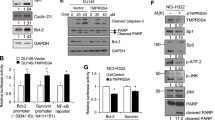
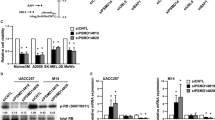
Abstract
CD147, also known as extracellular matrix metalloproteinase inducer (EMMPRIN), is a transmembrane glycoprotein that is highly expressed in tumor cells, particularly melanoma cells, and plays critical roles in tumor cell metastasis through the regulation of matrix metalloprotease (MMP) expression. In this study, we identified Fyn as a novel interacting protein of CD147. Fyn is a member of the Src family of nonreceptor tyrosine kinases that regulates diverse physiological processes, such as T lymphocyte differentiation, through the TCR signaling pathway. Our findings demonstrated that Fyn directly phosphorylates CD147 at Y140 and Y183. Two phosphospecific antibodies against Y140 and Y183 were developed to validate the phosphorylation of CD147 by Fyn. Moreover, the CD147-FF (Y140F/Y183F) mutation impaired the interaction between CD147 and GnT-V, leading to decreased CD147 glycosylation and membrane recruitment. In addition, CD147-FF significantly blocked MMP-9 expression as well as cell migration. Moreover, we found that Fyn is overexpressed in clinical melanoma tissues as well as in melanoma cell lines. Knockdown of Fyn expression markedly attenuated the malignant phenotype of melanoma cells in vitro and in vivo through downregulation of CD147 phosphorylation, indicating that Fyn/CD147 is a potential target molecule in melanoma treatment. Finally, through virtual screening, we identified amodiaquine as a potential inhibitor targeting the Fyn/CD147 axis. Amodiaquine treatment dramatically inhibited the phosphorylation of CD147 by Fyn, thus attenuating melanoma cell growth and invasion in vitro and in vivo, suggesting that amodiaquine is a promising inhibitor for melanoma treatment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
22 February 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41388-021-01641-8
References
Watanabe A, Hoshino D, Koshikawa N, Seiki M, Suzuki T, Ichikawa K. Critical role of transient activity of MT1-MMP for ECM degradation in invadopodia. PLoS Comput Biol. 2013;9:e1003086.
Zhang J, Huang X, Wang L. Pioglitazone inhibits the expression of matrix metalloproteinase-9, a protein involved in diabetes-associated wound healing. Mol Med Rep. 2014;10:1084–8.
Moro N, Mauch C, Zigrino P. Metalloproteinases in melanoma. Eur J Cell Biol. 2014;93:23–9.
Villanueva J, Herlyn M. Melanoma and the tumor microenvironment. Curr Oncol Rep. 2008;10:439–46.
Kuphal S, Bosserhoff A. Recent progress in understanding the pathology of malignant melanoma. J Pathol. 2009;219:400–9.
Vultur A, Herlyn M. SnapShot: melanoma. Cancer Cell 2013;23:706–e1.
Su J, Gao T, Jiang M, Wu L, Zeng W, Zhao S, et al. CD147 silencing inhibits tumor growth by suppressing glucose transport in melanoma. Oncotarget 2016;7:64778–84.
Huang W, Luo WJ, Zhu P, Tang J, Yu XL, Cui HY, et al. Modulation of CD147-induced matrix metalloproteinase activity: role of CD147 N-glycosylation. Biochem J. 2013;449:437–48.
Saito YD, Jensen AR, Salgia R, Posadas EM. Fyn: a novel molecular target in cancer. Cancer 2010;116:1629–37.
Susa M, Rohner D, Bichsel S. Differences in binding of PI 3-kinase to the src-homology domains 2 and 3 of p56 lck and p59 fyn tyrosine kinases. Biochem Biophys Res Commun. 1996;220:729–34.
Raab M, Cai YC, Bunnell SC, Heyeck SD, Berg LJ, Rudd CE. p56Lck and p59Fyn regulate CD28 binding to phosphatidylinositol 3-kinase, growth factor receptor-bound protein GRB-2, and T cell-specific protein-tyrosine kinase ITK: implications for T-cell costimulation. Proc Natl Acad Sci USA. 1995;92:8891–5.
Zeng W, Su J, Wu L, Yang D, Long T, Li D, et al. CD147 promotes melanoma progression through hypoxia-induced MMP2 activation. Curr Mol Med. 2014;14:163–73.
Zhou J, Zhu P, Jiang JL, Zhang Q, Wu ZB, Yao XY, et al. Involvement of CD147 in overexpression of MMP-2 and MMP-9 and enhancement of invasive potential of PMA-differentiated THP-1. BMC Cell Biol. 2005;6:25.
Su J, Chen X, Kanekura T. A CD147-targeting siRNA inhibits the proliferation, invasiveness, and VEGF production of human malignant melanoma cells by down-regulating glycolysis. Cancer Lett. 2009;273:140–7.
Mauris J, Woodward AM, Cao Z, Panjwani N, Argueso P. Molecular basis for MMP9 induction and disruption of epithelial cell-cell contacts by galectin-3. J Cell Sci. 2014;127(Pt 14):3141–8.
Wu JB, Shen L, Qiu L, Duan QW, Luo ZG, Dong XX. Reversal effect of GnT-V on the radioresistance of human nasopharyngeal carcinoma cells by alteration beta1, 6-GlcNAc branched N-glycans. Int J Clin Exp Pathol. 2015;8:9901–11.
Cui J, Huang W, Wu B, Jin J, Jing L, Shi WP, et al. N-glycosylation by N-acetylglucosaminyltransferase V enhances the interaction of CD147/basigin with integrin beta1 and promotes HCC metastasis. J Pathol. 2018;245:41–52.
Gruszewska E, Chrostek L. The alterations of glycosylation in malignant diseases. Pol Merkur Lekarski. 2013;34:58–61.
Mehner C, Hockla A, Miller E, Ran S, Radisky DC, Radisky ES. Tumor cell-produced matrix metalloproteinase 9 (MMP-9) drives malignant progression and metastasis of basal-like triple negative breast cancer. Oncotarget 2014;5:2736–49.
Liao CG, Kong LM, Song F, Xing JL, Wang LX, Sun ZJ, et al. Characterization of basigin isoforms and the inhibitory function of basigin-3 in human hepatocellular carcinoma proliferation and invasion. Mol Cell Biol. 2011;31:2591–604.
Kanekura T, Chen X. CD147/basigin promotes progression of malignant melanoma and other cancers. J Dermatol Sci. 2010;57:149–54.
Kanekura T, Chen X, Kanzaki T. Basigin (CD147) is expressed on melanoma cells and induces tumor cell invasion by stimulating production of matrix metalloproteinases by fibroblasts. Int J Cancer. 2002;99:520–8.
Long T, Su J, Tang W, Luo Z, Liu S, Liu Z, et al. A novel interaction between calcium-modulating cyclophilin ligand and Basigin regulates calcium signaling and matrix metalloproteinase activities in human melanoma cells. Cancer Lett. 2013;339:93–101.
Rey MC, Bonamigo RR, Cartell A, Furian R, Bonfa R, Bonfa R. MMP-2 and TIMP-2 in cutaneous melanoma: association with prognostic factors and description in cutaneous metastases. Am J Dermatopathol. 2011;33:413–4.
Cotignola J, Reva B, Mitra N, Ishill N, Chuai S, Patel A, et al. Matrix Metalloproteinase-9 (MMP-9) polymorphisms in patients with cutaneous malignant melanoma. BMC Med Genet. 2007;8:10.
Liang X, Lu Y, Wilkes M, Neubert TA, Resh MD. The N-terminal SH4 region of the Src family kinase Fyn is modified by methylation and heterogeneous fatty acylation: role in membrane targeting, cell adhesion, and spreading. J Biol Chem. 2004;279:8133–9.
Elias D, Ditzel HJ. Fyn is an important molecule in cancer pathogenesis and drug resistance. Pharm Res. 2015;100:250–4.
Miyamoto Y, Tamano M, Torii T, Kawahara K, Nakamura K, Tanoue A, et al. Data supporting the role of Fyn in initiating myelination in the peripheral nervous system. Data Brief. 2016;7:1098–105.
Lang V, Semichon M, Michel F, Brossard C, Gary-Gouy H, Bismuth G. Fyn membrane localization is necessary to induce the constitutive tyrosine phosphorylation of Sam68 in the nucleus of T lymphocytes. J Immunol. 1999;162:7224–32.
Salmond RJ, Filby A, Qureshi I, Caserta S, Zamoyska R. T-cell receptor proximal signaling via the Src-family kinases, Lck and Fyn, influences T-cell activation, differentiation, and tolerance. Immunol Rev. 2009;228:9–22.
Schaeuble K, Hauser MA, Singer E, Groettrup M, Legler DF. Cross-talk between TCR and CCR7 signaling sets a temporal threshold for enhanced T lymphocyte migration. J Immunol. 2011;187:5645–52.
Yadav V, Denning MF. Fyn is induced by Ras/PI3K/Akt signaling and is required for enhanced invasion/migration. Mol Carcinog. 2011;50:346–52.
Li K, Zhang Y, Zhang Y, Jiang W, Shen J, Xu S, et al. Tyrosine kinase Fyn promotes osteoarthritis by activating the beta-catenin pathway. Ann Rheum Dis. 2018;77:935–43.
Baillat G, Siret C, Delamarre E, Luis J. Early adhesion induces interaction of FAK and Fyn in lipid domains and activates raft-dependent Akt signaling in SW480 colon cancer cells. Biochim Biophys Acta. 2008;1783:2323–31.
Yu XL, Jiang JL, Li L, Feng Q, Xu J, Chen ZN. The glycosylation characteristic of hepatoma-associated antigen HAb18G/CD147 in human hepatoma cells. Int J Biochem Cell Biol. 2006;38:1939–45.
Li JH, Huang W, Lin P, Wu B, Fu ZG, Shen HM, et al. N-linked glycosylation at Asn152 on CD147 affects protein folding and stability: promoting tumour metastasis in hepatocellular carcinoma. Sci Rep. 2016;6:35210.
Fan J, Wang S, Yu S, He J, Zheng W, Zhang J. N-acetylglucosaminyltransferase IVa regulates metastatic potential of mouse hepatocarcinoma cells through glycosylation of CD147. Glycoconj J. 2012;29:323–34.
Kato N, Yuzawa Y, Kosugi T, Hobo A, Sato W, Miwa Y, et al. The E-selectin ligand basigin/CD147 is responsible for neutrophil recruitment in renal ischemia/reperfusion. J Am Soc Nephrol. 2009;20:1565–76.
Tang Y, Cong X, Wang S, Fang S, Dong X, Yuan Y, et al. GnT-V promotes chemosensitivity to gemcitabine in bladder cancer cells through beta1,6 GlcNAc branch modification of human equilibrative nucleoside transporter 1. Biochem Biophys Res Commun. 2018;503:3142–8.
Huang B, Wu Q, Ge Y, Zhang J, Sun L, Zhang Y, et al. Expression of N-acetylglucosaminyltransferase V in gastric cancer correlates with metastasis and prognosis. Int J Oncol. 2014;44:849–57.
Li B, Su S, Zhang MY, He L, Wang QD, He K. Effect of GnT-V knockdown on the proliferation, migration and invasion of the SMMC7721/R human hepatocellular carcinoma drug-resistant cell line. Mol Med Rep. 2016;13:469–76.
Huang B, Sun L, Cao J, Zhang Y, Wu Q, Zhang J, et al. Downregulation of the GnT-V gene inhibits metastasis and invasion of BGC823 gastric cancer cells. Oncol Rep. 2013;29:2392–400.
Acknowledgements
This work was financially supported by the Major Projects of International Cooperation and Exchanges NSFC Grant No. 81620108024; Grant No. 81572679, 81773341, 31770774, 81572677, 81830096 from the National Natural Science Foundation; the Strategy-Oriented Special Project of Central South University in China (ZLXD2017003); the Provincial Major Project of basic or Applied Research in Natural Science, Guangdong Provincial, Education Department (2016KZDXM038) and the China Postdoctoral Science Foundation (2019M652808).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, X., Huang, Z., Guo, Y. et al. The phosphorylation of CD147 by Fyn plays a critical role for melanoma cells growth and metastasis. Oncogene 39, 4183–4197 (2020). https://doi.org/10.1038/s41388-020-1287-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-020-1287-3
This article is cited by
-
FYN: emerging biological roles and potential therapeutic targets in cancer
Journal of Translational Medicine (2023)
-
Advances in the expression and function of Fyn in different human tumors
Clinical and Translational Oncology (2023)
-
NETO2 promotes melanoma progression via activation of the Ca2+/CaMKII signaling pathway
Frontiers of Medicine (2023)
-
PA2G4 promotes the metastasis of hepatocellular carcinoma by stabilizing FYN mRNA in a YTHDF2-dependent manner
Cell & Bioscience (2022)
-
Hypoxia-dependent drivers of melanoma progression
Journal of Experimental & Clinical Cancer Research (2021)