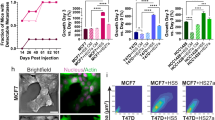
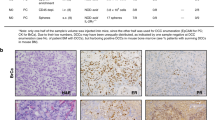
Abstract
Estrogen receptor-positive (ER+) breast cancer can recur up to 20 years after initial diagnosis. Delayed recurrences arise from disseminated tumors cells (DTCs) in sites such as bone marrow that remain quiescent during endocrine therapy and subsequently proliferate to produce clinically detectable metastases. Identifying therapies that eliminate DTCs and/or effectively target cells transitioning to proliferation promises to reduce risk of recurrence. To tackle this problem, we utilized a 3D co-culture model incorporating ER+ breast cancer cells and bone marrow mesenchymal stem cells to represent DTCs in a bone marrow niche. 3D co-cultures maintained cancer cells in a quiescent, viable state as measured by both single-cell and population-scale imaging. Single-cell imaging methods for metabolism by fluorescence lifetime (FLIM) of NADH and signaling by kinases Akt and ERK revealed that breast cancer cells utilized oxidative phosphorylation and signaling by Akt to a greater extent both in 3D co-cultures and a mouse model of ER+ breast cancer cells in bone marrow. Using our 3D co-culture model, we discovered that combination therapies targeting oxidative phosphorylation via the thioredoxin reductase (TrxR) inhibitor, D9, and the Akt inhibitor, MK-2206, preferentially eliminated breast cancer cells without altering viability of bone marrow stromal cells. Treatment of mice with disseminated ER+ human breast cancer showed that D9 plus MK-2206 blocked formation of new metastases more effectively than tamoxifen. These data establish an integrated experimental system to investigate DTCs in bone marrow and identify combination therapy against metabolic and kinase targets as a promising approach to effectively target these cells and reduce risk of recurrence in breast cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Code availability
All custom MATLAB code including the image processing files require a material transfer agreement from the University of Michigan.
References
Pan H, Gray R, Braybrooke J, Davies C, Taylor C, McGale P, et al. 20-year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N. Engl J Med. 2017;377:1836–46.
Zhang Y, Schnabel CA, Schroeder BE, Jerevall P-L, Jankowitz RC, Fornander T, et al. Breast cancer index identifies early-stage estrogen receptor–positive breast cancer patients at risk for early- and late-distant recurrence. Clin Cancer Res. 2013;19:4196.
Zhang XHF, Giuliano M, Trivedi MV, Schiff R, Kent OC. Metastasis dormancy in estrogen receptor-positive breast cancer. Clin. Cancer Res. 2013. https://doi.org/10.1158/078-0432.CCR-13-838.
Pantel K, Alix-Panabieres C. Bone marrow as a reservoir for disseminated tumor cells: a special source for liquid biopsy in cancer patients. Bonekey Rep. 2014;3:584.
Chambers A, Groom A, MacDonald I. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2:563–72.
Kennecke H, Yerushalmi R, Woods R, Cheang MCU, Voduc D, Speers CH, et al. Metastatic behavior of breast cancer subtypes. J Clin Oncol. 2010;28:3271–7.
Bartkowiak K, Riethdorf S, Pantel K. The interrelating dynamics of hypoxic tumor microenvironments and cancer cell phenotypes in cancer metastasis. Cancer Microenviron. 2012;5:59–72.
Luengo A, Gui DY, Vander Heiden MG. Targeting metabolism for cancer therapy. Cell Chem Biol. 2017;24:1161–80.
Gross S, Rahal R, Stransky N, Lengauer C, Hoeflich KP. Targeting cancer with kinase inhibitors. J Clin Investig. 2015;125:1780–9.
Kohno M, Pouyssegur J. Targeting the ERK signaling pathway in cancer therapy. Ann Med. 2006;38:200–11.
Martini M, De Santis MC, Braccini L, Gulluni F, Hirsch E. PI3K/AKT signaling pathway and cancer: an updated review. Ann Med. 2014;46:372–83.
Pradhan S, Sperduto JL, Farino CJ, Slater JH. Engineered in vitro models of tumor dormancy and reactivation. J Biol Eng. 2018;12:37.
Mehta G, Hsiao A, Ingram M, Luker G, Takayama S. Opportunities and challenges for use of tumor spheroids as models to test drug delivery and efficacy. J Control Release. 2012;164:192–204.
Widner DB, Park SH, Eber MR, Shiozawa Y. Interactions between disseminated tumor cells and bone marrow stromal cells regulate tumor dormancy. Curr Osteoporos Rep. 2018;16:596–602.
Cavnar S, Rickelmann A, Meguiar K, Xiao A, Dosch J, Leung B, et al. Modeling selective elimination of quiescent cancer cells from bone marrow. Neoplasia 2015;17:625–33.
Iwata M, Sandstrom R, Delrow J, Stamatoyannopoulos J, Torok-Storb B. Functionally and phenotypically distinct subpopulations of marrow stromal cells are fibroblast in origin and induce different fates in peripheral blood monocytes. Stem Cells Dev. 2014;23:729–40.
Sakaue-Sawano A, Kuorkawa H, Morimura T, Hanyu A, Hama H, Osawa H, et al. Visualizing spatiotemporal dynamics of multicellular cell-cycle progression. Cell 2008;132:487–98.
Pozarowski P, Darzynkiewicz Z. Analysis of cell cycle by flow cytometry. Methods Mol Biol. 2004;281:301–11.
Humphries BA, Buschhaus JM, Chen YC, Haley HR, Qyli T, Chiang B, et al. Plasminogen Activator Inhibitor 1 (PAI1) promotes actin cytoskeleton reorganization and glycolytic metabolism in triple-negative breast cancer. Mol Cancer Res. 2019;17:1142–1154.
Stringari C, Nourse JL, Flanagan LA, Gratton E. Phasor fluorescence lifetime microscopy of free and protein-bound nadh reveals neural stem cell differentiation potential. PLOS ONE 2012;7:e48014.
Maryu G, Matsuda M, Aoki K. Multiplexed fluorescence imaging of ERK and Akt activities and cell-cycle progression. Cell Struct Funct. 2016;41:81–92.
Spinosa PC, Humphries BA, Lewin Mejia D, Buschhaus JM, Linderman JJ, Luker GD, et al. Short-term cellular memory tunes the signaling responses of the chemokine receptor CXCR4. Science Signaling. 2019;12:eaaw4204. https://stke.sciencemag.org/content/12/589/eaaw4204.long.
Regot S, Hughey JJ, Bajar BT, Carrasco S, Covert MW. High-sensitivity measurements of multiple kinase activities in live single cells. Cell 2014;157:1724–34.
Aka JA, Lin S-X. Comparison of functional proteomic analyses of human breast cancer cell lines T47D and MCF7. PloS one 2012;7:e31532-e.
Wang H, Yu C, Gao X, Welte T, Muscarella A, Tian L, et al. The osteogenic niche promotes early-stage bone colonization of disseminated breast cancer cells. Cancer Cell 2015;27:193–210.
Haley HR, Shen N, Qyli T, Buschhaus JM, Pirone ME, Luker KE, et al., editors. Enhanced bone metastases in skeletally immature mice. Tomography. 2018;4:84–93.
Walsh AJ, Poole KM, Duvall CL, Skala MC. Ex vivo optical metabolic measurements from cultured tissue reflect in vivo tissue status. J Biomed Opt. 2012;17:116015.
Arnér ESJ, Holmgren A. Physiological functions of thioredoxin and thioredoxin reductase. Eur J Biochem. 2000;267:6102–9.
LeBleu VS, O’Connell JT, Gonzalez Herrera KN, Wikman H, Pantel K, Haigis Marcia C, et al. PGC-1α mediates mitochondrial biogenesis and oxidative phosphorylation in cancer cells to promote metastasis. Nat Cell Biol. 2014;16:992.
Weinberg SE, Chandel NS. Targeting mitochondria metabolism for cancer therapy. Nat Chem Biol. 2014;11:9.
Mustacich D, Powis G. Thioredoxin reductase. Biochemical J. 2000;346:1–8. Pt 1
Scalcon V, Bindoli A, Rigobello M. Significance of the mitochondrial thioredoxin reductase in cancer cells: an update on role, targets and inhibitors. Free Radic. Biol. Med. 2018;127:62–79.
Zhang D, Xu Z, Yuan J, Zhao Y-X, Qiao Z-Y, Gao Y-J, et al. Synthesis and molecular recognition studies on small-molecule inhibitors for thioredoxin reductase. J Medicinal Chem. 2014;57:8132–9.
Luo M, Shang L, Brooks MD, Jiagge E, Zhu Y, Buschhaus JM, et al. Targeting breast cancer stem cell state equilibrium through modulation of redox signaling. Cell Metab 2018;28:69–86.e6.
Yarosz EL, Chang C-H. The role of reactive oxygen species in regulating T cell-mediated immunity and disease. Immune Netw 2018;18:e14-e.
Patel CH, Leone RD, Horton MR, Powell JD. Targeting metabolism to regulate immune responses in autoimmunity and cancer. Nat Rev Drug Discov. 2019;18:669–88.
Yin Z, Bai L, Li W, Zeng T, Tian H, Cui J. Targeting T cell metabolism in the tumor microenvironment: an anti-cancer therapeutic strategy. J Exp Clin Cancer Res. 2019;38:403.
Sosa MS, Avivar-Valderas A, Bragado P, Wen H-C, Aguirre-Ghiso JA. ERK1/2 and p38α/β signaling in tumor cell quiescence: opportunities to control dormant residual disease. Clin Cancer Res. 2011;17:5850–7.
Shi J, Wang L, Zou C, Xia Y, Qin S, Keller E, et al. Tumor microenvironment promotes prostate cancer cell dissemination via the Akt/mTOR pathway. Oncotarget 2018;9:9206–18.
Zhang XHF, Wang Q, Gerald W, Hudis CA, Norton L, Smid M, et al. Latent bone metastasis in breast cancer tied to Src-dependent survival signals. Cancer Cell 2009;16:67–78.
Grabinski N, Bartkowiak K, Grupp K, Brandt B, Pantel K, Jücker M. Distinct functional roles of Akt isoforms for proliferation, survival, migration and EGF-mediated signalling in lung cancer derived disseminated tumor cells. Cell Signal 2011;23:1952–60.
Jabbarzadeh Kaboli P, Salimian F, Aghapour S, Xiang S, Zhao Q, Li M, et al. Akt-targeted therapy as a promising strategy to overcome drug resistance in breast cancer—a comprehensive review from chemotherapy to immunotherapy. Pharmacol Res 2020;156:104806.
Abu-Eid R, Samara RN, Ozbun L, Abdalla MY, Berzofsky JA, Friedman KM, et al. Selective inhibition of regulatory T cells by targeting the PI3K-Akt pathway. Cancer Immunol Res. 2014;2:1080–9.
Ding W, Shanafelt TD, Lesnick CE, Erlichman C, Leis JF, Secreto C, et al. Akt inhibitor MK2206 selectively targets CLL B-cell receptor induced cytokines, mobilizes lymphocytes and synergizes with bendamustine to induce CLL apoptosis. Br J Haematol. 2014;164:146–50.
Xue G, Zippelius A, Wicki A, Mandalà M, Tang F, Massi D, et al. Integrated Akt/PKB signaling in immunomodulation and its potential role in cancer immunotherapy. J Natl Cancer Inst. 2015;107:djv171.
Tripathy D, Chien AJ, Hylton N, Buxton MB, Ewing CA, Wallace AM, et al. Adaptively randomized trial of neoadjuvant chemotherapy with or without the Akt inhibitor MK-2206: graduation results from the I-SPY 2 Trial. J Clin Oncol. 2015;33:524
Heinz S, Freyberger A, Lawrenz B, Schladt L, Schmuck G, Ellinger-Ziegelbauer H. Mechanistic investigations of the mitochondrial complex I inhibitor rotenone in the context of pharmacological and safety evaluation. Sci Rep. 2017;7:45465-.
Thakur S, Daley B, Gaskins K, Vasko VV, Boufraqech M, Patel D, et al. Metformin targets mitochondrial glycerophosphate dehydrogenase to control rate of oxidative phosphorylation and growth of thyroid cancer in vitro and in vivo. Clin Cancer Res. 2018;24:4030–43
Melzer C, Yang Y, Hass R. Interaction of MSC with tumor cells. Cell Commun Signal: Ccs 2016;14:20-.
Plava J, Cihova M, Burikova M, Matuskova M, Kucerova L, Miklikova S. Recent advances in understanding tumor stroma-mediated chemoresistance in breast cancer. Mol cancer 2019;18:67-.
Vallabhaneni KC, Penfornis P, Dhule S, Guillonneau F, Adams KV,Yuan Mo Y, et al. Extracellular vesicles from bone marrow mesenchymal stem/stromal cells transport tumor regulatory microRNA, proteins, and metabolites. Oncotarget. 2015;6:4953–67
Zhong W, Tong Y, Li Y, Yuan J, Hu S, Hu T, et al. Mesenchymal stem cells in inflammatory microenvironment potently promote metastatic growth of cholangiocarcinoma via activating Akt/NF-κB signaling by paracrine CCL5. Oncotarget 2017;8:73693–704.
Özdemir B, Sflomos G, Brisken C. The challenges of modeling hormone receptor-positive breast cancer in mice. Endocr-Relat Cancer. 2018;25:ERC-18.
Ottewell PD, Wang N, Brown HK, Reeves KJ, Fowles CA, Croucher PI, et al. Zoledronic acid has differential antitumor activity in the pre- and postmenopausal bone microenvironment in vivo. Clin Cancer Res. 2014;20:2922–32.
Lu X, Mu E, Wei Y, Riethdorf S, Yang Q, Yuan M, et al. VCAM-1 promotes osteolytic expansion of indolent bone micrometastasis of breast cancer by engaging α4β1-positive osteoclast progenitors. Cancer cell 2011;20:701–14.
Ogba N, Manning NG, Bliesner BS, Ambler SK, Haughian JM, Pinto MP, et al. Luminal breast cancer metastases and tumor arousal from dormancy are promoted by direct actions of estradiol and progesterone on the malignant cells. Breast Cancer Res. 2014;16:489.
Holen I, Walker M, Nutter F, Fowles A, Evans CA, Eaton CL, et al. Oestrogen receptor positive breast cancer metastasis to bone: inhibition by targeting the bone microenvironment in vivo. Clin Exp Metastasis. 2016;33:211–24.
Buschhaus JM, Luker KE, Luker GD. A Facile, In Vitro 384-Well Plate System to Model Disseminated Tumor Cells in the Bone Marrow Microenvironment. In: Lacorazza HD, editor. Cellular Quiescence: Methods and Protocols. New York: Springer New York; 2018. p. 201–13.
Cavnar S, Xiao A, Gibbons A, Rickelmann A, Neely T, Luker K, et al. Imaging sensitivity of quiescent cancer cells to metabolic perturbations in bone marrow spheroids. Tomography. 2016;2:146–57.
Cavnar S, Salomonsson E, Luker K, Luker G, Takayama S. Transfer, imaging, and analysis plate for facile handling of 384 hanging drop 3D tissue spheroids. J Lab Autom. 2014;19:208–14.
Phansalkar N, More S, Sabale A, Joshi MS. Adaptive local thresholding for detection of nuclei in diversity stained cytology images. In Proc 2011 International Conference on Communications and Signal Processing. IEEE (Institute of Electrical and Electronics Engineers) 2011 p. 218–20. https://ieeexplore.ieee.org/document/5739305.
Eckley SS, Buschhaus JM, Humphries BA, Robison TH, Luker KE, Luker GD. Short-term environmental conditioning generates cellular memory that enhances tumorigenic potential of triple-negative breast cancer cells. Tomography. 2019;5:346–357
Hirai H, Sootome H, Nakatsuru Y, Miyama K, Taguchi S, Tsujioka K, et al. MK-2206, an allosteric Akt inhibitor, enhances antitumor efficacy by standard chemotherapeutic agents or molecular targeted drugs in vitro and in vivo. Mol Cancer Ther. 2010;9:1956
Ma G, He J, Yu Y, Xu Y, Yu X, Martinez J, et al. Tamoxifen inhibits ER-negative breast cancer cell invasion and metastasis by accelerating Twist1 degradation. Int J Biol Sci. 2015;11:618–28.
Acknowledgements
We thank Ayşe J. Muñiz and Max Wicha for paper feedback. We thank Michael Pihalja for assistance with flow cytometry. We acknowledge funding from United States National Institutes of Health grants R01CA238042, R01CA196018, U01CA210152, R01CA238023, R33CA225549, R50CA221807, and R37CA222563. Brock Humphries, Ph.D., was supported by an American Cancer Society - Michigan Cancer Research Fund Postdoctoral Fellowship, PF-18-236-01-CCG. We acknowledge support to the University of Michigan Rogel Cancer Center through National Institutes of Health grant P30CA046592 for flow cytometry and animal imaging studies. This material is based upon work supported by the National Science Foundation Graduate Research Fellowship under Grant No. DGE 1256260 to Johanna Buschhaus.
Author information
Authors and Affiliations
Contributions
JMB, KEL, and GDL conceptualized and designed the study. BAH and KEL provided reagents. JMB, SSE, SR, BAH, THR, HRH, ASB, and ACC performed experiments. JMB and KEL wrote MATLAB code. JMB, BAH, KEL, and GDL acquired funding. JMB, SR, and THR analyzed data. JMB and GDL wrote the paper. All authors reviewed the paper before submission.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Buschhaus, J.M., Humphries, B.A., Eckley, S.S. et al. Targeting disseminated estrogen-receptor-positive breast cancer cells in bone marrow. Oncogene 39, 5649–5662 (2020). https://doi.org/10.1038/s41388-020-01391-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-020-01391-z
This article is cited by
-
The Role of Breast Cancer Cells in Bone Metastasis: Suitable Seeds for Nourishing Soil
Current Osteoporosis Reports (2024)
-
Three-Dimensional Tumor Models to Study Cancer Stemness-Mediated Drug Resistance
Cellular and Molecular Bioengineering (2024)
-
The role of cancer cell bioenergetics in dormancy and drug resistance
Cancer and Metastasis Reviews (2023)
-
Recent insights into the effects of metabolism on breast cancer cell dormancy
British Journal of Cancer (2022)
-
Epithelial-mesenchymal transition in cancer stemness and heterogeneity: updated
Medical Oncology (2022)