Abstract
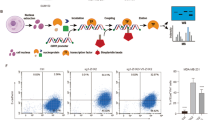
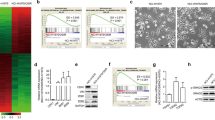
Cancer cells with mesenchymal attributes potentially display chemoresistance. Cancer stem cells (CSCs), which are intrinsically resistant to most chemotherapy agents, exhibit considerable phenotypic heterogeneity in their epithelial versus mesenchymal states. However, the drug response of CSCs in the epithelial and mesenchymal states has not been completely investigated. In this study, we found that epithelial-type (E-cadherinhigh/CD133high) CSCs displayed a higher sphere formation ability and chemoresistance than mesenchymal-type (E-cadherinlowCD133high) CSCs. Gene expression profiling of the CSC and non-CSC subpopulations with distinct epithelial-to-mesenchymal transition (EMT) states showed that MyoD family inhibitor domain-containing (MDFIC) was selectively upregulated in epithelial-type CSCs. Knockdown of MDFIC sensitized epithelial-type CSCs to chemotherapy agents. Ectopic expression of MDFIC increased the chemoresistance of mesenchymal-type CSCs. In a tissue microarray, high MDFIC expression was associated with poor prognosis of non-small cell lung cancer (NSCLC) patients. A mechanistic study showed that the MDFIC p32 isoform, which is located in the cytoplasm, interacted with the destruction complex, Axin/GSK-3/β-catenin. This interaction stabilized β-catenin by inhibiting β-catenin phosphorylation at S33/37 and increased the nuclear translocation and transcriptional activity of β-catenin. Knockdown of β-catenin decreased MDFIC-enhanced chemoresistance. These results suggested that the upregulation of MDFIC enhanced the chemoresistance of epithelial-type CSCs by elevating β-catenin activity. Thus, targeting MDFIC-regulated β-catenin signaling of epithelial-type CSCs may be a potential strategy to overcome chemoresistance in NSCLC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34.
Reck M, Rabe KF. Precision diagnosis and treatment for advanced non-small-cell lung cancer. N Engl J Med. 2017;377:849–61.
Gandhi L, Rodriguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378:2078–92.
Wu DM, Zhang T, Liu YB, Deng SH, Han R, Liu T, et al. The PAX6-ZEB2 axis promotes metastasis and cisplatin resistance in non-small cell lung cancer through PI3K/AKT signaling. Cell Death Dis. 2019;10:349.
Yadav AK, Desai NS. Cancer stem cells: acquisition, characteristics, therapeutic implications, targeting strategies and future prospects. Stem Cell Rev Rep. 2019;15:331–55.
Heng WS, Gosens R, Kruyt FAE. Lung cancer stem cells: origin, features, maintenance mechanisms and therapeutic targeting. Biochem Pharmacol. 2019;160:121–33.
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–15.
Gupta PB, Pastushenko I, Skibinski A, Blanpain C, Kuperwasser C. Phenotypic plasticity: driver of cancer initiation, progression, and therapy resistance. Cell Stem Cell. 2019;24:65–78.
Mittal V. Epithelial mesenchymal transition in aggressive lung cancers. Adv Exp Med Biol. 2016;890:37–56.
Beck TN, Korobeynikov VA, Kudinov AE, Georgopoulos R, Solanki NR, Andrews-Hoke M, et al. Anti-Mullerian hormone signaling regulates epithelial plasticity and chemoresistance in lung cancer. Cell Rep. 2016;16:657–71.
Collisson EA, Sadanandam A, Olson P, Gibb WJ, Truitt M, Gu S, et al. Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy. Nat Med. 2011;17:500–3.
Ocana OH, Corcoles R, Fabra A, Moreno-Bueno G, Acloque H, Vega S, et al. Metastatic colonization requires the repression of the epithelial-mesenchymal transition inducer Prrx1. Cancer Cell. 2012;22:709–24.
Yu M, Bardia A, Wittner BS, Stott SL, Smas ME, Ting DT, et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science. 2013;339:580–4.
Luo M, Shang L, Brooks MD, Jiagge E, Zhu Y, Buschhaus JM, et al. Targeting breast cancer stem cell state equilibrium through modulation of redox signaling. Cell Metab. 2018;28:69–86.e6.
Vijay GV, Zhao N, Den Hollander P, Toneff MJ, Joseph R, Pietila M, et al. GSK3beta regulates epithelial-mesenchymal transition and cancer stem cell properties in triple-negative breast cancer. Breast Cancer Res. 2019;21:37.
Chang YW, Su YJ, Hsiao M, Wei KC, Lin WH, Liang CL, et al. Diverse targets of beta-catenin during the epithelial-mesenchymal transition define cancer stem cells and predict disease relapse. Cancer Res. 2015;75:3398–410.
Song C, Lu R, Bienzle D, Liu HC, Yoo D. Interaction of the porcine reproductive and respiratory syndrome virus nucleocapsid protein with the inhibitor of MyoD family-a domain-containing protein. Biol Chem. 2009;390:215–23.
Szklarczyk D, Morris JH, Cook H, Kuhn M, Wyder S, Simonovic M, et al. The STRING database in 2017: quality-controlled protein-protein association networks, made broadly accessible. Nucleic Acids Res. 2017;45:D362–8.
Szklarczyk D, Gable AL, Lyon D, Junge A, Wyder S, Huerta-Cepas J, et al. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019;47:D607–13.
Tiran V, Lindenmann J, Brcic L, Heitzer E, Stanzer S, Tabrizi-Wizsy NG, et al. Primary patient-derived lung adenocarcinoma cell culture challenges the association of cancer stem cells with epithelial-to-mesenchymal transition. Sci Rep. 2017;7:10040.
Pore M, Meijer C, de Bock GH, Boersma-van EkW, Terstappen LW, Groen HJ, et al. Cancer stem cells, epithelial to mesenchymal markers, and circulating tumor cells in small cell lung cancer. Clin Lung Cancer. 2016;17:535–42.
Sullivan JP, Spinola M, Dodge M, Raso MG, Behrens C, Gao B, et al. Aldehyde dehydrogenase activity selects for lung adenocarcinoma stem cells dependent on notch signaling. Cancer Res. 2010;70:9937–48.
Huang X, Zhu H, Gao Z, Li J, Zhuang J, Dong Y, et al. Wnt7a activates canonical Wnt signaling, promotes bladder cancer cell invasion, and is suppressed by miR-370-3p. J Biol Chem. 2018;293:6693–706.
Li G, Su Q, Liu H, Wang D, Zhang W, Lu Z, et al. Frizzled7 promotes epithelial-to-mesenchymal transition and stemness via activating canonical Wnt/beta-catenin pathway in gastric cancer. Int J Biol Sci. 2018;14:280–93.
Yakisich JS, Azad N, Kaushik V, O’Doherty GA, Iyer AK. Nigericin decreases the viability of multidrug-resistant cancer cells and lung tumorspheres and potentiates the effects of cardiac glycosides. Tumour Biol. 2017;39:1010428317694310.
Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y, et al. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell. 2002;108:837–47.
MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17:9–26.
Chen CM, Kraut N, Groudine M, Weintraub H. I-mf, a novel myogenic repressor, interacts with members of the MyoD family. Cell. 1996;86:731–41.
Thebault S, Gachon F, Lemasson I, Devaux C, Mesnard JM. Molecular cloning of a novel human I-mfa domain-containing protein that differently regulates human T-cell leukemia virus type I and HIV-1 expression. J Biol Chem. 2000;275:4848–57.
Oakley RH, Busillo JM, Cidlowski JA. Cross-talk between the glucocorticoid receptor and MyoD family inhibitor domain-containing protein provides a new mechanism for generating tissue-specific responses to glucocorticoids. J Biol Chem. 2017;292:5825–44.
Wang Q, Young TM, Mathews MB, Pe’ery T. Developmental regulators containing the I-mfa domain interact with T cyclins and Tat and modulate transcription. J Mol Biol. 2007;367:630–46.
Kusano S, Raab-Traub N. I-mfa domain proteins interact with Axin and affect its regulation of the Wnt and c-Jun N-terminal kinase signaling pathways. Mol Cell Biol. 2002;22:6393–405.
Snider L, Thirlwell H, Miller JR, Moon RT, Groudine M, Tapscott SJ. Inhibition of Tcf3 binding by I-mfa domain proteins. Mol Cell Biol. 2001;21:1866–73.
Novak A, Dedhar S. Signaling through beta-catenin and Lef/Tcf. Cell Mol Life Sci. 1999;56:523–37.
Thebault S, Mesnard JM. How the sequestration of a protein interferes with its mechanism of action: example of a new family of proteins characterized by a particular cysteine-rich carboxy-terminal domain involved in gene expression regulation. Curr Protein Pept Sci. 2001;2:155–67.
Thu KL, Becker-Santos DD, Radulovich N, Pikor LA, Lam WL, Tsao MS. SOX15 and other SOX family members are important mediators of tumorigenesis in multiple cancer types. Oncoscience 2014;1:326–35.
Xu YR, Yang WX. SOX-mediated molecular crosstalk during the progression of tumorigenesis. Semin Cell Dev Biol. 2017;63:23–34.
Zhang M, Wang J, Gao T, Chen X, Xu Y, Yu X, et al. Inhibition of SOX15 sensitizes esophageal squamous carcinoma cells to paclitaxel. Curr Mol Med. 2019;19:349–56.
Dongre A, Weinberg RA. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat Rev Mol Cell Biol. 2019;20:69–84.
Tsoukalas N, Aravantinou-Fatorou E, Tolia M, Giaginis C, Galanopoulos M, Kiakou M, et al. Epithelial-mesenchymal transition in non small-cell lung cancer. Anticancer Res. 2017;37:1773–8.
Wang D, Wen GM, Hou W, Xia P. The roles of CD133 expression in the patients with non-small cell lung cancer. Cancer Biomark. 2018;22:385–94.
Sculier JP, Chansky K, Crowley JJ, Van Meerbeeck J, Goldstraw P, International Staging C, et al. The impact of additional prognostic factors on survival and their relationship with the anatomical extent of disease expressed by the 6th Edition of the TNM Classification of Malignant Tumors and the proposals for the 7th edition. J Thorac Oncol. 2008;3:457–66.
Liu YP, Yang CJ, Huang MS, Yeh CT, Wu AT, Lee YC, et al. Cisplatin selects for multidrug-resistant CD133+ cells in lung adenocarcinoma by activating Notch signaling. Cancer Res. 2013;73:406–16.
Acknowledgements
This study was financially supported by the Ministry of Science and Technology of Taiwan (MOST 107-2320-B-037-025-) and Kaohsiung Medical University Hospital (KMUH106-6M63). This work was also financially supported by the Research Center for Environmental Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan. We thank Dr Michael Hsiao for providing the tissue microarray chips. We thank the assistance of the Department of Pathology and the Biobank in Kaohsiung Medical University Hospital, Kaohsiung, Taiwan for the clinical sample collection. We thank the Center for Research Resources and Development in Kaohsiung Medical University for the instrumental support for the confocal microscope and TissueFAX system. The authors thank the Immunobiology core facility of Clinical Medicine Research Center in National Cheng Kung University Hospital for assisting with the fluorescence-activated cell sorting.
Funding
This study was financially supported by the Ministry of Science and Technology of Taiwan (MOST 107-2320-B-037-025-) and Kaohsiung Medical University Hospital (KMUH105-5M58).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Chen, CJ., Yang, CJ., Yang, SF. et al. The MyoD family inhibitor domain-containing protein enhances the chemoresistance of cancer stem cells in the epithelial state by increasing β-catenin activity. Oncogene 39, 2377–2390 (2020). https://doi.org/10.1038/s41388-019-1152-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-019-1152-4