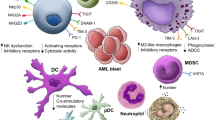
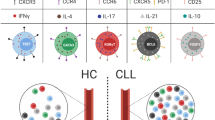
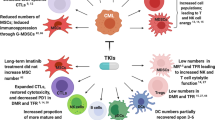
Abstract
Acute myeloid leukemia (AML) is a systemic, heterogeneous hematologic malignancy with poor overall survival. While some malignancies have seen improvements in clinical outcomes with immunotherapy, success of these agents in AML remains elusive. Despite limited progress, stem cell transplantation and donor lymphocyte infusions show that modulation of the immune system can improve overall survival of AML patients. Understanding the causes of immune evasion and disease progression will identify potential immune-mediated targets in AML. This review explores immunosuppressive mechanisms that alter T-cell-mediated immunity in AML.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Howlader NNA, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, et al. SEER Cancer Statistics Review, 1975–2016. Bethesda, MD: National Cancer Institute.
Papaemmanuil E, Gerstung M, Bullinger L, Gaidzik VI, Paschka P, Roberts ND, et al. Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med. 2016;374:2209–21. https://doi.org/10.1056/NEJMoa1516192.
Pollyea DA. New drugs for acute myeloid leukemia inspired by genomics and when to use them. Hematology. 2018;2018:45–50. https://doi.org/10.1182/asheducation-2018.1.45.
Cerrano M, Itzykson R. New treatment options for acute myeloid leukemia in 2019. Curr Oncol Rep. 2019;21:16. https://doi.org/10.1007/s11912-019-0764-8.
Lamble AJ, Lind EF. Targeting the immune microenvironment in acute myeloid leukemia: a focus on T cell immunity. Front Oncol. 2018;8:213. https://doi.org/10.3389/fonc.2018.00213.
Austin R, Smyth MJ, Lane SW. Harnessing the immune system in acute myeloid leukaemia. Crit Rev Oncol/Hematol. 2016;103:62–77. https://doi.org/10.1016/j.critrevonc.2016.04.020.
Horowitz MM, Gale RP, Sondel PM, Goldman JM, Kersey J, Kolb HJ, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75:555–62.
Boddu P, Kantarjian H, Garcia-Manero G, Allison J, Sharma P, Daver N. The emerging role of immune checkpoint based approaches in AML and MDS. Leuk lymphoma. 2018;59:790–802. https://doi.org/10.1080/10428194.2017.1344905.
Liu Y, Bewersdorf JP, Stahl M, Zeidan AM. Immunotherapy in acute myeloid leukemia and myelodysplastic syndromes: the dawn of a new era? Blood Rev. 2019;34:67–83. https://doi.org/10.1016/j.blre.2018.12.001.
Buggins AG, Milojkovic D, Arno MJ, Lea NC, Mufti GJ, Thomas NS. et al. Microenvironment produced by acute myeloid leukemia cells prevents T cell activation and proliferation by inhibition of NF-kappaB, c-Myc, and pRb pathways. J Immunol. 2001;167:6021–30.
Le Dieu R, Taussig DC, Ramsay AG, Mitter R, Miraki-Moud F, Fatah R, et al. Peripheral blood T cells in acute myeloid leukemia (AML) patients at diagnosis have abnormal phenotype and genotype and form defective immune synapses with AML blasts. Blood. 2009;114:3909–16. https://doi.org/10.1182/blood-2009-02-206946.
Barosi G. An immune dysregulation in MPN. Curr Hematol Malig Rep. 2014;9:331–9. https://doi.org/10.1007/s11899-014-0227-0.
Fozza C, Crobu V, Isoni MA, Dore F. The immune landscape of myelodysplastic syndromes. Crit Rev Oncol/Hematol. 2016;107:90–9. https://doi.org/10.1016/j.critrevonc.2016.08.016.
Chalmers ZR, Connelly CF, Fabrizio D, Gay L, Ali SM, Ennis R, et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 2017;9:34 https://doi.org/10.1186/s13073-017-0424-2.
Klco JM, Spencer DH, Miller CA, Griffith M, Lamprecht TL, O’Laughlin M, et al. Functional heterogeneity of genetically defined subclones in acute myeloid leukemia. Cancer Cell. 2014;25:379–92. https://doi.org/10.1016/j.ccr.2014.01.031.
Hersh EM, Whitecar JP Jr., McCredie KB, Bodey GP Sr., Freireich EJ. Chemotherapy, immunocompetence, immunosuppression and prognosis in acute leukemia. N Engl J Med 1971;285:1211–6. https://doi.org/10.1056/nejm197111252852201.
Hersh EM, Gutterman JU, Mavligit GM, McCredie KB, Burgess MA, Matthews A, et al. Serial studies of immunocompetence of patients undergoing chemotherapy for acute leukemia. J Clin Investig. 1974;54:401–8. https://doi.org/10.1172/jci107775.
Rashidi A, Fisher SI. Spontaneous remission of acute myeloid leukemia. Leuk lymphoma. 2015;56:1727–34. https://doi.org/10.3109/10428194.2014.970545.
Muller-Schmah C, Solari L, Weis R, Pfeifer D, Scheibenbogen C, Trepel M, et al. Immune response as a possible mechanism of long-lasting disease control in spontaneous remission of MLL/AF9-positive acute myeloid leukemia. Ann Hematol. 2012;91:27–32. https://doi.org/10.1007/s00277-011-1332-y.
Hasegawa K, Tanaka S, Fujiki F, Morimoto S, Nakajima H, Tatsumi N, et al. An immunocompetent mouse model for MLL/AF9 leukemia reveals the potential of spontaneous cytotoxic T-cell response to an antigen expressed in leukemia cells. PLoS ONE. 2015;10:e0144594. https://doi.org/10.1371/journal.pone.0144594.
Zhang L, Chen X, Liu X, Kline DE, Teague RM, Gajewski TF, et al. CD40 ligation reverses T cell tolerance in acute myeloid leukemia. J Clin Investig. 2013;123:1999–2010. https://doi.org/10.1172/jci63980.
Almosailleakh M, Schwaller J. Murine models of acute myeloid leukaemia. Int J Mol Sci. 2019;20. https://doi.org/10.3390/ijms20020453.
Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. https://doi.org/10.1038/nri1806.
Teague RM, Kline J. Immune evasion in acute myeloid leukemia: current concepts and future directions. J Immunother Cancer. 2013;1. https://doi.org/10.1186/2051-1426-1-13.
Wang X, Zheng J, Liu J, Yao J, He Y, Li X, et al. Increased population of CD4(+)CD25(high), regulatory T cells with their higher apoptotic and proliferating status in peripheral blood of acute myeloid leukemia patients. Eur J Haematol. 2005;75:468–76. https://doi.org/10.1111/j.1600-0609.2005.00537.x. e-pub ahead of print.
Tian T, Yu S, Liu L, Xue F, Yuan C, Wang M, et al. The profile of T helper subsets in bone marrow microenvironment is distinct for different stages of acute myeloid leukemia patients and chemotherapy partly ameliorates these variations. PLoS ONE. 2015; 10. https://doi.org/10.1371/journal.pone.0131761.
Williams P, Basu S, Garcia-Manero G, Hourigan CS, Oetjen KA, Cortes JE, et al. The distribution of T-cell subsets and the expression of immune checkpoint receptors and ligands in patients with newly diagnosed and relapsed acute myeloid leukemia. Cancer. 2018. https://doi.org/10.1002/cncr.31896.
van Galen P, Hovestadt V, Wadsworth Ii MH, Hughes TK, Griffin GK, Battaglia S, et al. Single-cell RNA-Seq reveals AML hierarchies relevant to disease progression and immunity. Cell. 2019;176:1265–81.e1224. https://doi.org/10.1016/j.cell.2019.01.031.
Ustun C, Miller JS, Munn DH, Weisdorf DJ, Blazar BR. Regulatory T cells in acute myelogenous leukemia: is it time for immunomodulation? Blood. 2011;118:5084–95. https://doi.org/10.1182/blood-2011-07-365817.
Ersvaer E, Liseth K, Skavland J, Gjertsen BT, Bruserud O. Intensive chemotherapy for acute myeloid leukemia differentially affects circulating TC1, TH1, TH17 and TREG cells. BMC Immunol. 2010;11:38. https://doi.org/10.1186/1471-2172-11-38.
Kanakry CG, Hess AD, Gocke CD, Thoburn C, Kos F, Meyer C, et al. Early lymphocyte recovery after intensive timed sequential chemotherapy for acute myelogenous leukemia: peripheral oligoclonal expansion of regulatory T cells. Blood. 2011;117:608–17. https://doi.org/10.1182/blood-2010-04-277939.
Lichtenegger FS, Lorenz R, Gellhaus K, Hiddemann W, Beck B, Subklewe M. Impaired NK cells and increased T regulatory cell numbers during cytotoxic maintenance therapy in AML. Leuk Res. 2014;38:964–9. https://doi.org/10.1016/j.leukres.2014.05.014.
Shenghui Z, Yixiang H, Jianbo W, Kang Y, Laixi B, Yan Z, et al. Elevated frequencies of CD4(+) CD25(+) CD127lo regulatory T cells is associated to poor prognosis in patients with acute myeloid leukemia. Int J Cancer. 2011;129:1373–81. https://doi.org/10.1002/ijc.25791.
Szczepanski MJ, Szajnik M, Czystowska M, Mandapathil M, Strauss L, Welsh A. et al. Increased frequency and suppression by regulatory T cells in patients with acute myelogenous leukemia. Clin Cancer Res. 2009;15:3325–32. https://doi.org/10.1158/1078-0432.Ccr-08-3010.
Zhou Q, Bucher C, Munger ME, Highfill SL, Tolar J, Munn DH, et al. Depletion of endogenous tumor-associated regulatory T cells improves the efficacy of adoptive cytotoxic T-cell immunotherapy in murine acute myeloid leukemia. Blood. 2009;114:3793–802. https://doi.org/10.1182/blood-2009-03-208181.
Kittang AO, Kordasti S, Sand KE, Costantini B, Kramer AM, Perezabellan P, et al. Expansion of myeloid derived suppressor cells correlates with number of T regulatory cells and disease progression in myelodysplastic syndrome. Oncoimmunology. 2016;5:e1062208. https://doi.org/10.1080/2162402x.2015.1062208.
Menter T, Kuzmanic B, Bucher C, Medinger M, Halter J, Dirnhofer S, et al. Beneficial role of increased FOXP3(+) regulatory T-cells in acute myeloid leukaemia therapy response. Br J Haematol. 2018;182:581–3. https://doi.org/10.1111/bjh.14819.
Schnorfeil FM, Lichtenegger FS, Emmerig K, Schlueter M, Neitz JS, Draenert R, et al. T cells are functionally not impaired in AML: increased PD-1 expression is only seen at time of relapse and correlates with a shift towards the memory T cell compartment. J Hematol Oncol. 2015;8:93. https://doi.org/10.1186/s13045-015-0189-2.
Sun YX, Kong HL, Liu CF, Yu S, Tian T, Ma DX, et al. The imbalanced profile and clinical significance of T helper associated cytokines in bone marrow microenvironment of the patients with acute myeloid leukemia. Hum Immunol. 2014;75:113–8. https://doi.org/10.1016/j.humimm.2013.11.014.
Musuraca G, De Matteis S, Napolitano R, Papayannidis C, Guadagnuolo V, Fabbri F, et al. IL-17/IL-10 double-producing T cells: new link between infections, immunosuppression and acute myeloid leukemia. J Transl Med. 2015;13:229. https://doi.org/10.1186/s12967-015-0590-1.
Tian T, Yu S, Wang M, Yuan C, Zhang H, Ji C. et al. Aberrant T helper 17 cells and related cytokines in bone marrow microenvironment of patients with acute myeloid leukemia. Clin Dev Immunol. 2013;2013:915873. https://doi.org/10.1155/2013/915873.
Han Y, Ye A, Bi L, Wu J, Yu K, Zhang S. Th17 cells and interleukin-17 increase with poor prognosis in patients with acute myeloid leukemia. Cancer Sci. 2014;105:933–42. https://doi.org/10.1111/cas.12459.
Wu C, Wang S, Wang F, Chen Q, Peng S, Zhang Y, et al. Increased frequencies of T helper type 17 cells in the peripheral blood of patients with acute myeloid leukaemia. Clin Exp Immunol. 2009;158:199–204. https://doi.org/10.1111/j.1365-2249.2009.04011.x.
Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492–9.
Zhou Q, Munger ME, Veenstra RG, Weigel BJ, Hirashima M, Munn DH, et al. Coexpression of Tim-3 and PD-1 identifies a CD8+ T-cell exhaustion phenotype in mice with disseminated acute myelogenous leukemia. Blood. 2011;117:4501–10. https://doi.org/10.1182/blood-2010-10-310425.
Deng M, Gui X, Kim J, Xie L, Chen W, Li Z, et al. LILRB4 signalling in leukaemia cells mediates T cell suppression and tumour infiltration. Nature. 2018;562:605–9. https://doi.org/10.1038/s41586-018-0615-z.
LaBelle JL, Hanke CA, Blazar BR, Truitt RL. Negative effect of CTLA-4 on induction of T-cell immunity in vivo to B7-1+, but not B7-2+, murine myelogenous leukemia. Blood. 2002;99:2146–53.
Knaus HA, Berglund S, Hackl H, Blackford AL, Zeidner JF, Montiel-Esparza R, et al. Signatures of CD8+ T cell dysfunction in AML patients and their reversibility with response to chemotherapy. JCI Insight. 2018;3. https://doi.org/10.1172/jci.insight.120974.
Wang M, Bu J, Zhou M, Sido J, Lin Y, Liu G. et al. CD8(+)T cells expressing both PD-1 and TIGIT but not CD226 are dysfunctional in acute myeloid leukemia (AML) patients. Clin Immunol. 2018;190:64–73. https://doi.org/10.1016/j.clim.2017.08.021.
Dama P, Tang M, Fulton N, Kline J, Liu H. Gal9/Tim-3 expression level is higher in AML patients who fail chemotherapy. J Immunother Cancer. 2019;7:175. https://doi.org/10.1186/s40425-019-0611-3.
Paley MA, Kroy DC, Odorizzi PM, Johnnidis JB, Dolfi DV, Barnett BE. et al. Progenitor and terminal subsets of CD8+ T cells cooperate to contain chronic viral infection. Science. 2012;338:1220–5. https://doi.org/10.1126/science.1229620.
Jia B, Wang L, Claxton DF, Ehmann WC, Rybka WB, Mineishi S, et al. Bone marrow CD8 T cells express high frequency of PD-1 and exhibit reduced anti-leukemia response in newly diagnosed AML patients. Blood Cancer J. 2018;8:34. https://doi.org/10.1038/s41408-018-0069-4.
Jia B, Zhao C, Rakszawski KL, Claxton DF, Ehmann WC, Rybka WB, et al. Eomes+T-betlow CD8+ T cells are functionally impaired and are associated with poor clinical outcome in patients with acute myeloid leukemia (AML). Cancer Res. 2019. https://doi.org/10.1158/0008-5472.Can-18-3107.
Daver N, Garcia-Manero G, Basu S, Boddu PC, Alfayez M, Cortes JE, et al. Efficacy, safety, and biomarkers of response to azacitidine and nivolumab in relapsed/refractory acute myeloid leukemia: a nonrandomized, open-label, phase II study. Cancer Discov. 2019;9:370–83. https://doi.org/10.1158/2159-8290.Cd-18-0774.
Masarova L, Kantarjian H, Ravandi F, Sharma P, Garcia-Manero G, Daver N. Update on immunotherapy in AML and MDS: monoclonal antibodies and checkpoint inhibitors paving the road for clinical practice. In: Naing A, Hajjar J, editors. Immunotherapy. Cham: Springer International Publishing; 2018. p. 97–116.
Lee JB, Chen B, Vasic D, Law AD, Zhang L. Cellular immunotherapy for acute myeloid leukemia: how specific should it be? Blood Rev. 2019;35:18–31. https://doi.org/10.1016/j.blre.2019.02.001.
Wouters BJ, Delwel R. Epigenetics and approaches to targeted epigenetic therapy in acute myeloid leukemia. Blood. 2016;127:42–52. https://doi.org/10.1182/blood-2015-07-604512.
Kelderman S, Schumacher TN, Haanen JB. Acquired and intrinsic resistance in cancer immunotherapy. Mol Oncol. 2014;8:1132–9. https://doi.org/10.1016/j.molonc.2014.07.011.
Philip M, Fairchild L, Sun L, Horste EL, Camara S, Shakiba M, et al. Chromatin states define tumour-specific T cell dysfunction and reprogramming. Nature. 2017;545:452–6. https://doi.org/10.1038/nature22367.
Radpour R, Riether C, Simillion C, Hopner S, Bruggmann R, Ochsenbein AF CD8(+) T cells expand stem and progenitor cells in favorable but not adverse risk acute myeloid leukemia. Leukemia 2019. https://doi.org/10.1038/s41375-019-0441-9.
Frumento G, Rotondo R, Tonetti M, Damonte G, Benatti U, Ferrara GB. Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3-dioxygenase. J Exp Med. 2002;196:459–68.
Curti A, Aluigi M, Pandolfi S, Ferri E, Isidori A, Salvestrini V, et al. Acute myeloid leukemia cells constitutively express the immunoregulatory enzyme indoleamine 2,3-dioxygenase. Leukemia. 2007;21:353–5. https://doi.org/10.1038/sj.leu.2404485.
Fukuno K, Hara T, Tsurumi H, Shibata Y, Mabuchi R, Nakamura N. et al. Expression of indoleamine 2,3-dioxygenase in leukemic cells indicates an unfavorable prognosis in acute myeloid leukemia patients with intermediate-risk cytogenetics. Leuk Lymphoma. 2015;56:1398–405. https://doi.org/10.3109/10428194.2014.953150.
Chamuleau ME, van de Loosdrecht AA, Hess CJ, Janssen JJ, Zevenbergen A, Delwel R, et al. High INDO (indoleamine 2,3-dioxygenase) mRNA level in blasts of acute myeloid leukemic patients predicts poor clinical outcome. Haematologica. 2008;93:1894–8. https://doi.org/10.3324/haematol.13113.
Corm S, Berthon C, Imbenotte M, Biggio V, Lhermitte M, Dupont C, et al. Indoleamine 2,3-dioxygenase activity of acute myeloid leukemia cells can be measured from patients’ sera by HPLC and is inducible by IFN-gamma. Leuk Res. 2009;33:490–4. https://doi.org/10.1016/j.leukres.2008.06.014.
Curti A, Pandolfi S, Valzasina B, Aluigi M, Isidori A, Ferri E, et al. Modulation of tryptophan catabolism by human leukemic cells results in the conversion of CD25- into CD25+ T regulatory cells. Blood. 2007;109:2871–7. https://doi.org/10.1182/blood-2006-07-036863.
Arandi N, Ramzi M, Safaei F, Monabati A. Overexpression of indoleamine 2,3-dioxygenase correlates with regulatory T cell phenotype in acute myeloid leukemia patients with normal karyotype. Blood Res. 2018;53:294–8. https://doi.org/10.5045/br.2018.53.4.294.
Mussai F, De Santo C, Abu-Dayyeh I, Booth S, Quek L, McEwen-Smith RM, et al. Acute myeloid leukemia creates an arginase-dependent immunosuppressive microenvironment. Blood. 2013;122:749–58. https://doi.org/10.1182/blood-2013-01-480129.
Mussai F, Wheat R, Sarrou E, Booth S, Stavrou V, Fultang L, et al. Targeting the arginine metabolic brake enhances immunotherapy for leukaemia. Int J Cancer. 2018. https://doi.org/10.1002/ijc.32028.
Orleans-Lindsay JK, Barber LD, Prentice HG, Lowdell MW. Acute myeloid leukaemia cells secrete a soluble factor that inhibits T and NK cell proliferation but not cytolytic function–implications for the adoptive immunotherapy of leukaemia. Clin Exp Immunol. 2001;126:403–11.
Losman JA, Looper RE, Koivunen P, Lee S, Schneider RK, McMahon C, et al. (R)-2-hydroxyglutarate is sufficient to promote leukemogenesis and its effects are reversible. Science. 2013;339:1621–5.
Medeiros BC, Fathi AT, DiNardo CD, Pollyea DA, Chan SM, Swords R. Isocitrate dehydrogenase mutations in myeloid malignancies. Leukemia. 2017;31:272–81. https://doi.org/10.1038/leu.2016.275.
DiNardo CD, Propert KJ, Loren AW, Paietta E, Sun Z, Levine RL, et al. Serum 2-hydroxyglutarate levels predict isocitrate dehydrogenase mutations and clinical outcome in acute myeloid leukemia. Blood. 2013;121:4917–24. https://doi.org/10.1182/blood-2013-03-493197.
Bunse L, Pusch S, Bunse T, Sahm F, Sanghvi K, Friedrich M, et al. Suppression of antitumor T cell immunity by the oncometabolite (R)-2-hydroxyglutarate. Nat Med. 2018;24:1192–203. https://doi.org/10.1038/s41591-018-0095-6.
Zhang L, Sorensen MD, Kristensen BW, Reifenberger G, McIntyre TM, Lin F. D-2-hydroxyglutarate is an intercellular mediator in IDH-mutant gliomas inhibiting complement and T cells. Clin Cancer Res. 2018;24:5381–91. https://doi.org/10.1158/1078-0432.Ccr-17-3855.
Xu T, Stewart KM, Wang X, Liu K, Xie M, Ryu JK, et al. Metabolic control of TH17 and induced Treg cell balance by an epigenetic mechanism. Nature. 2017;548:228–33. https://doi.org/10.1038/nature23475.
Kang X, Kim J, Deng M, John S, Chen H, Wu G. et al. Inhibitory leukocyte immunoglobulin-like receptors: Immune checkpoint proteins and tumor sustaining factors. Cell Cycle. 2016;15:25–40. https://doi.org/10.1080/15384101.2015.1121324.
Dobrowolska H, Gill KZ, Serban G, Ivan E, Li Q, Qiao P, et al. Expression of immune inhibitory receptor ILT3 in acute myeloid leukemia with monocytic differentiation. Cytom Part B Clin Cytom. 2013;84:21–9. https://doi.org/10.1002/cyto.b.21050.
Tonks A, Hills R, White P, Rosie B, Mills KI, Burnett AK, et al. CD200 as a prognostic factor in acute myeloid leukaemia. Leukemia. 2007;21:566–8. https://doi.org/10.1038/sj.leu.2404559.
Damiani D, Tiribelli M, Raspadori D, Sirianni S, Meneghel A, Cavalllin M, et al. Clinical impact of CD200 expression in patients with acute myeloid leukemia and correlation with other molecular prognostic factors. Oncotarget. 2015;6:30212–21. https://doi.org/10.18632/oncotarget.4901.
Zahran AM, Mohammed Saleh MF, Sayed MM, Rayan A, Ali AM, Hetta HF. Up-regulation of regulatory T cells, CD200 and TIM3 expression in cytogenetically normal acute myeloid leukemia. Cancer Biomark. 2018;22:587–95. https://doi.org/10.3233/cbm-181368.
Coles SJ, Hills RK, Wang EC, Burnett AK, Man S, Darley RL, et al. Increased CD200 expression in acute myeloid leukemia is linked with an increased frequency of FoxP3+ regulatory T cells. Leukemia. 2012;26:2146–8. https://doi.org/10.1038/leu.2012.75.
Coles SJ, Hills RK, Wang EC, Burnett AK, Man S, Darley RL, et al. Expression of CD200 on AML blasts directly suppresses memory T-cell function. Leukemia. 2012;26:2148–51.
Moreaux J, Hose D, Reme T, Jourdan E, Hundemer M, Legouffe E, et al. CD200 is a new prognostic factor in multiple myeloma. Blood. 2006;108:4194–7. https://doi.org/10.1182/blood-2006-06-029355.
Moreaux J, Veyrune JL, Reme T, De Vos J, Klein B. CD200: a putative therapeutic target in cancer. Biochem Biophys Res Commun. 2008;366:117–22. https://doi.org/10.1016/j.bbrc.2007.11.103.
Kretz-Rommel A, Qin F, Dakappagari N, Ravey EP, McWhirter J, Oltean D. et al. CD200 expression on tumor cells suppresses antitumor immunity: new approaches to cancer immunotherapy. J Immunol. 2007;178:5595–605. https://doi.org/10.4049/jimmunol.178.9.5595.
Kawasaki BT, Farrar WL. Cancer stem cells, CD200 and immunoevasion. Trends Immunol. 2008;29:464–8. https://doi.org/10.1016/j.it.2008.07.005.
Christopher MJ, Petti AA, Rettig MP, Miller CA, Chendamarai E, Duncavage EJ, et al. Immune Escape of Relapsed AML Cells after Allogeneic Transplantation. N. Engl J Med. 2018;379:2330–41. https://doi.org/10.1056/NEJMoa1808777.
Tikhonova AN, Dolgalev I, Hu H, Sivaraj KK, Hoxha E, Cuesta-Domínguez Á, et al. The bone marrow microenvironment at single-cell resolution. Nature 2019. https://doi.org/10.1038/s41586-019-1104-8.
Acknowledgements
The authors are supported by K23HL138291(PBF) and T32CA217834 (ZL).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
PBF receives research funding support from Incyte, Forma Therapeutics, and Astex Pharmaceuticals and has consulted for Agios Pharmaceuticals. ZL and MP have no competing interests to declare.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, Z., Philip, M. & Ferrell, P.B. Alterations of T-cell-mediated immunity in acute myeloid leukemia. Oncogene 39, 3611–3619 (2020). https://doi.org/10.1038/s41388-020-1239-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-020-1239-y
This article is cited by
-
Single-cell transcriptomic profiling reveals immune cell heterogeneity in acute myeloid leukaemia peripheral blood mononuclear cells after chemotherapy
Cellular Oncology (2024)
-
MAIT cells numbers and frequencies in patients with acute myeloid leukemia at diagnosis: association with cytogenetic profile and gene mutations
Cancer Immunology, Immunotherapy (2022)
-
Single-cell map of diverse immune phenotypes in the acute myeloid leukemia microenvironment
Biomarker Research (2021)
-
PER2: a potential molecular marker for hematological malignancies
Molecular Biology Reports (2021)