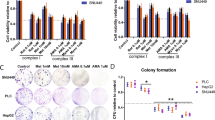
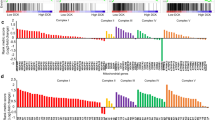
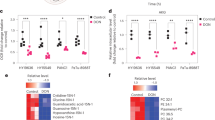
Abstract
Pancreatic ductal adenocarcinoma (PDA) is aggressive cancer characterized by rapid progression, metastatic recurrence, and highly resistant to treatment. PDA cells exhibit aerobic glycolysis, or the Warburg effect, which reduces the flux of pyruvate into mitochondria. As a result, more glycolytic metabolites are shunted to pathways for the production of building blocks (e.g., ribose) and reducing agents (e.g., NADPH) for biosynthesis that are necessary for cell proliferation. In addition, PDA cells are highly addicted to glutamine for both maintaining biosynthetic pathways and achieving redox balance. Mitochondrial uncoupling facilitates proton influx across the mitochondrial inner membrane without generating ATP, leading to a futile cycle that consumes glucose metabolites and glutamine. We synthesized a new mitochondrial uncoupler MB1-47 and tested its effect on cancer cell metabolism and the anticancer activity in pancreatic cancer cell models and murine tumor transplantation models. MB1-47 uncouples mitochondria in the pancreatic cancer cells, resulting in: (1) the acceleration of pyruvate oxidation and TCA turnover; (2) increases in AMP/ATP and ADP/AMP ratios; and (3) a decrease in the synthesis rate of nucleotides and sugar nucleotides. Moreover, MB1-47 arrests cell cycle at G0–G1 phase, reduces clonogenicity, and inhibits cell growth of murine and human pancreatic cancer cells. In vivo studies showed that MB1-47 inhibits tumor growth in murine tumor transplantation models, and inhibits the hepatic metastasis when tumor cells were transplanted intrasplenically. Our results provide proof of concept for a potentially new strategy of treating PDA, and a novel prototype experimental drug for future studies and development.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Siegel R, Naishadham D, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29.
Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–21.
Mimeault M, Brand RE, Sasson AA, Batra SK. Recent advances on the molecular mechanisms involved in pancreatic cancer progression and therapies. Pancreas. 2005;31:301–16.
Stathis A, Moore MJ. Advanced pancreatic carcinoma: current treatment and future challenges. Nat Rev Clin Oncol. 2010;7:163–72.
Gillen S, Schuster T, Meyer zum Büschenfelde C, Friess H, Kleeff J. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLOS Med. 2010;7:e1000267.
Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–25.
Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605–17.
Maitra A, Hruban RH. Pancreatic cancer. Annu Rev Pathol. 2008;3:157–88.
Efthimiou E, Crnogorac-Jurcevic T, Lemoine NR. Pancreatic cancer genetics. Pancreatology. 2001;1:571–5.
Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801–6.
Zhou W, Capello M, Fredolini C, Racanicchi L, Piemonti L, Liotta LA, et al. Proteomic analysis reveals Warburg effect and anomalous metabolism of glutamine in pancreatic cancer cells. J Proteome Res. 2012;11:554–63.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74.
Blum R, Kloog Y. Metabolism addiction in pancreatic cancer. Cell Death Dis. 2014;5:e1065.
Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–33.
DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7:11–20.
Faubert B, Boily G, Izreig S, Griss T, Samborska B, Dong Z, et al. AMPK is a negative regulator of the Warburg effect and suppresses tumor growth in vivo. Cell Metab. 2013;17:113–24.
Lunt SY, Vander Heiden MG. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu Rev Cell Dev Biol. 2011;27:441–64.
Levine B. Cell biology: autophagy and cancer. Nature. 2007;446:745–7.
Levine AJ, Puzio-Kuter AM. The control of the metabolic switch in cancers by oncogenes and tumor suppressor genes. Science. 2010;330:1340–4.
Wise DR, DeBerardinis RJ, Mancuso A, Sayed N, Zhang XY, Pfeiffer HK, et al. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc Natl Acad Sci USA. 2008;105:18782–7.
Livingstone LR, White A, Sprouse J, Livanos E, Jacks T, Tlsty TD. Altered cell cycle arrest and gene amplification potential accompany loss of wild-type p53. Cell. 1992;70:923–35.
DeBerardinis RJ, Mancuso A, Daikhin E, Nissim I, Yudkoff M, Wehrli S, et al. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci. 2007;104:19345–50.
Son J, Lyssiotis CA, Ying H, Wang X, Hua S, Ligorio M, et al. Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature. 2013;496:101–5.
Jastroch M, Divakaruni AS, Mookerjee S, Treberg JR, Brand MD. Mitochondrial proton and electron leaks. Essays Biochem. 2010;47:53–67.
Harper J, Dickinson K, Brand M. Mitochondrial uncoupling as a target for drug development for the treatment of obesity. Obes Rev. 2001;2:255–65.
Parascandola J. Dinitrophenol and bioenergetics: an historical perspective. Mol Cell Biochem. 1974;5:69–77.
Frayha GJ, Smyth JD, Gobert JG, Savel J. The mechanisms of action of antiprotozoal and anthelmintic drugs in man. Gen Pharm. 1997;28:273–99.
Tao H, Zhang Y, Zeng X, Shulman GI, Jin S. Niclosamide ethanolamine-induced mild mitochondrial uncoupling improves diabetic symptoms in mice. Nat Med. 2014;20:1263–9.
Alasadi A, Chen M, Swapna GVT, Tao H, Guo J, Collantes J, et al. Effect of mitochondrial uncouplers niclosamide ethanolamine (NEN) and oxyclozanide on hepatic metastasis of colon cancer. Cell Death Dis. 2018;9:215.
Wang Y, Nasiri AR, Damsky WE, Perry CJ, Zhang XM, Rabin-Court A, et al. Uncoupling hepatic oxidative phosphorylation reduces tumor growth in two murine models of colon cancer. Cell Rep. 2018;24:47–55.
Shimizu M, Yano E. Mutagenicity of mono-nitrobenzene derivatives in the Ames test and rec assay. Mutat Res. 1986;170:11–22.
Terada H. Uncouplers of oxidative phosphorylation. Environ Health Perspect. 1990;87:213–8.
Sun S-Y, Yue P, Dawson MI, Shroot B, Michel S, Lamph WW, et al. Differential effects of synthetic nuclear retinoid receptor-selective retinoids on the growth of human non-small cell lung carcinoma cells. Cancer Res. 1997;57:4931–9.
Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet. 2004;363:1049–57.
Wang W, Guan KL. AMP-activated protein kinase and cancer. Acta Physiol. 2009;196:55–63.
Fryer LG, Parbu-Patel A, Carling D. The anti-diabetic drugs rosiglitazone and metformin stimulate AMP-activated protein kinase through distinct signaling pathways. J Biol Chem. 2002;277:25226–32.
Xu J, Ji J, Yan X-H. Cross-talk between AMPK and mTOR in regulating energy balance. Crit Rev Food Sci Nutr. 2012;52:373–81.
Acknowledgements
The authors want to thank Frank Leu for reviewing and editing the paper. The project was partially funded by Rutgers Busch Biomedical Award (2014), HCED Iraq Scholarship program, New Jersey Cancer Commission Research (NJCCR) Fellowship, and Mito BioPharma, LLC. AA is supported by HCED Iraq Scholarship program. HT was supported in part by NJCCR. AA, HT, JG, and SJ are partially supported by NIH (1R21CA216604 and 1R21 AA027050).
Author information
Authors and Affiliations
Contributions
Conception and design: SJ and AA. Development of methodology: AA, SJ, BC, and DA. Acquisition of data: BC synthesized the compound, AA conducted most of the biological experiments, AA and HT conducted OCR Seahorse experiment, AA, JG, and XS conducted the LC-MS experiment. Analysis and interpretation of data (e.g., statistical analysis, LC-MS): AA, XS, and SJ. Writing, review, and/or revision of the paper: AA and SJ. Study supervision: SJ.
Corresponding author
Ethics declarations
Conflict of interest
SJ is a founder of Mito BioPharma, which has a license right from Rutgers University for developing safe mitochondrial uncouplers for treating cancer and metabolic diseases. BC, HT, and DA are co-inventors of the patents covering the rights of MB1-47 and its derivatives. The rest of the authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Alasadi, A., Cao, B., Guo, J. et al. Mitochondrial uncoupler MB1-47 is efficacious in treating hepatic metastasis of pancreatic cancer in murine tumor transplantation models. Oncogene 40, 2285–2295 (2021). https://doi.org/10.1038/s41388-021-01688-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-021-01688-7