Abstract
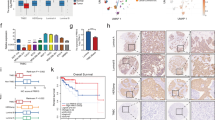
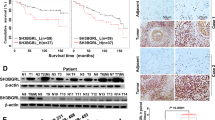
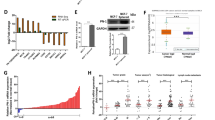
Metastasis is responsible for the death of most breast cancer patients. Robo1 has been implicated as a tumor suppressor for various cancers including breast cancer. However, it is not well understood how Robo1 expression is regulated during tumorigenesis. In this study, we uncovered that the transmembrane proline rich γ-carboxyglutamic acid protein 4 (PRRG4) promotes breast cancer metastasis by downregulating Robo1. Analysis of mRNA expression data in The Cancer Genome Atlas and immunohistochemistry assay on breast tumor samples showed that PRRG4 expression was higher in breast tumors than in normal breast tissues. Experiments with PRRG4 knockdown and overexpression revealed that PRRG4 promoted migration and invasion of breast cancer cells, and enhanced metastasis in an experimental metastasis model. Mechanistically, we found that PRRG4 via its LPSY and PPPY motifs recruited the E3 ubiquitin ligase NEDD4, which induced ubiquitination and degradation of Robo1, thus contributing to migration and invasion of breast cancer cells. In addition, PRRG4 interacted with and enhanced protein tyrosine kinase Src and FAK activation. Overall, our data support a model that PRRG4 via NEDD4 downregulates the Robo1, resulting in the activation of Src and FAK and promoting breast cancer metastasis. PRRG4 may be a novel target for treating metastatic breast cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Fitzmaurice C, Abate D, Abbasi N, Abbastabar H, Abd-Allah F, Abdel-Rahman O, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: a systematic analysis for the global burden of disease study. JAMA Oncol. 2019;5:1749–68.
Nayar U, Cohen O, Kapstad C, Cuoco MS, Waks AG, Wander SA, et al. Acquired HER2 mutations in ER(+) metastatic breast cancer confer resistance to estrogen receptor-directed therapies. Nat Genet. 2019;51:207–16.
Waks AG, Winer EP. Breast cancer treatment: a review. JAMA. 2019;321:288–300.
Kulman JD, Harris JE, Xie L, Davie EW. Identification of two novel transmembrane gamma-carboxyglutamic acid proteins expressed broadly in fetal and adult tissues. Proc Natl Acad Sci USA. 2001;98:1370–5.
Kulman JD, Harris JE, Haldeman BA, Davie EW. Primary structure and tissue distribution of two novel proline-rich gamma-carboxyglutamic acid proteins. Proc Natl Acad Sci USA. 1997;94:9058–62.
Yazicioglu MN, Monaldini L, Chu K, Khazi FR, Murphy SL, Huang H, et al. Cellular localization and characterization of cytosolic binding partners for Gla domain-containing proteins PRRG4 and PRRG2. J Biol Chem. 2013;288:25908–14.
Xu S, Han JC, Morales A, Menzie CM, Williams K, Fan YS. Characterization of 11p14-p12 deletion in WAGR syndrome by array CGH for identifying genes contributing to mental retardation and autism. Cytogenet Genome Res. 2008;122:181–7.
Yamamoto T, Togawa M, Shimada S, Sangu N, Shimojima K, Okamoto N. Narrowing of the responsible region for severe developmental delay and autistic behaviors in WAGR syndrome down to 1.6 Mb including PAX6, WT1, and PRRG4. Am J Med Genet A. 2014;164A:634–8.
Yip KT, Das PK, Suria D, Lim CR, Ng GH, Liew CC. A case-controlled validation study of a blood-based seven-gene biomarker panel for colorectal cancer in Malaysia. J Exp Clin Cancer Res. 2010;29:128.
Wang SZ, Ibrahim LA, Kim YJ, Gibson DA, Leung HC, Yuan W, et al. Slit/Robo signaling mediates spatial positioning of spiral ganglion neurons during development of cochlear innervation. J Neurosci. 2013;33:12242–54.
Whitford KL, Marillat V, Stein E, Goodman CS, Tessier-Lavigne M, Chedotal A, et al. Regulation of cortical dendrite development by Slit-Robo interactions. Neuron. 2002;33:47–61.
Blockus H, Chedotal A. The multifaceted roles of Slits and Robos in cortical circuits: from proliferation to axon guidance and neurological diseases. Curr Opin Neurobiol. 2014;27:82–8.
Blockus H, Chedotal A. Slit-Robo signaling. Development. 2016;143:3037–44.
Dickinson RE, Duncan WC. The SLIT-ROBO pathway: a regulator of cell function with implications for the reproductive system. Reproduction. 2010;139:697–704.
Ballard MS, Hinck L. A roundabout way to cancer. Adv Cancer Res. 2012;114:187–235.
Gara RK, Kumari S, Ganju A, Yallapu MM, Jaggi M, Chauhan SC. Slit/Robo pathway: a promising therapeutic target for cancer. Drug Disco Today. 2015;20:156–64.
Dallol A, Forgacs E, Martinez A, Sekido Y, Walker R, Kishida T, et al. Tumour specific promoter region methylation of the human homologue of the Drosophila Roundabout gene DUTT1 (ROBO1) in human cancers. Oncogene. 2002;21:3020–8.
Schmid BC, Rezniczek GA, Fabjani G, Yoneda T, Leodolter S, Zeillinger R. The neuronal guidance cue Slit2 induces targeted migration and may play a role in brain metastasis of breast cancer cells. Breast Cancer Res Treat. 2007;106:333–42.
Qin F, Zhang H, Ma L, Liu X, Dai K, Li W, et al. Low expression of Slit2 and Robo1 is associated with poor prognosis and brain-specific metastasis of breast cancer patients. Sci Rep. 2015;5:14430.
Bhattacharya R, Mukherjee N, Dasgupta H, Islam MS, Alam N, Roy A, et al. Frequent alterations of SLIT2-ROBO1-CDC42 signalling pathway in breast cancer: clinicopathological correlation. J Genet. 2016;95:551–63.
Wang J, Wang L, Liu FF, Ma YJ, Fu L, Li WL, et al. [Robo1 expression in breast cancer and its relationship to brain metastasis]. Zhonghua Zhong Liu Za Zhi. 2011;33:447–51.
Chang PH, Hwang-Verslues WW, Chang YC, Chen CC, Hsiao M, Jeng YM, et al. Activation of Robo1 signaling of breast cancer cells by Slit2 from stromal fibroblast restrains tumorigenesis via blocking PI3K/Akt/beta-catenin pathway. Cancer Res. 2012;72:4652–61.
Yuasa-Kawada J, Kinoshita-Kawada M, Rao Y, Wu JY. Deubiquitinating enzyme USP33/VDU1 is required for Slit signaling in inhibiting breast cancer cell migration. Proc Natl Acad Sci USA. 2009;106:14530–5.
Marlow R, Strickland P, Lee JS, Wu X, Pebenito M, Binnewies M, et al. SLITs suppress tumor growth in vivo by silencing Sdf1/Cxcr4 within breast epithelium. Cancer Res. 2008;68:7819–27.
Justice ED, Barnum SJ, Kidd T. The WAGR syndrome gene PRRG4 is a functional homologue of the commissureless axon guidance gene. PLoS Genet. 2017;13:e1006865.
Keleman K, Rajagopalan S, Cleppien D, Teis D, Paiha K, Huber LA, et al. Comm sorts robo to control axon guidance at the Drosophila midline. Cell. 2002;110:415–27.
Boase NA, Kumar S. NEDD4: the founding member of a family of ubiquitin-protein ligases. Gene. 2015;557:113–22.
Zhao X, Guan JL. Focal adhesion kinase and its signaling pathways in cell migration and angiogenesis. Adv Drug Deliv Rev. 2011;63:610–5.
Juarez-Cruz JC, Zuniga-Eulogio MD, Olea-Flores M, Castaneda-Saucedo E, Mendoza-Catalan MA, Ortuno-Pineda C, et al. Leptin induces cell migration and invasion in a FAK-Src-dependent manner in breast cancer cells. Endocr Connect. 2019;8:1539–52.
Ke H, Zhao L, Zhang H, Feng X, Xu H, Hao J, et al. Loss of TDP43 inhibits progression of triple-negative breast cancer in coordination with SRSF3. Proc Natl Acad Sci USA. 2018;115:E3426–35.
Nieto MA. The ins and outs of the epithelial to mesenchymal transition in health and disease. Annu Rev Cell Dev Biol. 2011;27:347–76.
Lu W, Kang Y. Epithelial-mesenchymal plasticity in cancer progression and metastasis. Dev Cell. 2019;49:361–74.
Yang N, Morrison CD, Liu P, Miecznikowski J, Bshara W, Han S, et al. TAZ induces growth factor-independent proliferation through activation of EGFR ligand amphiregulin. Cell Cycle. 2012;11:2922–30.
Diepenbruck M, Waldmeier L, Ivanek R, Berninger P, Arnold P, van Nimwegen E, et al. Tead2 expression levels control the subcellular distribution of Yap and Taz, zyxin expression and epithelial-mesenchymal transition. J Cell Sci. 2014;127:1523–36.
Gilestro GF. Redundant mechanisms for regulation of midline crossing in Drosophila. PLoS ONE. 2008;3:e3798.
Myat A, Henry P, McCabe V, Flintoft L, Rotin D, Tear G. Drosophila Nedd4, a ubiquitin ligase, is recruited by Commissureless to control cell surface levels of the roundabout receptor. Neuron. 2002;35:447–59.
Gorla M, Santiago C, Chaudhari K, Layman AAK, Oliver PM, Bashaw GJ. Ndfip proteins target robo receptors for degradation and allow commissural axons to cross the midline in the developing spinal cord. Cell Rep. 2019;26:3298–312.
Xia Y, Wang L, Xu Z, Kong R, Wang F, Yin K, et al. Reduced USP33 expression in gastric cancer decreases inhibitory effects of Slit2-Robo1 signalling on cell migration and EMT. Cell Prolif. 2019;52:e12606.
Nakatsukasa K, Huyer G, Michaelis S, Brodsky JL. Dissecting the ER-associated degradation of a misfolded polytopic membrane protein. Cell. 2008;132:101–12.
Kanelis V, Bruce MC, Skrynnikov NR, Rotin D, Forman-Kay JD. Structural determinants for high-affinity binding in a Nedd4 WW3* domain-Comm PY motif complex. Structure. 2006;14:543–53.
Pluskey S, Wandless TJ, Walsh CT, Shoelson SE. Potent stimulation of SH-PTP2 phosphatase activity by simultaneous occupancy of both SH2 domains. J Biol Chem. 1995;270:2897–900.
Ottinger EA, Botfield MC, Shoelson SE. Tandem SH2 domains confer high specificity in tyrosine kinase signaling. J Biol Chem. 1998;273:729–35.
Naser R, Aldehaiman A, Diaz-Galicia E, Arold ST. Endogenous control mechanisms of FAK and PYK2 and their relevance to cancer development. Cancers. 2018;10:196.
Summy JM, Gallick GE. Src family kinases in tumor progression and metastasis. Cancer Metastasis Rev. 2003;22:337–58.
Patel A, Sabbineni H, Clarke A, Somanath PR. Novel roles of Src in cancer cell epithelial-to-mesenchymal transition, vascular permeability, microinvasion and metastasis. Life Sci. 2016;157:52–61.
Ngan E, Stoletov K, Smith HW, Common J, Muller WJ, Lewis JD, et al. LPP is a Src substrate required for invadopodia formation and efficient breast cancer lung metastasis. Nat Commun. 2017;8:15059.
Calalb MB, Polte TR, Hanks SK. Tyrosine phosphorylation of focal adhesion kinase at sites in the catalytic domain regulates kinase activity: a role for Src family kinases. Mol Cell Biol. 1995;15:954–63.
Wong K, Ren XR, Huang YZ, Xie Y, Liu G, Saito H, et al. Signal transduction in neuronal migration: roles of GTPase activating proteins and the small GTPase Cdc42 in the Slit-Robo pathway. Cell. 2001;107:209–21.
Kong R, Yi F, Wen P, Liu J, Chen X, Ren J, et al. Myo9b is a key player in SLIT/ROBO-mediated lung tumor suppression. J Clin Invest. 2015;125:4407–20.
Wallace AG, Raduwan H, Carlet J, Soto MC. The RhoGAP HUM-7/Myo9 integrates signals to modulate RHO-1/RhoA during embryonic morphogenesis in Caenorhabditis elegans. Development. 2018;145:dev168724.
Yuan T, Ma H, Du Z, Xiong X, Gao H, Fang Z, et al. Shp1 positively regulates EGFR signaling by controlling EGFR protein expression in mammary epithelial cells. Biochem Biophys Res Commun. 2017;488:439–44.
Fang Z, Li T, Chen W, Wu D, Qin Y, Liu M, et al. Gab2 promotes cancer stem cell like properties and metastatic growth of ovarian cancer via downregulation of miR-200c. Exp Cell Res. 2019;382:111462.
He L, Du Z, Xiong X, Ma H, Zhu Z, Gao H, et al. Targeting androgen receptor in treating HER2 positive breast cancer. Sci Rep. 2017;7:14584.
Timms JF, Carlberg K, Gu H, Chen H, Kamatkar S, Nadler MJ, et al. Identification of major binding proteins and substrates for the SH2-containing protein tyrosine phosphatase SHP-1 in macrophages. Mol Cell Biol. 1998;18:3838–50.
Acknowledgements
We thank Dr. Weibao Jiao (Kunming Institute of Zoology, Academia Sinica) for kindly providing us the MDA-MB-231-luciferase cells, and Helen Gu (Department of Economics, Massachusetts Institute of Technology) for proofreading the paper. This work was supported by grants (81972463 and 81772819) from the National Natural Science Foundation of China, and grants (LY20C080001 and LY18H080003) from Natural Science Foundation of Zhejiang Province, China, and was supported in part by Wenzhou Medical University Startup funds and the Key Discipline of Zhejiang Province in Medical Technology (First Class, Category A).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, L., Qin, Y., Wu, G. et al. PRRG4 promotes breast cancer metastasis through the recruitment of NEDD4 and downregulation of Robo1. Oncogene 39, 7196–7208 (2020). https://doi.org/10.1038/s41388-020-01494-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-020-01494-7
This article is cited by
-
Oncoprotein SET-associated transcription factor ZBTB11 triggers lung cancer metastasis
Nature Communications (2024)
-
PRRG4 regulates mitochondrial function and promotes migratory behaviors of breast cancer cells through the Src-STAT3-POLG axis
Cancer Cell International (2023)
-
Circ_0056618 enhances PRRG4 expression by competitively binding to miR-411-5p to promote the malignant progression of colorectal cancer
Molecular and Cellular Biochemistry (2023)
-
Adaptors as the regulators of HECT ubiquitin ligases
Cell Death & Differentiation (2021)