Abstract
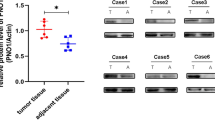
Osteosarcoma (OS) is the most common primary malignancy of the bone that predominantly affects children and adolescents. Hippo pathway is a crucial regulator of organ size and tumorigenesis. However, how Hippo pathway regulates the occurrence of osteosarcoma is largely unknown. Here, we reported the regulator of G protein signaling protein 12 (RGS12) is a novel Hippo pathway regulator and tumor suppressor of osteosarcoma. Depletion of Rgs12 promotes osteosarcoma progression and lung metastasis in an orthotopic xenograft mouse model. Our data showed that the knockdown of RGS12 upregulates Ezrin expression through promoting the GNA12/13-RhoA-YAP pathway. Moreover, RGS12 negatively regulates the transcriptional activity of YAP/TEAD1 complex through its PDZ domain function to inhibit the expression and function of the osteosarcoma marker Ezrin. PDZ domain peptides of RGS12 can inhibit the development of intratibial tumor and lung metastases. Collectively, this study identifies that the RGS12 is a novel tumor suppressor in osteosarcoma through inhibiting YAP-TEAD1-Ezrin signaling pathway and provides a proof of principle that targeting RGS12 may be a therapeutic strategy for osteosarcoma.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Kansara M, Teng MW, Smyth MJ, Thomas DM. Translational biology of osteosarcoma. Nat Rev Cancer. 2014;14:722–35.
Tang QL, Lu JC, Zou CY, Shao Y, Chen Y, Narala S, et al. CDH4 is a novel determinant of osteosarcoma tumorigenesis and metastasis. Oncogene. 2018;37:3617–30.
Wang DY, Wu YN, Huang JQ, Wang W, Xu M, Jia JP, et al. Hippo/YAP signaling pathway is involved in osteosarcoma chemoresistance. Chin J Cancer. 2016;35:1–8.
Harvey KF, Zhang XM, Thomas DM. The Hippo pathway and human cancer. Nat Rev Cancer. 2013;13:246–57.
Yu FX, Guan KL. The Hippo pathway: regulators and regulations. Genes Dev. 2013;27:355–71.
Zhao B, Tumaneng K, Guan KL. The Hippo pathway in organ size control, tissue regeneration and stem cell self-renewal. Nat cell Biol. 2011;13:877–83.
Chai JW, Xu SJ, Guo FB. TEAD1 mediates the oncogenic activities of Hippo-YAP1 signaling in osteosarcoma. Biochem Bioph Res Co. 2017;488:297–302.
Abramow-Newerly M, Roy AA, Nunn C, Chidiac P. RGS proteins have a signalling complex: Interactions between RGS proteins and GPCRs, effectors, and auxiliary proteins. Cell Signal. 2006;18:579–91.
Stewart A, Fisher RA. Introduction: G protein-coupled receptors and RGS proteins. Prog Mol Biol Transl Sci. 2015;133:1–11.
Yang SY, Li YP. RGS12 is essential for RANKL-evoked signaling for terminal differentiation of osteoclasts in vitro. J Bone Miner Res. 2007;22:45–54.
Keinan D, Yang SY, Cohen RE, Yuan X, Liu TJ, Li YP. Role of regulator of G protein signaling proteins in bone. Front Biosci-Landmrk. 2014;19:634–48.
Li Z, Liu T, Gilmore A, Gomez NM, Fu C, Lim J, et al. Regulator of G protein signaling protein 12 (Rgs12) controls mouse osteoblast differentiation via calcium channel/oscillation and Galphai-ERK signaling. J Bone Min Res. 2019;34:752–64.
Yu FX, Zhao B, Panupinthu N, Jewell JL, Lian I, Wang LH, et al. Regulation of the hippo-YAP pathway by G-protein-coupled receptor signaling. Cell. 2012;150:780–91.
Wang YQ, Wang JH, Zhang L, Karatas OF, Shao LJ, Zhang YQ, et al. RGS12 is a novel tumor-suppressor gene in african american prostate cancer that represses AKT and MNX1 expression. Cancer Res. 2017;77:4247–57.
Chatterjee TK, Fisher RA. RGS12TS-S localizes at nuclear matrix-associated subnuclear structures and represses transcription: structural requirements for subnuclear targeting and transcriptional repression. Mol Cell Biol. 2002;22:4334–45.
Yuan X, Cao J, Liu T, Li YP, Scannapieco F, He X, et al. Regulators of G protein signaling 12 promotes osteoclastogenesis in bone remodeling and pathological bone loss. Cell Death Differ. 2015;22:2046–57.
Yang S, Li YP, Liu T, He X, Yuan X, Li C, et al. Mx1-cre mediated Rgs12 conditional knockout mice exhibit increased bone mass phenotype. Genesis. 2013;51:201–9.
Ng AYH, Li Z, Jones MM, Yang S, Li C, Fu C et al. Regulator of G protein signaling 12 enhances osteoclastogenesis by suppressing Nrf2-dependent antioxidant proteins to promote the generation of reactive oxygen species. Elife. 2019;8:e42591.
Walkley CR, Qudsi R, Sankaran VG, Perry JA, Gostissa M, Roth SI, et al. Conditional mouse osteosarcoma, dependent on p53 loss and potentiated by loss of Rb, mimics the human disease. Genes Dev. 2008;22:1662–76.
Berman SD, Calo E, Landman AS, Danielian PS, Miller ES, West JC, et al. Metastatic osteosarcoma induced by inactivation of Rb and p53 in the osteoblast lineage. P Natl Acad Sci USA. 2008;105:11851–6.
Ji Q, Hao X, Meng Y, Zhang M, Desano J, Fan D, et al. Restoration of tumor suppressor miR-34 inhibits human p53-mutant gastric cancer tumorspheres. BMC Cancer. 2008;8:266.
Liu JC, Deng T, Lehal RS, Kim J, Zacksenhaus E. Identification of tumorsphere- and tumor-initiating cells in HER2/Neu-Induced mammary tumors. Cancer Res. 2007;67:8671–81.
Mittal V. Epithelial mesenchymal transition in tumor metastasis. Annu Rev Pathol. 2018;13:395–412.
Lamar JM, Stern P, Liu H, Schindler JW, Jiang ZG, Hynes RO. The Hippo pathway target, YAP, promotes metastasis through its TEAD-interaction domain. P Natl Acad Sci USA. 2012;109:E2441–E2450.
Fromigue O, Hamidouche Z, Vaudin P, Lecanda F, Patino A, Barbry P, et al. CYR61 downregulation reduces osteosarcoma cell invasion, migration, and metastasis. J Bone Miner Res. 2011;26:1533–42.
Zhang WJ, Gao YJ, Li PX, Shi ZB, Guo T, Li F, et al. VGLL4 functions as a new tumor suppressor in lung cancer by negatively regulating the YAP-TEAD transcriptional complex. Cell Res. 2014;24:331–43.
Holden JK, Cunningham CN. Targeting the hippo pathway and cancer through the TEAD family of transcription factors. Cancers. 2018;10:81–96.
Mohri Z, Del Rio Hernandez A, Krams R. The emerging role of YAP/TAZ in mechanotransduction. J Thorac Dis. 2017;9:E507–E509.
Zhang L, Yue T, Jiang J. Hippo signaling pathway and organ size control. Fly. 2009;3:68–73.
Yuan X, Cao J, He XN, Serra R, Qu J, Cao X. et al. Ciliary IFT80 balances canonical versus non-canonical hedgehog signalling for osteoblast differentiation. Nat Commun. 2016;7:1024–38.
Zhang WQ, Dai YY, Hsu PC, Wang H, Cheng L, Yang YL, et al. Targeting YAP in malignant pleural mesothelioma. J Cell Mol Med. 2017;21:2663–76.
Ren L, Khanna C. Role of ezrin in osteosarcoma metastasis. Adv Exp Med Biol. 2014;804:181–201.
Meng Z, Moroishi T, Guan KL. Mechanisms of Hippo pathway regulation. Genes Dev. 2016;30:1–17.
Mao J, Yuan H, Xie W, Wu D. Guanine nucleotide exchange factor GEF115 specifically mediates activation of Rho and serum response factor by the G protein alpha subunit Galpha13. Proc Natl Acad Sci USA. 1998;95:12973–6.
Jules J, Yang SY, Chen W, Li YP. Role of regulators of G protein signaling proteins in bone physiology and pathophysiology. Prog Mol Biol Transl. 2015;133:47–75.
Cho H, Harrison K, Kehrl JH. Regulators of G protein signaling: potential drug targets for controlling cardiovascular and immune function. Curr drug targets Immune, Endocr Metab Disord. 2004;4:107–18.
Lee HJ, Zheng JJ. PDZ domains and their binding partners: structure, specificity, and modification. Cell Commun Signal. 2010;8:1–18.
Meyers PA, Schwartz CL, Krailo M, Kleinerman ES, Betcher D, Bernstein ML, et al. Osteosarcoma: a randomized, prospective trial of the addition of ifosfamide and/or muramyl tripeptide to cisplatin, doxorubicin, and high-dose methotrexate. J Clin Oncol. 2005;23:2004–11.
Hurst JH, Hooks SB. Regulator of G-protein signaling (RGS) proteins in cancer biology. Biochemical Pharmacol. 2009;78:1289–97.
Gross JD, Kaski SW, Schroer AB, Wix KA, Siderovski DP, Setola V. Regulator of G protein signaling-12 modulates the dopamine transporter in ventral striatum and locomotor responses to psychostimulants. J Psychopharmacol. 2018;32:191–203.
Buckbinder L, Velasco-Miguel S, Chen Y, Xu N, Talbott R, Gelbert L, et al. The p53 tumor suppressor targets a novel regulator of G protein signaling. Proc Natl Acad Sci USA. 1997;94:7868–72.
Bouvier C, Macagno N, Nguyen Q, Loundou A, Jiguet-Jiglaire C, Gentet JC, et al. Prognostic value of the Hippo pathway transcriptional coactivators YAP/TAZ and beta1-integrin in conventional osteosarcoma. Oncotarget. 2016;7:64702–10.
Yang Z, Zhang M, Xu K, Liu L, Hou WK, Cai YZ, et al. Knockdown of YAP1 inhibits the proliferation of osteosarcoma cells in vitro and in vivo. Oncol Rep. 2014;32:1265–72.
Zhao B, Lei QY, Guan KL. The Hippo-YAP pathway: new connections between regulation of organ size and cancer. Curr Opin Cell Biol. 2008;20:638–46.
Wang G, Beier F. Rac1/Cdc42 and RhoA GTPases antagonistically regulate chondrocyte proliferation, hypertrophy, and apoptosis. J Bone Miner Res. 2005;20:1022–31.
Zhang YC, Xia HW, Ge XJ, Chen QJ, Yuan DD, Chen Q, et al. CD44 acts through RhoA to regulate YAP signaling. Cell Signal. 2014;26:2504–13.
Fukuda H, Nakamura S, Chisaki Y, Takada T, Toda Y, Murata H, et al. Daphnetin inhibits invasion and migration of LM8 murine osteosarcoma cells by decreasing RhoA and Cdc42 expression. Biochem Biophys Res Commun. 2016;471:63–67.
Vogt S, Grosse R, Schultz G, Offermanns S. Receptor-dependent RhoA activation in G(12)/G(13)-deficient cells - Genetic evidence for an involvement of G(q)/G(11). J Biol Chem. 2003;278:28743–9.
Bradley SJ, Wiegman CH, Iglesias MM, Kong KC, Butcher AJ, Plouffe B, et al. Mapping physiological G protein-coupled receptor signaling pathways reveals a role for receptor phosphorylation in airway contraction. Proc Natl Acad Sci USA. 2016;113:4524–9.
Khanna C, Wan XL, Bose S, Cassaday R, Olomu O, Mendoza A, et al. The membrane-cytoskeleton linker ezrin is necessary for osteosarcoma metastasis. Nat Med. 2004;10:182–6.
Rozengurt E, Sinnett-Smith J, Eibl G. Yes-associated protein (YAP) in pancreatic cancer: at the epicenter of a targetable signaling network associated with patient survival. Signal Transduct Tar. 2018;3:1038–48.
Sabile AA, Arlt MJ, Muff R, Bode B, Langsam B, Bertz J, et al. Cyr61 expression in osteosarcoma indicates poor prognosis and promotes intratibial growth and lung metastasis in mice. J Bone Miner Res. 2012;27:58–67.
Igarashi K, Kawaguchi K, Murakami T, Miyake K, Kiyuna T, Miyake M, et al. Patient-derived orthotopic xenograft models of sarcoma. Cancer Lett. 2020;469:332–9.
Brusgard JL, Choe M, Chumsri S, Renoud K, MacKerell AD Jr, Sudol M, et al. RUNX2 and TAZ-dependent signaling pathways regulate soluble E-Cadherin levels and tumorsphere formation in breast cancer cells. Oncotarget. 2015;6:28132–50.
Li Y, Hu N, Yang D, Oxenkrug G, Yang Q. Regulating the balance between the kynurenine and serotonin pathways of tryptophan metabolism. FEBS J. 2017;284:948–66.
Xu MZ, Chan SW, Liu AM, Wong KF, Fan ST, Chen J, et al. AXL receptor kinase is a mediator of YAP-dependent oncogenic functions in hepatocellular carcinoma. Oncogene. 2011;30:1229–40.
Acknowledgements
This work was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS, AR061052), the National Institute on Aging (NIA, AG048388) awarded to SY.
Author information
Authors and Affiliations
Contributions
SY-Y and YL conceived this study, generated hypotheses, and designed the experiments. YL, ML, ST-Y, AMF, and TSKE performed the experiments and analyzed the data. SY-Y and YL wrote, reviewed, and edited the paper. SY-Y supervised the project.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Li, Y., Liu, M., Yang, S. et al. RGS12 is a novel tumor suppressor in osteosarcoma that inhibits YAP-TEAD1-Ezrin signaling. Oncogene 40, 2553–2566 (2021). https://doi.org/10.1038/s41388-020-01599-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-020-01599-z
This article is cited by
-
Long non-coding HOXA-AS3 contributes to osteosarcoma progression through the miR-1286/TEAD1 axis
Journal of Orthopaedic Surgery and Research (2023)
-
Regulator of G protein signaling protein 6 alleviates acute lung injury by inhibiting inflammation and promoting cell self-renewal in mice
Cellular & Molecular Biology Letters (2023)
-
Function and regulation of RGS family members in solid tumours: a comprehensive review
Cell Communication and Signaling (2023)
-
RGS proteins and their roles in cancer: friend or foe?
Cancer Cell International (2023)
-
A DNA methylation signature for the prediction of tumour recurrence in stage II colorectal cancer
British Journal of Cancer (2023)