Abstract
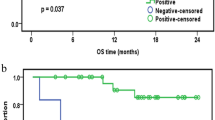
Extramedullary infiltration (EMI), as a concomitant symptom of acute myeloid leukemia (AML), is associated with low complete remission and poor prognosis in AML. However, the mechanism of EMI remains indistinct. Clinical trials showed that increased miR-29s were associated with a poor overall survival in AML [14]. Nevertheless, they were proved to work as tumor suppressor genes by encouraging apoptosis and inhibiting proliferation in vitro. These contradictory results led us to the hypothesis that miR-29s may play a notable role in the prognosis of AML rather than leukemogenesis. Thus, we explored the specimens of AML patients and addressed this issue into miR-29c&b2 knockout mice. As a result, a poor overall survival and invasive blast cells were observed in high miR-29c&b2-expression patients, and the wildtype mice presented a shorter survival with heavier leukemia infiltration in extramedullary organs. Subsequently, we found that the miR-29c&b2 inside leukemia cells promoted EMI, but not the one in the microenvironment. The analysis of signal pathway revealed that miR-29c&b2 could target HMG-box transcription factor 1 (Hbp1) directly, then reduced Hbp1 bound to the promoter of non-muscle myosin IIB (Myh10) as a transcript inhibitor. Thus, increased Myh10 encouraged the migration of leukemia cells. Accordingly, AML patients with EMI were confirmed to have high miR-29c&b2 and MYH10 with low HBP1. Therefore, we identify that miR-29c&b2 contribute to the poor prognosis of AML patients by promoting EMI, and related genes analyses are prospectively feasible in assessment of AML outcome.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Dohner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Buchner T, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129:424–47.
Estey EH. Acute myeloid leukemia: 2019 update on risk-stratification and management. Am J Hematol. 2018;93:1267–91.
Junge A, Bacher U, Mueller BU, Keller P, Solenthaler M, Pabst T. Adverse outcome of AML with aberrant CD16 and CD56 NK cell marker expression. Hematol Oncol. 2018. [Online ahead of print].
Patel SS, Kuo FC, Gibson CJ, Steensma DP, Soiffer RJ, Alyea EP, et al. High NPM1-mutant allele burden at diagnosis predicts unfavorable outcomes in de novo AML. Blood. 2018;131:2816–25.
Byrd JC, Mrozek K, Dodge RK, Carroll AJ, Edwards CG, Arthur DC, et al. Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse and overall survival in adult patients with de novo acute myeloid leukemia. Blood. 2002;100:4325–36.
Chang H, Brandwein J, Yi QL, Chun K, Patterson B, Brien B. Extramedullary infiltrates of AML are associated with CD56 expression, 11q23 abnormalities and inferior clinical outcome. Leuk Res. 2004;28:1007–11.
Grimwade D, Walker H, Oliver F, Wheatley K, Harrison C, Harrison G, et al. The importance of diagnostic cytogenetics on outcome in AML: analysis of 1,612 patients entered into the MRC AML 10 trial. Blood. 1998;92:2322–33.
Zhou T, Bloomquist MS, Ferguson LS, Reuther J, Marcogliese AN, Elghetany MT, et al. Pediatric myeloid sarcoma: a single institution clinicopathologic and molecular analysis. Pediatr Hematol Oncol. 2020;37:76–89.
Hu GH, Lu AD, Jia YP, Zuo YX, Wu J, Zhang LP. Prognostic impact of extramedullary infiltration in pediatric low-risk acute myeloid leukemia: a retrospective single-center study over 10 years. Clin Lymphoma Myeloma Leuk. 2020;20:e813–20.
Kobayashi R, Tawa A, Hanada R, Horibe K, Tsuchida M, Tsukimoto I, et al. Extramedullary infiltration at diagnosis and prognosis in children with acute myelogenous leukemia. Pediatr Blood Cancer. 2007;48:393–8.
Xu J, Zhang W, Yan XJ, Lin XQ, Li W, Mi JQ, et al. DNMT3A mutation leads to leukemic extramedullary infiltration mediated by TWIST1. J Hematol Oncol. 2016;9:106.
Garzon R, Heaphy CE, Havelange V, Fabbri M, Volinia S, Tsao T, et al. MicroRNA 29b functions in acute myeloid leukemia. Blood. 2009;114:5331–41.
Gong JN, Yu J, Lin HS, Zhang XH, Yin XL, Xiao Z, et al. The role, mechanism and potentially therapeutic application of microRNA-29 family in acute myeloid leukemia. Cell Death Differ. 2014;21:100–12.
Butrym A, Rybka J, Baczynska D, Poreba R, Kuliczkowski K, Mazur G. Clinical response to azacitidine therapy depends on microRNA-29c (miR-29c) expression in older acute myeloid leukemia (AML) patients. Oncotarget. 2016;7:30250–7.
Li Y, Cai BL, Shen LL, Dong Y, Lu Q, Sun SK, et al. MiRNA-29b suppresses tumor growth through simultaneously inhibiting angiogenesis and tumorigenesis by targeting Akt3. Cancer Lett. 2017;397:111–9.
Xu WW, Li B, Guan XY, Chung SK, Wang Y, Yip YL, et al. Cancer cell-secreted IGF2 instigates fibroblasts and bone marrow-derived vascular progenitor cells to promote cancer progression. Nat Commun. 2017;8:14399.
Zhu J, Xiong G, Fu H, Evers BM, Zhou BP, Xu R. Chaperone Hsp47 drives malignant growth and invasion by modulating an ECM gene network. Cancer Res. 2015;75:1580–91.
Zhu LH, Miao XT, Wang NY. Integrated miRNA-mRNA analysis of Epstein-Barr virus-positive nasopharyngeal carcinoma. Genet Mol Res. 2015;14:6028–36.
Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–5.
Chen W, Qin H, Chesebro B, Cheever MA. Identification of a gag-encoded cytotoxic T-lymphocyte epitope from FBL-3 leukemia shared by Friend, Moloney, and Rauscher murine leukemia virus-induced tumors. J Virol. 1996;70:7773–82.
Dawson PJ, Fieldsteel AH, Bostick WL. Pathologic studies of Friend virus leukemia and the development of a transplantable tumor in BALB/c mice. Cancer Res. 1963;23:349–54.
De Harven E, Friend C. Further electron microscope studies of a mouse leukemia induced by cell-free filtrates. J Biophys Biochem Cytol. 1960;7:747–52.
Liu JH, Zhou F, ZX L, Zhang SF. Pathological findings of multi-organ invasion during autopsy of 101 patients with acute leukemia. Chin J Practical Intern Med. 2014;034:1112–3.
Thiele J, Laubert A, Vykoupil KF, Georgii A. Autopsy and clinical findings in acute leukemia and chronic myeloproliferative diseases-an evaluation of 104 patients. Pathol Res Pr. 1985;179:328–36.
Viadana E, Bross ID, Pickren JW. An autopsy study of the metastatic patterns of human leukemias. Oncology. 1978;35:87–96.
Zhu C, Wang Y, Kuai W, Sun X, Chen H, Hong Z. Prognostic value of miR-29a expression in pediatric acute myeloid leukemia. Clin Biochem. 2013;46:49–53.
Cochrane DR, Jacobsen BM, Connaghan KD, Howe EN, Bain DL, Richer JK. Progestin regulated miRNAs that mediate progesterone receptor action in breast cancer. Mol Cell Endocrinol. 2012;355:15–24.
Rostas JW III, Pruitt HC, Metge BJ, Mitra A, Bailey SK, Bae S, et al. microRNA-29 negatively regulates EMT regulator N-myc interactor in breast cancer. Mol Cancer. 2014;13:200.
Sun XJ, Liu BY, Yan S, Jiang TH, Cheng HQ, Jiang HS, et al. MicroRNA-29a promotes pancreatic cancer growth by inhibiting tristetraprolin. Cell Physiol Biochem. 2015;37:707–18.
Fabbri M, Garzon R, Cimmino A, Liu Z, Zanesi N, Callegari E, et al. MicroRNA-29 family reverts aberrant methylation in lung cancer by targeting DNA methyltransferases 3A and 3B. Proc Natl Acad Sci USA. 2007;104:15805–10.
Mott JL, Kobayashi S, Bronk SF, Gores GJ. mir-29 regulates Mcl-1 protein expression and apoptosis. Oncogene. 2007;26:6133–40.
Robaina MC, Mazzoccoli L, Arruda VO, Reis FR, Apa AG, de Rezende LM, et al. Deregulation of DNMT1, DNMT3B and miR-29s in Burkitt lymphoma suggests novel contribution for disease pathogenesis. Exp Mol Pathol. 2015;98:200–7.
Zhao JJ, Lin JH, Lwin T, Yang H, Guo JP, Kong W, et al. microRNA expression profile and identification of miR-29 as a prognostic marker and pathogenetic factor by targeting CDK6 in mantle cell lymphoma. Blood. 2010;115:2630–9.
Hugo HJ, Gunasinghe N, Hollier BG, Tanaka T, Blick T, Toh A, et al. Epithelial requirement for in vitro proliferation and xenograft growth and metastasis of MDA-MB-468 human breast cancer cells: oncogenic rather than tumor-suppressive role of E-cadherin. Breast Cancer Res. 2017;19:86.
Padmanaban V, Krol I, Suhail Y, Szczerba BM, Aceto N, Bader JS, et al. E-cadherin is required for metastasis in multiple models of breast cancer. Nature. 2019;573:439.
Sun R, Xie HY, Qian JX, Huang YN, Yang F, Zhang FL, et al. FBXO22 possesses both protumorigenic and antimetastatic roles in breast cancer progression. Cancer Res. 2018;78:5274–86.
Kwon JJ, Factora TD, Dey S, Kota J. A systematic review of miR-29 in cancer. Mol Ther Oncolytics. 2019;12:173–94.
Chen X, Yue B, Zhang C, Qi M, Qiu J, Wang Y, et al. MiR-130a-3p inhibits the viability, proliferation, invasion, and cell cycle, and promotes apoptosis of nasopharyngeal carcinoma cells by suppressing BACH2 expression. Biosci Rep. 2017;37:BSR20160576.
Le Goff C, Cormier-Daire V. The ADAMTS(L) family and human genetic disorders. Hum Mol Genet. 2011;20:R163–7.
Paulson KE, Rieger-Christ K, McDevitt MA, Kuperwasser C, Kim J, Unanue VE, et al. Alterations of the HBP1 transcriptional repressor are associated with invasive breast cancer. Cancer Res. 2007;67:6136–45.
Qu X, Gao D, Ren Q, Jiang X, Bai J, Sheng L. miR-211 inhibits proliferation, invasion and migration of cervical cancer via targeting SPARC. Oncol Lett. 2018;16:853–60.
Bollaert E, Serra AD, Demoulin JB. The HMG box transcription factor HBP1: a cell cycle inhibitor at the crossroads of cancer signaling pathways. Cell Mol Life Sci. 2019;76:1529–39.
Han YC, Park CY, Bhagat G, Zhang J, Wang Y, Fan JB, et al. microRNA-29a induces aberrant self-renewal capacity in hematopoietic progenitors, biased myeloid development, and acute myeloid leukemia. J Exp Med. 2010;207:475–89.
Sun X, Geng X, Zhang J, Zhao H, Liu Y. miR-155 promotes the growth of osteosarcoma in a HBP1-dependent mechanism. Mol Cell Biochem. 2015;403:139–47.
Wu H, Ng R, Chen X, Steer CJ, Song G. MicroRNA-21 is a potential link between non-alcoholic fatty liver disease and hepatocellular carcinoma via modulation of the HBP1-p53-Srebp1c pathway. Gut. 2016;65:1850–60.
Yan Z, Wang J, Wang C, Jiao Y, Qi W, Che S. miR-96/HBP1/Wnt/β-catenin regulatory circuitry promotes glioma growth. FEBS Lett. 2014;588:3038–46.
Yang Z, Wu L, Zhu X, Xu J, Jin R, Li G, et al. MiR-29a modulates the angiogenic properties of human endothelial cells. Biochem Biophys Res Commun. 2013;434:143–9.
Su CY, Cheng X, Li YS, Han Y, Song XY, Yu DP, et al. MiR-21 improves invasion and migration of drug-resistant lung adenocarcinoma cancer cell and transformation of EMT through targeting HBP1. Cancer Med. 2018;7:2485–503.
Lackie JM. Crawling movement. Cell movement and cell behaviour. Berlin: Springer Netherlands.1986. pp 145–74.
Robert-Paganin J, Pylypenko O, Kikuti C, Sweeney HL, Houdusse A. Force generation by myosin motors: a structural perspective. Chem Rev. 2020;120:5–35.
Kiani FA, Fischer S. Catalytic strategy used by the myosin motor to hydrolyze ATP. Proc Natl Acad Sci USA. 2014;111:E2947–56.
Gutzman JH, Sahu SU, Kwas C. Non-muscle myosin IIA and IIB differentially regulate cell shape changes during zebrafish brain morphogenesis. Dev Biol. 2015;397:103–15.
Ridge LA, Mitchell K, Al-Anbaki A, Qureshi WMS, Stephen LA, Tenin G, et al. Non-muscle myosin IIB (Myh10) is required for epicardial function and coronary vessel formation during mammalian development. Plos Genet. 2017;13:e1007068.
Wang Y, Yang Q, Cheng Y, Gao M, Kuang L, Wang C. Myosin heavy chain 10 (MYH10) gene silencing reduces cell migration and invasion in the glioma cell lines U251, T98G, and SHG44 by inhibiting the Wnt/β-Catenin pathway. Med Sci Monit. 2018;24:9110–9.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (No. 81870132, 81261120568) and the Science and Technology Commission of Shanghai Municipality (18DZ2290700,18DZ2293500).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval and consent to participate
All human bone marrow specimens were collected from patients diagnosed with AML at the Shanghai Ninth People’s Hospital, China, from 2016 to 2020. Informed consent was obtained from all patients and the project was approved by the Ethics Committee of Shanghai Ninth People’s Hospital. Six- to eight-week-old C57BL/6 or miR-29c&b2 knockout C57BL/6 mice were purchased from the Shanghai Model Organisms Center, Inc (Shanghai, China). Six- to eight-week-old NOD-SCID mice were purchased from Shanghai SLAC Laboratory Animal Co., Ltd (Shanghai, China). All experiments with mice were performed strictly in accordance with a protocol approved by the Administrative Panel on Laboratory Animal Care of the Shanghai Ninth People’s Hospital.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Wei, Y., Lu, W., Yu, Y. et al. miR-29c&b2 encourage extramedullary infiltration resulting in the poor prognosis of acute myeloid leukemia. Oncogene 40, 3434–3448 (2021). https://doi.org/10.1038/s41388-021-01775-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-021-01775-9