Abstract
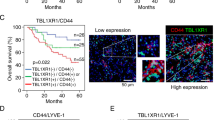
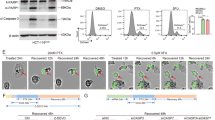
Anoikis is a type of programmed cell death induced by loss of anchorage to the extracellular matrix (ECM). Anoikis resistance (AR) is crucial for the survival of metastatic cancer cells in blood, lymphatic circulation and distant organs. Compared to ordinary cancer cells, anoikis resistant cancer cells undergo various cellular and molecular alterations, probably characterizing the cells with unique features not limited to anoikis resistance. However, the molecular mechanisms connecting anoikis resistance to other metastatic properties are still poorly understood. Here, the biological interaction between anoikis resistance and angiogenesis as well as their involvement into peritoneal metastasis of gastric cancer (GC) were investigated in vitro and in vivo. The prognostic value of key components involved in this interaction was evaluated in the GC cohort. Compared to ordinary GC cells, GCAR cells exhibited stronger metastatic and pro-angiogenic traits corresponding to elevated PDGFB secretion. Mechanistically, transcription factor C/EBPβ facilitated PDGFB transcription by directly binding to and interacting with PDGFB promoter elements, subsequently increasing PDGFB secretion. Secreted PDGFB promoted the survival of detached GC cells through a C/EBPβ-dependent self-feedback loop. Moreover, secreted PDGFB promoted angiogenesis in metastases via activation of the MAPK/ERK signaling pathway in vascular endothelial cells. Both C/EBPβ activation level and PDGFB expression were significantly elevated in GC and correlated with metastatic progression and poor prognosis of patients with GC. Overall, interaction between GCAR cells and vascular endothelial cells promotes angiogenesis and peritoneal metastasis of GC based on C/EBPβ-mediated PDGFB autocrine and paracrine signaling. C/EBPβ-PDGFB-PDGFRβ-MAPK axis promises to be potential prognostic biomarkers and therapeutic targets for peritoneal metastasis of GC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
All raw and processed RNA sequencing data of HUVEC was deposited to NCBI Genome Expression Omnibus (GEO; https://www.ncbi.nlm.nih.gov/geo/) under the accession numbers: GSE176441.
Change history
19 January 2022
A Correction to this paper has been published: https://doi.org/10.1038/s41388-021-02096-7
References
McLean MH, El-Omar EM. Genetics of gastric cancer. Nat Rev Gastroenterol Hepatol. 2014;11:664–74.
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108.
Shah MA. Update on metastatic gastric and esophageal cancers. J Clin Oncol. 2015;33:1760–9.
Yonemura Y, Bandou E, Kinoshita K, Kawamura T, Takahashi S, Endou Y, et al. Effective therapy for peritoneal dissemination in gastric cancer. Surg Oncol Clin N. Am. 2003;12:635–48.
Thomassen I, van Gestel YR, van Ramshorst B, Luyer MD, Bosscha K, Nienhuijs SW, et al. Peritoneal carcinomatosis of gastric origin: a population-based study on incidence, survival and risk factors. Int J Cancer. 2014;134:622–8.
Hong SH, Shin YR, Roh SY, Jeon EK, Song KY, Park CH, et al. Treatment outcomes of systemic chemotherapy for peritoneal carcinomatosis arising from gastric cancer with no measurable disease: retrospective analysis from a single center. Gastric Cancer. 2013;16:290–300.
Strilic B, Offermanns S. Intravascular survival and extravasation of tumor cells. Cancer Cell. 2017;32:282–93.
Jin K, Li T, van Dam H, Zhou F, Zhang L. Molecular insights into tumour metastasis: tracing the dominant events. J Pathol. 2017;241:567–77.
Haemmerle M, Taylor ML, Gutschner T, Pradeep S, Cho MS, Sheng J, et al. Platelets reduce anoikis and promote metastasis by activating YAP1 signaling. Nat Commun. 2017;8:310.
Buchheit CL, Weigel KJ, Schafer ZT. Cancer cell survival during detachment from the ECM: multiple barriers to tumour progression. Nat Rev Cancer. 2014;14:632–41.
Du S, Miao J, Zhu Z, Xu E, Shi L, Ai S, et al. NADPH oxidase 4 regulates anoikis resistance of gastric cancer cells through the generation of reactive oxygen species and the induction of EGFR. Cell Death Dis. 2018;9:948.
Eddy RJ, Weidmann MD, Sharma VP, Condeelis JS. Tumor cell invadopodia: invasive protrusions that orchestrate metastasis. Trends Cell Biol. 2017;27:595–607.
Wang S, Wu X, Zhang J, Chen Y, Xu J, Xia X, et al. CHIP functions as a novel suppressor of tumour angiogenesis with prognostic significance in human gastric cancer. Gut.2013;62:496–508.
Weis SM, Cheresh DA. Tumor angiogenesis: molecular pathways and therapeutic targets. Nat Med. 2011;17:1359–70.
Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62.
La Porta S, Roth L, Singhal M, Mogler C, Spegg C, Schieb B, et al. Endothelial Tie1-mediated angiogenesis and vascular abnormalization promote tumor progression and metastasis. J Clin Invest. 2018;128:834–45.
Kirsch M, Schackert G, Black PM. Metastasis and angiogenesis. Cancer Treat Res. 2004;117:285–304.
Kuczynski EA, Vermeulen PB, Pezzella F, Kerbel RS, Reynolds AR. Vessel co-option in cancer. Nat Rev Clin Oncol. 2019;16:469–93.
Xue Y, Lim S, Yang Y, Wang Z, Jensen LD, Hedlund EM, et al. PDGF-BB modulates hematopoiesis and tumor angiogenesis by inducing erythropoietin production in stromal cells. Nat Med. 2011;18:100–10.
Lindahl P, Johansson BR, Levéen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science.1997;277:242–5.
Kitadai Y, Kodama M, Shinagawa K. Stroma-directed molecular targeted therapy in gastric cancer. Cancers (Basel). 2011;3:4245–57.
Zhang Y, Lu H, Dazin P, Kapila Y. Squamous cell carcinoma cell aggregates escape suspension-induced, p53-mediated anoikis: fibronectin and integrin alphav mediate survival signals through focal adhesion kinase. J Biol Chem. 2004;279:48342–9.
Chung AS, Ferrara N. Developmental and pathological angiogenesis. Annu Rev Cell Dev Biol. 2011;27:563–84.
Kipps E, Tan DS, Kaye SB. Meeting the challenge of ascites in ovarian cancer: new avenues for therapy and research. Nat Rev Cancer. 2013;13:273–82.
Arnaoutova I, Kleinman HK. In vitro angiogenesis: endothelial cell tube formation on gelled basement membrane extract. Nat Protoc. 2010;5:628–35.
Kastana P, Zahra FT, Ntenekou D, Katraki-Pavlou S, Beis D, Lionakis MS, et al. Matrigel plug assay for in vivo evaluation of angiogenesis. Methods Mol Biol. 2019;1952:219–32.
Mazzone M, Bergers G. Regulation of blood and lymphatic vessels by immune cells in tumors and metastasis. Annu Rev Physiol. 2019;81:535–60.
Toubal A, Clément K, Fan R, Ancel P, Pelloux V, Rouault C, et al. SMRT-GPS2 corepressor pathway dysregulation coincides with obesity-linked adipocyte inflammation. J Clin Invest. 2013;123:362–79.
Guo L, Li X, Tang QQ. Transcriptional regulation of adipocyte differentiation: a central role for CCAAT/enhancer-binding protein (C/EBP) β. J Biol Chem. 2015;290:755–61.
Kim EK, Choi EJ. Pathological roles of MAPK signaling pathways in human diseases. Biochim Biophys Acta. 2010;1802:396–405.
Hosaka K, Yang Y, Seki T, Fischer C, Dubey O, Fredlund E, et al. Pericyte-fibroblast transition promotes tumor growth and metastasis. Proc Natl Acad Sci U.S.A. 2016;113:E5618–27.
Paoli P, Giannoni E, Chiarugi P. Anoikis molecular pathways and its role in cancer progression. Biochim Biophys Acta. 2013;1833:3481–98.
Avivar-Valderas A, Bobrovnikova-Marjon E, Alan Diehl J, Bardeesy N, Debnath J, Aguirre-Ghiso JA. Regulation of autophagy during ECM detachment is linked to a selective inhibition of mTORC1 by PERK. Oncogene.2013;32:4932–40.
Chen N, Debnath J. IκB kinase complex (IKK) triggers detachment-induced autophagy in mammary epithelial cells independently of the PI3K-AKT-MTORC1 pathway. Autophagy.2013;9:1214–27.
Locasale JW, Grassian AR, Melman T, Lyssiotis CA, Mattaini KR, Bass AJ, et al. Phosphoglycerate dehydrogenase diverts glycolytic flux and contributes to oncogenesis. Nat Genet. 2011;43:869–74.
Schafer ZT, Grassian AR, Song L, Jiang Z, Gerhart-Hines Z, Irie HY, et al. Antioxidant and oncogene rescue of metabolic defects caused by loss of matrix attachment. Nature.2009;461:109–13.
Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002;29:15–8.
De Palma M, Biziato D, Petrova TV. Microenvironmental regulation of tumour angiogenesis. Nat Rev Cancer. 2017;17:457–74.
Liu Y, Cao X. Characteristics and significance of the pre-metastatic niche. Cancer Cell. 2016;30:668–81.
Nissen LJ, Cao R, Hedlund EM, Wang Z, Zhao X, Wetterskog D, et al. Angiogenic factors FGF2 and PDGF-BB synergistically promote murine tumor neovascularization and metastasis. J Clin Invest. 2007;117:2766–77.
Hosaka K, Yang Y, Seki T, Nakamura M, Andersson P, Rouhi P, et al. Tumour PDGF-BB expression levels determine dual effects of anti-PDGF drugs on vascular remodelling and metastasis. Nat Commun. 2013;4:2129.
Liu T, Ma W, Xu H, Huang M, Zhang D, He Z, et al. PDGF-mediated mesenchymal transformation renders endothelial resistance to anti-VEGF treatment in glioblastoma. Nat Commun. 2018;9:3439.
Jitariu AA, Raica M, Cîmpean AM, Suciu SC. The role of PDGF-B/PDGFR-BETA axis in the normal development and carcinogenesis of the breast. Crit Rev Oncol Hematol. 2018;131:46–52.
Tamura A, Hirai H, Yokota A, Kamio N, Sato A, Shoji T, et al. C/EBPβ is required for survival of Ly6C(-) monocytes. Blood.2017;130:1809–18.
Cheng P, Chen Y, He TL, Wang C, Guo SW, Hu H, et al. Menin coordinates C/EBPβ-mediated TGF-β signaling for epithelial-mesenchymal transition and growth inhibition in pancreatic cancer. Mol Ther Nucleic Acids. 2019;18:155–65.
Carro MS, Lim WK, Alvarez MJ, Bollo RJ, Zhao X, Snyder EY, et al. The transcriptional network for mesenchymal transformation of brain tumours. Nature.2010;463:318–25.
Gomis RR, Alarcón C, Nadal C, Van Poznak C, Massagué J. C/EBPbeta at the core of the TGFbeta cytostatic response and its evasion in metastatic breast cancer cells. Cancer Cell. 2006;10:203–14.
Zhang YY, Li SF, Qian SW, Zhang YY, Liu Y, Tang QQ, et al. Phosphorylation prevents C/EBPβ from the calpain-dependent degradation. Biochem Biophys Res Commun. 2012;419:550–5.
Wagner EF, Nebreda AR. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat Rev Cancer. 2009;9:537–49.
Ma W, Xu Z, Wang Y, Li W, Wei Z, Chen T, et al. A positive feedback loop of SLP2 activates MAPK signaling pathway to promote gastric cancer progression. Theranostics.2018;8:5744–57.
Wang S, Wang X, Gao Y, Peng Y, Dong N, Xie Q, et al. RN181 is a tumour suppressor in gastric cancer by regulation of the ERK/MAPK-cyclin D1/CDK4 pathway. J Pathol. 2019;248:204–16.
Chen X, Chen X, Zhang X, Wang L, Cao P, Rajamanickam V, et al. Curcuminoid B63 induces ROS-mediated paraptosis-like cell death by targeting TrxR1 in gastric cells. Redox Biol. 2019;21:101061.
Wang X, Liang Q, Zhang L, Gou H, Li Z, Chen H, et al. C8orf76 promotes gastric tumorigenicity and metastasis by directly inducing lncRNA DUSP5P1 and associates with patient outcomes. Clin Cancer Res. 2019;25:3128–40.
Pontes-Quero S, Fernández-Chacón M, Luo W, Lunella FF, Casquero-Garcia V, Garcia-Gonzalez I, et al. High mitogenic stimulation arrests angiogenesis. Nat Commun. 2019;10:2016.
Zabielska-Koczywąs K, Wojtkowska A, Dolka I, Małek A, Walewska M, Wojtalewicz A, et al. 3D chick embryo chorioallantoic membrane model as an in vivo model to study morphological and histopathological features of feline fibrosarcomas. BMC Vet Res. 2017;13:201.
Xu YZ, Kanagaratham C, Jancik S, Radzioch D. Promoter deletion analysis using a dual-luciferase reporter system. Methods Mol Biol. 2013;977:79–93.
Shen X, Pasha MA, Hidde K, Khan A, Liang M, Guan W, et al. Group 2 innate lymphoid cells promote airway hyperresponsiveness through production of VEGFA. J Allergy Clin Immunol. 2018;141:1929–31.e4.
Jin L, Chun J, Pan C, Kumar A, Zhang G, Ha Y, et al. The PLAG1-GDH1 axis promotes anoikis resistance and tumor metastasis through CamKK2-AMPK signaling in LKB1-deficient lung cancer. Mol Cell. 2018;69:87–99.e7.
Acknowledgements
We are particularly grateful to Dr. Chong Sun (German Cancer Research Center, Heidelberg, Germany) for rigorous guidance.
Author information
Authors and Affiliations
Contributions
SD, ZY, EX, MZ, WL, JM, XW and HY performed the experiments; XZ, HC and LS analysed data; WG, XX and XL provided the samples; SD and SY wrote the paper; WG and SY commented on the study and revised the paper; SD, WG, XX and XL designed the research.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The use of human samples was approved by the Ethics Committee of the Nanjing Drum Tower Hospital of Nanjing University Medical School. Written informed consents were obtained from all patients. The study was performed according to the guideline expressed in the Declaration of Helsinki.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Du, S., Yang, Z., Lu, X. et al. Anoikis resistant gastric cancer cells promote angiogenesis and peritoneal metastasis through C/EBPβ-mediated PDGFB autocrine and paracrine signaling. Oncogene 40, 5764–5779 (2021). https://doi.org/10.1038/s41388-021-01988-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-021-01988-y
This article is cited by
-
Deciphering the significance of anoikis in bladder cancer and systematic analysis of S100A7 as a potential therapeutic target
European Journal of Medical Research (2024)
-
Biodegradable nanoplatform upregulates tumor microenvironment acidity for enhanced cancer therapy via synergistic induction of apoptosis, ferroptosis, and anti-angiogenesis
Journal of Nanobiotechnology (2023)
-
Extracellular matrix remodeling in tumor progression and immune escape: from mechanisms to treatments
Molecular Cancer (2023)
-
Development of a prognostic model to predict BLCA based on anoikis-related gene signature: preliminary findings
BMC Urology (2023)
-
PDGFB-targeted functional MRI nanoswitch for activatable T1–T2 dual-modal ultra-sensitive diagnosis of cancer
Journal of Nanobiotechnology (2023)