Abstract
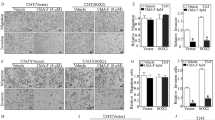
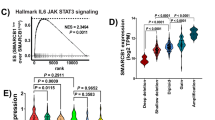
There are unmet clinical needs for novel therapeutic targets and drugs for bladder cancer. Majority of previous work relied on limited bladder cancer cell lines, which could not well represent the tumor heterogeneity and pathology of this disease. Recently, it has been shown that cancer organoids can recapitulate pathological and molecular properties of bladder cancer. Here, we report, by our knowledge, the first bladder cancer organoid-based small molecule screening for epigenetic drugs. We found that SRT1720, a Sirtuin 1 (SIRT1) activator, significantly inhibits the growth of both mouse and human bladder cancer organoids. And it also restrains the development of mouse in situ bladder cancer and human PDX bladder cancer. Mutation of Sirt1 promotes the growth of cancer organoids and decreases their sensitivity to SRT1720, which validate Sirt1 as the target of SRT1720 in bladder cancer. Mechanistically, SRT1720 treatment represses the hypoxia pathway through deacetylating HIF1α by activating Sirt1. Genetic or pharmaceutic inhibitions of HIF mimic the anti-tumor effect of SRT1720. Furthermore, the SIRT1-repressed gene signature is associated with the hypoxia target gene signature and poor prognosis in human bladder cancers. Thus, our study demonstrates the power of cancer organoid-based drug discovery and, in principle, identifies SRT1720 as a new treatment for bladder cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
All bulk RNA data generated by this study have been deposited in the GEO data sets (https://www.ncbi.nlm.nih.gov/gds). The data can be accessed under the accession number GSE155525. The code is available from the authors on request.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
Berdik C. Unlocking bladder cancer. Nature. 2017;551:S34–S35.
Vlachostergios PJ, Faltas BM. Treatment resistance in urothelial carcinoma: an evolutionary perspective. Nat Rev Clin Oncol. 2018;15:495–509.
Loriot Y, Necchi A, Park SH, Garcia-Donas J, Huddart R, Burgess E, et al. Erdafitinib in locally advanced or metastatic urothelial carcinoma. N Engl J Med. 2019;381:338–48.
Gillet J-P, Calcagno AM, Varma S, Marino M, Green LJ, Vora MI, et al. Redefining the relevance of established cancer cell lines to the study of mechanisms of clinical anti-cancer drug resistance. Proc Natl Acad Sci USA. 2011;108:18708–13.
Willyard C. The boom in mini stomachs, brains, breasts, kidneys and more. Nature. 2015;523:520–2.
Huch M, Boj SF, Clevers H. Lgr5(+) liver stem cells, hepatic organoids and regenerative medicine. Regen Med. 2013;8:385–7.
Van De Wetering M, Francies HE, Francis JM, Bounova G, Iorio F, Pronk A, et al. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell. 2015;161:933–45.
Lee SH, Hu W, Matulay JT, Silva MV, Owczarek TB, Kim K, et al. Tumor evolution and drug response in patient-derived organoid models of bladder cancer. Cell. 2018;173:515–28.e517.
Drost J, Clevers H. Translational applications of adult stem cell-derived organoids. Development. 2017;144:968–75.
Mullenders J, De Jongh E, Brousali A, Roosen M, Blom JP, Begthel H, et al. Mouse and human urothelial cancer organoids: a tool for bladder cancer research. Proc Natl Acad Sci USA. 2019;116:4567–74.
Robertson AG, Kim J, Al-Ahmadie H, Bellmunt J, Guo G, Cherniack AD, et al. Comprehensive molecular characterization of muscle-invasive bladder cancer. Cell. 2017;171:540–56.e525.
Network CGAR. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature. 2014;507:315–22.
Wolff EM, Liang G, Jones PA. Mechanisms of disease: genetic and epigenetic alterations that drive bladder cancer. Nat Clin Pract Urol. 2005;2:502–10.
Knowles MA, Hurst CD. Molecular biology of bladder cancer: new insights into pathogenesis and clinical diversity. Nat Rev Cancer. 2015;15:25–41.
Choi W, Porten S, Kim S, Willis D, Plimack ER, Hoffman-Censits J, et al. Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer Cell. 2014;25:152–65.
Yates DR, Rehman I, Abbod MF, Meuth M, Cross SS, Linkens DA, et al. Promoter hypermethylation identifies progression risk in bladder cancer. Clin Cancer Res. 2007;13:2046–53.
Dudziec E, Miah S, Choudhry HM, Owen HC, Blizard S, Glover M, et al. Hypermethylation of CpG islands and shores around specific microRNAs and mirtrons is associated with the phenotype and presence of bladder cancer. Clin Cancer Res. 2011;17:1287–96.
Milne JC, Lambert PD, Schenk S, Carney DP, Smith JJ, Gagne DJ, et al. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450:712–6.
Wu B, Pan X, Chen X, Chen M, Shi K, Xu J, et al. Epigenetic drug library screening identified an LSD1 inhibitor to target UTX-deficient cells for differentiation therapy. Signal Transduct Target Ther. 2019;4:11.
Chen J, Zhao L, Peng H, Dai S, Quan Y, Wang M, et al. An organoid-based drug screening identified a menin-MLL inhibitor for endometrial cancer through regulating the HIF pathway. Cancer Gene Ther. 2021;28:112–5.
Groselj B, Kerr M, Kiltie AE. Radiosensitisation of bladder cancer cells by panobinostat is modulated by Ku80 expression. Radiother Oncol. 2013;108:429–33.
Yang Z, He L, Lin K, Zhang Y, Deng A, Liang Y, et al. The KMT1A-GATA3-STAT3 circuit is a novel self-renewal signaling of human bladder cancer stem cells. Clin Cancer Res. 2017;23:6673–85.
Süssmuth SD, Haider S, Landwehrmeyer GB, Farmer R, Frost C, Tripepi G, et al. An exploratory double‐blind, randomized clinical trial with selisistat, a SirT1 inhibitor, in patients with H untington’s disease. Br J Clin Pharmacol. 2015;79:465–76.
Van Der Meer AJ, Scicluna BP, Moerland PD, Lin J, Jacobson EW, Vlasuk GP, et al. The selective sirtuin 1 activator SRT2104 reduces endotoxin-induced cytokine release and coagulation activation in humans. Crit Care Med. 2015;43:e199–202.
Julien C, Tremblay C, Émond V, Lebbadi M, Salem N Jr, Bennett DA, et al. Sirtuin 1 reduction parallels the accumulation of tau in Alzheimer disease. J Neuropathol Exp Neurol. 2009;68:48–58.
Deng C-X. SIRT1, is it a tumor promoter or tumor suppressor? Int J Biol Sci. 2009;5:147.
Weinstein JN, Collisson EA, Mills GB, Shaw KRM, Ozenberger BA, Ellrott K, et al. The cancer genome atlas pan-cancer analysis project. Nat Genet. 2013;45:1113.
Saunders L, Verdin E. Sirtuins: critical regulators at the crossroads between cancer and aging. Oncogene. 2007;26:5489–504.
Lee JT, Gu W. SIRT1: regulator of p53 deacetylation. Genes Cancer. 2013;4:112–7.
Li H-T, Duymich CE, Weisenberger DJ, Liang G. Genetic and epigenetic alterations in bladder cancer. Int Neurourol J. 2016;20:S84.
Faleiro I, Leão R, Binnie A, De Mello RA, Maia A-T, Castelo-Branco P. Epigenetic therapy in urologic cancers: an update on clinical trials. Oncotarget. 2017;8:12484.
Mitchell SJ, Martin-Montalvo A, Mercken EM, Palacios HH, Ward TM, Abulwerdi G, et al. The SIRT1 activator SRT1720 extends lifespan and improves health of mice fed a standard diet. Cell Rep. 2014;6:836–43.
Zeng L, Chen R, Liang F, Tsuchiya H, Murai H, Nakahashi T, et al. Silent information regulator, Sirtuin 1, and age‐related diseases. Geriatrics Gerontol Int. 2009;9:7–15.
Carafa V, Altucci L, Nebbioso A. Dual tumor suppressor and tumor promoter action of sirtuins in determining malignant phenotype. Front Pharmacol. 2019;10:38.
Hu Q, Wang G, Peng J, Qian G, Jiang W, Xie C, et al. Knockdown of SIRT1 suppresses bladder cancer cell proliferation and migration and induces cell cycle arrest and antioxidant response through FOXO3a-mediated pathways. Biomed Res Int. 2017;2017:3781904.
Chen J, Cao L, Li Z, Li Y. SIRT1 promotes GLUT1 expression and bladder cancer progression via regulation of glucose uptake. Hum Cell. 2019;32:193–201.
Dioum EM, Chen R, Alexander MS, Zhang Q, Hogg RT, Gerard RD, et al. Regulation of hypoxia-inducible factor 2alpha signaling by the stress-responsive deacetylase sirtuin 1. Science. 2009;324:1289–93.
Lim JH, Lee YM, Chun YS, Chen J, Kim JE, Park JW. Sirtuin 1 modulates cellular responses to hypoxia by deacetylating hypoxia-inducible factor 1alpha. Mol Cell. 2010;38:864–78.
Laemmle A, Lechleiter A, Roh V, Schwarz C, Portmann S, Furer C, et al. Inhibition of SIRT1 impairs the accumulation and transcriptional activity of HIF-1α protein under hypoxic conditions. PLoS One. 2012;7:e33433.
Joo HY, Yun M, Jeong J, Park ER, Shin HJ, Woo SR, et al. SIRT1 deacetylates and stabilizes hypoxia-inducible factor-1α (HIF-1α) via direct interactions during hypoxia. Biochem Biophys Res Commun. 2015;462:294–300.
Shin DH, Choi YJ, Park JW. SIRT1 and AMPK mediate hypoxia-induced resistance of non-small cell lung cancers to cisplatin and doxorubicin. Cancer Res. 2014;74:298–308.
Majmundar AJ, Wong WJ, Simon MC. Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell. 2010;40:294–309.
Chen R, Dioum EM, Hogg RT, Gerard RD, Garcia JA. Hypoxia increases sirtuin 1 expression in a hypoxia-inducible factor-dependent manner. J Biol Chem. 2011;286:13869–78.
Geng H, Liu Q, Xue C, David LL, Beer TM, Thomas GV, et al. HIF1α protein stability is increased by acetylation at lysine 709. J Biol Chem. 2012;287:35496–505.
Farghali H, Kemelo MK, Canová NK. SIRT1 modulators in experimentally induced liver injury. Oxid Med Cell Longev. 2019;2019:8765954.
Sun J, He X, Zhu Y, Ding Z, Dong H, Feng Y, et al. SIRT1 activation disrupts maintenance of myelodysplastic syndrome stem and progenitor cells by restoring TET2 function. Cell Stem Cell. 2018;23:355–69.e359.
Chini CC, Espindola-Netto JM, Mondal G, Guerrico AM, Nin V, Escande C, et al. SIRT1-activating compounds (STAC) negatively regulate pancreatic cancer cell growth and viability through a SIRT1 lysosomal-dependent pathway. Clin Cancer Res. 2016;22:2496–507.
Wang Y, Bi Y, Chen X, Li C, Li Y, Zhang Z, et al. Histone deacetylase SIRT1 negatively regulates the differentiation of interleukin-9-producing CD4(+) T cells. Immunity. 2016;44:1337–49.
Zhao H, Yan C, Hu Y, Mu L, Huang K, Li Q, et al. Sphere‑forming assay vs. organoid culture: Determining long‑term stemness and the chemoresistant capacity of primary colorectal cancer cells. Int J Oncol. 2019;54:893–904.
Lahusen TJ, Deng CX. SRT1720 induces lysosomal-dependent cell death of breast cancer cells. Mol Cancer Ther. 2015;14:183–92.
Acknowledgements
We thank all Chen-Liu laboratory members for their invaluable suggestions and technical support, and Wei team members for patient counseling.
Funding
This work was supported by the National Key R&D Program of China (Grant No. 2017YFA0505600), National Natural Science Foundation of China (Grant No. 82002692), 1.3.5. Project for Disciplines of Excellence, West China Hospital, Sichuan University (Grant No. ZYGD18011 and ZY2016104), Post-Doctor Research Project of Sichuan University (Grant No. 2021SCU12016), and Post-Doctor Research Project, West China Hospital, Sichuan University (Grant No. 19HXBH057).
Author information
Authors and Affiliations
Contributions
PT, MW, YL, CC, and QW conceived the project, designed experiments, and wrote the paper. PT, MW, YW, JD, JW, LQ, ZB, JZ, and JC performed experiments and analyzed data. PZ, TL, PH, LY, and QG did patient counseling. AZ performed RNA-seq and bioinformatics analysis.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study was approved by the Ethical Research Committee of the West China Hospital (2019-933/2020-330). Written informed consent was obtained from all patients involved. All patients had a confirmed pathologic diagnosis of MIBC and underwent radical cystectomy.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Tan, P., Wang, M., Zhong, A. et al. SRT1720 inhibits the growth of bladder cancer in organoids and murine models through the SIRT1-HIF axis. Oncogene 40, 6081–6092 (2021). https://doi.org/10.1038/s41388-021-01999-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-021-01999-9
This article is cited by
-
Targeting histone modifiers in bladder cancer therapy — preclinical and clinical evidence
Nature Reviews Urology (2024)
-
The strict regulation of HIF-1α by non-coding RNAs: new insight towards proliferation, metastasis, and therapeutic resistance strategies
Cancer and Metastasis Reviews (2024)
-
The role of organoids in cancer research
Experimental Hematology & Oncology (2023)