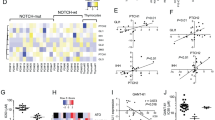
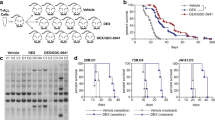
Abstract
Notwithstanding intensified therapy, a considerable fraction of T-cell acute lymphoblastic leukemia (T-ALL) patients face a dismal prognosis due to primary resistance to treatment and relapse, raising the need for more efficient and targeted therapies. Hedgehog (HH) signaling is a major developmental pathway frequently deregulated in cancer, for which a role in T-ALL is emerging. Mounting evidence suggests that ligand-independent activation of HH pathway occurs in cancer including T-ALL, emphasizing the necessity of dissecting the complex interplay between HH and other signaling pathways regulating activation. In this work, we present a therapeutically relevant crosstalk between HH signaling and the glucocorticoid receptor (NR3C1) pathway acting at the level of GLI1 transcription factor. GLI inhibitor GANT61 and dexamethasone were shown to exert a synergistic anti-leukemic effect in vitro in T-ALL cell lines and patient-derived xenografts. Mechanistically, dexamethasone-activated NR3C1 impaired GLI1 function by dynamically modulating the recruitment of PCAF acetyltransferase and HDAC1 deacetylase. Increased GLI1 acetylation was associated with compromised transcriptional activity and reduced protein stability. In summary, our study identifies a novel crosstalk between GLI1 and NR3C1 signaling pathway which could be exploited in HH-dependent malignancies to increase therapeutic efficacy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Belver L, Ferrando A. The genetics and mechanisms of T cell acute lymphoblastic leukaemia. Nat Rev Cancer 2016;16:494–507.
Hunger SP, Mullighan CG. Acute Lymphoblastic Leukemia in Children. N Engl J Med. 2015;373:1541–52.
Gallet A. Hedgehog morphogen: from secretion to reception. Trends Cell Biol. 2011;21:238–46.
Riddle RD, Johnson RL, Laufer E, Tabin C. Sonic hedgehog mediates the polarizing activity of the ZPA. Cell 1993;75:1401–16.
Fuccillo M, Joyner AL, Fishell G. Morphogen to mitogen: the multiple roles of hedgehog signalling in vertebrate neural development. Nat Rev Neurosci. 2006;7:772–83.
Nieuwenhuis E, Hui CC. Hedgehog signaling and congenital malformations. Clin Genet. 2005;67:193–208.
Crompton T, Outram SV, Hager-Theodorides AL. Sonic hedgehog signalling in T-cell development and activation. Nat Rev Immunol. 2007;7:726–35.
Petrova R, Joyner AL. Roles for Hedgehog signaling in adult organ homeostasis and repair. Development 2014;141:3445–57.
Ramsbottom SA, Pownall ME. Regulation of hedgehog signalling inside and outside the cell. J Dev Biol. 2016;4:23.
Pak E, Segal RA. Hedgehog signal transduction: key players, oncogenic drivers, and cancer therapy. Dev Cell 2016;38:333–44.
Wu F, Zhang Y, Sun B, McMahon AP, Wang Y. Hedgehog signaling: from basic biology to cancer therapy. Cell Chem Biol. 2017;24:252–80.
Ding Q, Motoyama J, Gasca S, Mo R, Sasaki H, Rossant J, et al. Diminished Sonic hedgehog signaling and lack of floor plate differentiation in Gli2 mutant mice. Development 1998;125:2533–43.
Matise MP, Epstein DJ, Park HL, Platt KA, Joyner AL. Gli2 is required for induction of floor plate and adjacent cells, but not most ventral neurons in the mouse central nervous system. Development 1998;125:2759–70.
Litingtung Y, Chiang C. Specification of ventral neuron types is mediated by an antagonistic interaction between Shh and Gli3. Nat Neurosci. 2000;3:979–85.
Persson M, Stamataki D, te Welscher P, Andersson E, Bose J, Ruther U, et al. Dorsal-ventral patterning of the spinal cord requires Gli3 transcriptional repressor activity. Genes Dev. 2002;16:2865–78.
Dai P, Akimaru H, Tanaka Y, Maekawa T, Nakafuku M, Ishii S. Sonic Hedgehog-induced activation of the Gli1 promoter is mediated by GLI3. J Biol Chem. 1999;274:8143–52.
Amakye D, Jagani Z, Dorsch M. Unraveling the therapeutic potential of the Hedgehog pathway in cancer. Nat Med. 2013;19:1410–22.
Rubin LL, de Sauvage FJ. Targeting the Hedgehog pathway in cancer. Nat Rev Drug Discov. 2006;5:1026–33.
Dierks C, Grbic J, Zirlik K, Beigi R, Englund NP, Guo GR, et al. Essential role of stromally induced hedgehog signaling in B-cell malignancies. Nat Med. 2007;13:944–51.
Hegde GV, Peterson KJ, Emanuel K, Mittal AK, Joshi AD, Dickinson JD, et al. Hedgehog-induced survival of B-cell chronic lymphocytic leukemia cells in a stromal cell microenvironment: a potential new therapeutic target. Mol Cancer Res. 2008;6:1928–36.
Pandolfi S, Stecca B. Cooperative integration between HEDGEHOG-GLI signalling and other oncogenic pathways: implications for cancer therapy. Expert Rev Mol Med. 2015;17:e5.
Pietrobono S, Gagliardi S, Stecca B. Non-canonical hedgehog signaling pathway in cancer: activation of GLI transcription factors beyond smoothened. Front Genet. 2019;10:556.
Burns MA, Liao ZW, Yamagata N, Pouliot GP, Stevenson KE, Neuberg DS, et al. Hedgehog pathway mutations drive oncogenic transformation in high-risk T-cell acute lymphoblastic leukemia. Leukemia 2018;32:2126–37.
Dagklis A, Demeyer S, De Bie J, Radaelli E, Pauwels D, Degryse S, et al. Hedgehog pathway activation in T-cell acute lymphoblastic leukemia predicts response to SMO and GLI1 inhibitors. Blood 2016;128:2642–54.
Dagklis A, Pauwels D, Lahortiga I, Geerdens E, Bittoun E, Cauwelier B, et al. Hedgehog pathway mutations in T-cell acute lymphoblastic leukemia. Haematologica 2015;100:e102–5.
Hou X, Chen X, Zhang P, Fan Y, Ma A, Pang T, et al. Inhibition of hedgehog signaling by GANT58 induces apoptosis and shows synergistic antitumor activity with AKT inhibitor in acute T cell leukemia cells. Biochimie 2014;101:50–9.
Chiang M, Xu L, Shestova O, Histen G, L’heureux S, Romany C, et al. Leukemia-associated NOTCH1 Alleles Are Weak Tumor Initiators but Accelerate K-ras-initiated Leukemia. J Clin Investig. 2008;118:3181–94.
Schreck K, Taylor P, Marchionni L, Gopalakrishnan V, Bar E, Gaiano N, et al. The notch target Hes1 directly modulates Gli1 expression and hedgehog signaling: a potential mechanism of therapeutic resistance. Clin Cancer Res. 2010;16:6060–70.
Ingram W, McCue K, Tran T, Hallahan A, Wainwright B. Sonic Hedgehog regulates Hes1 through a novel mechanism that is independent of canonical notch pathway signalling. Oncogene 2008;27:1489–500.
Inaba H, Pui C-H. Glucocorticoid use in acute lymphoblastic leukemia: comparison of prednisone and dexamethasone. Lancet Oncol. 2010;11:1096–106.
Schrappe M, Valsecchi MG, Bartram CR, Schrauder A, Panzer-Grumayer R, Moricke A, et al. Late MRD response determines relapse risk overall and in subsets of childhood T-cell ALL: results of the AIEOP-BFM-ALL 2000 study. Blood 2011;118:2077–84.
Schmidt T, Meyer A. Autoregulation of Corticosteroid Receptors. How, When, Where, and Why? Receptor 1994;4:229–57.
Hoeck W, Rusconi S. Groner B. Down-regulation and phosphorylation of glucocorticoid receptors in cultured cells. Investigations with a monospecific antiserum against a bacterially expressed receptor fragment. J Biol Chem. 1989;264:14396–402.
Schwartz J, Sarvaiya P, Vedeckis W. Glucocorticoid receptor knock down reveals a similar apoptotic threshold but differing gene regulation patterns in T-cell and pre-B-cell acute lymphoblastic leukemia. Mol Cell Endocrinol. 2010;320:76–86.
Niewiadomski P, Niedziolka SM, Markiewicz L, Uspienski T, Baran B, Chojnowska K. Gli proteins: regulation in development and cancer. Cells 2019;8:147.
Canettieri G, Di Marcotullio L, Greco A, Coni S, Antonucci L, Infante P, et al. Histone deacetylase and Cullin3-REN(KCTD11) ubiquitin ligase interplay regulates Hedgehog signalling through Gli acetylation. Nat Cell Biol. 2010;12:132–42.
Coni S, Antonucci L, D’Amico D, Di Magno L, Infante P, De Smaele E, et al. Gli2 acetylation at lysine 757 regulates hedgehog-dependent transcriptional output by preventing its promoter occupancy. PLoS ONE 2013;8:e65718.
Coni S, Mancuso AB, Di Magno L, Sdruscia G, Manni S, Serrao SM, et al. Selective targeting of HDAC1/2 elicits anticancer effects through Gli1 acetylation in preclinical models of SHH Medulloblastoma. Sci Rep. 2017;7:44079.
Kassel O, Herrlich P. Crosstalk between the glucocorticoid receptor and other transcription factors: molecular aspects. Mol Cell Endocrinol. 2007;275:13–29.
Newton R, Holden NS. Separating transrepression and transactivation: a distressing divorce for the glucocorticoid receptor? Mol Pharmacol. 2007;72:799–809.
Malatesta M, Steinhauer C, Mohammad F, Pandey DP, Squatrito M, Helin K. Histone acetyltransferase PCAF is required for Hedgehog-Gli-dependent transcription and cancer cell proliferation. Cancer Res. 2013;73:6323–33.
Gulino A, Di Marcotullio L, Canettieri G, De Smaele E, Screpanti I. Hedgehog/Gli control by ubiquitination/acetylation interplay. Vitam Horm. 2012;88:211–27.
Wang Y, Davidow L, Arvanites AC, Blanchard J, Lam K, Xu K, et al. Glucocorticoid compounds modify smoothened localization and hedgehog pathway activity. Chem Biol. 2012;19:972–82.
Wang J, Lu J, Bond MC, Chen M, Ren X-R, Lyerly HK, et al. Identification of select glucocorticoids as Smoothened agonists: Potential utility for regenerative medicine. Proc Natl Acad Sci USA. 2010;107:9323–8.
Heine VM, Rowitch DH. Hedgehog signaling has a protective effect in glucocorticoid-induced mouse neonatal brain injury through an 11betaHSD2-dependent mechanism. J Clin Investig. 2009;119:267–77.
Wang J, Barak LS, Mook RA Jr, Chen W. Glucocorticoid hedgehog agonists in neurogenesis. Vitam Horm. 2011;87:207–15.
Heine VM, Griveau A, Chapin C, Ballard PL, Chen JK, Rowitch DH. A small-molecule smoothened agonist prevents glucocorticoid-induced neonatal cerebellar injury. Sci Transl Med. 2011;3:105ra4.
Gruber W, Peer E, Elmer D, Sternberg C, Tesanovic S, Del Burgo P, et al. Targeting Class I histone deacetylases by the novel small molecule inhibitor 4SC-202 blocks oncogenic hedgehog-GLI signaling and overcomes smoothened inhibitor resistance. Int J Cancer 2018;142:968–75.
Tosello V, Milani G, Martines A, Macri N, Van Loocke W, Matthijssens F, et al. A Novel t(8;14)(q24;q11) rearranged human cell line as a model for mechanistic and drug discovery studies of NOTCH1-Independent Human T-Cell Leukemia. Cells 2018;7:160.
Acknowledgements
We are grateful to Prof Warren Pear (University of Pennsylvania, Philadelphia, U.S.A) for the MigR1vector, Prof Raphael Kopan (Washington University, St. Louis, U.S.A.) for MigR1 vectors expressing ΔE-NOTCH1 mutant allele, Prof J. Aster (Brigham and Women’s Hospital, Harvard Medical School, Boston, U.S.A.) for the MigR1 HD-ΔPEST (NOTCH1 L1601P-ΔPEST) vector. Histidine-HA-Ubiquitin vector was a kind gift from Richard Baer (Columbia University). We thank Sonia Minuzzo and Marica Pinazza for providing T-ALL xenografts, Elena Laura Mazzoldi for cell sorting, Elena Masiero for technical assistance. We are grateful to Pieter Van Vlierberghe and Giorgio Arrigoni for reagents and suggestions.
Funding
This work was supported by the Italian Foundation for Cancer Research (Fondazione AIRC) grants to EP (IG2018#22233); Progetto di Ricerca di Ateneo (SID19_01; Università di Padova) to EP. Ministero dell’Istruzione, dell’Università e della Ricerca (MIUR) Ex 60% to EP; Istituto Oncologico Veneto 5 × 1000 fund to EP.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bongiovanni, D., Tosello, V., Saccomani, V. et al. Crosstalk between Hedgehog pathway and the glucocorticoid receptor pathway as a basis for combination therapy in T-cell acute lymphoblastic leukemia. Oncogene 39, 6544–6555 (2020). https://doi.org/10.1038/s41388-020-01453-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-020-01453-2