mTOR signaling pathway in rat models with rheumatoid arthritis. Biomed Pharmacother. 2018;102:1209–20.
Jia X, Wen Z, Sun Q, Zhao X, Yang H, Shi X, et al. Apatinib suppresses the Proliferation and Apoptosis of Gastric Cancer Cells via the PI3K/Akt Signaling Pathway. J BUON. 2019;24:1985–91.
Bao RK, Zheng SF, Wang XY. Selenium protects against cadmium-induced kidney apoptosis in chickens by activating the PI3K/AKT/Bcl-2 signaling pathway. Environ Sci Pollut Res Int. 2017;24:20342–53.
Zhang Y, He W, Zhang S. Seeking for Correlative Genes and Signaling Pathways With Bone Metastasis From Breast Cancer by Integrated Analysis. Front Oncol. 2019;9:138.
Subramaniam D, Thombre R, Dhar A, Anant S. DNA methyltransferases: a novel target for prevention and therapy. Front Oncol. 2014;4:80.
Pathania R, Ramachandran S, Mariappan G, Thakur P, Shi H, Choi JH, et al. Combined Inhibition of DNMT and HDAC Blocks the Tumorigenicity of Cancer Stem-like Cells and Attenuates Mammary Tumor Growth. Cancer Res. 2016;76:3224–35.
Pathania R, Ramachandran S, Elangovan S, Padia R, Yang P, Cinghu S, et al. DNMT1 is essential for mammary and cancer stem cell maintenance and tumorigenesis. Nat Commun. 2015;6:6910.
Mirza S, Sharma G, Pandya P, Ralhan R. Demethylating agent 5-aza-2-deoxycytidine enhances susceptibility of breast cancer cells to anticancer agents. Mol Cell Biochem. 2010;342:101–9.
Borges S, Doppler H, Perez EA, Andorfer CA, Sun Z, Anastasiadis PZ, et al. Pharmacologic reversion of epigenetic silencing of the PRKD1 promoter blocks breast tumor cell invasion and metastasis. Breast Cancer Res. 2013;15:R66.
Schaller MD. Cellular functions of FAK kinases: insight into molecular mechanisms and novel functions. J Cell Sci. 2010;123:1007–13. Pt 7
Luo M, Guan JL. Focal adhesion kinase: a prominent determinant in breast cancer initiation, progression and metastasis. Cancer Lett. 2010;289:127–39.
Chandrashekar DS, Bashel B, Balasubramanya SAH, Creighton CJ, Ponce-Rodriguez I, Chakravarthi B, et al. UALCAN: a Portal for Facilitating Tumor Subgroup Gene Expression and Survival Analyses. Neoplasia. 2017;19:649–58.
Gyorffy B, Lanczky A, Eklund AC, Denkert C, Budczies J, Li Q, et al. An online survival analysis tool to rapidly assess the effect of 22,277 genes on breast cancer prognosis using microarray data of 1,809 patients. Breast Cancer Res Treat. 2010;123:725–31.
Specht E, Kaemmerer D, Sanger J, Wirtz RM, Schulz S, Lupp A. Comparison of immunoreactive score, HER2/neu score and H score for the immunohistochemical evaluation of somatostatin receptors in bronchopulmonary neuroendocrine neoplasms. Histopathology. 2015;67:368–77.
Liu H, Song Y, Qiu H, Liu Y, Luo K, Yi Y, et al. Downregulation of FOXO3a by DNMT1 promotes breast cancer stem cell properties and tumorigenesis. Cell Death Differ. 2020;27:966–83.
Ku JL, Jeon YK, Park JG. Methylation-specific PCR. Methods Mol Biol. 2011;791:23–32.
Acknowledgements
We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this paper. This study was supported by the National Natural Science Foundation of China (Grant No. 81872362 and 82072665) and the Taishan Scholars Program of Shandong Province (Grant No. ts201511096).
Author information
Authors and Affiliations
Contributions
PG, YXZ and YL conceived the study; YL, HTL, XC, YWW, YRT, RRM and LS performed the experiments; YL analyzed the data and wrote the paper; YL, HTL and PG revised the paper. All authors have read and approved the final paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study was approved by the Ethical Committee on Scientific Research of Shandong University.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Li, Y., Liu, HT., Chen, X. et al. Aberrant promoter hypermethylation inhibits RGMA expression and contributes to tumor progression in breast cancer. Oncogene 41, 361–371 (2022). https://doi.org/10.1038/s41388-021-02083-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-021-02083-y
This article is cited by
-
A Phase 1B open-label study of gedatolisib (PF-05212384) in combination with other anti-tumour agents for patients with advanced solid tumours and triple-negative breast cancer
British Journal of Cancer (2023)
-
Transfection with Plasmid-Encoding lncRNA-SLERCC nanoparticle-mediated delivery suppressed tumor progression in renal cell carcinoma
Journal of Experimental & Clinical Cancer Research (2022)