Abstract
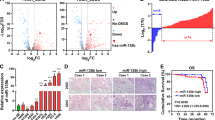
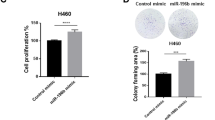
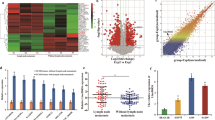
WNT5B glycoprotein belongs to the Wnt protein family. Limited investigations revealed a possible role of WNT5B in malignancies, such as triple-negative breast cancer and oral squamous cell carcinoma. However, whether WNT5B contributes to the progression of lung adenocarcinoma (LAD) remains unclear. Here, we initially determine that WNT5B is highly expressed in LAD and is positively correlated with lymph node metastasis and TNM stage. Consistently, clinical analysis reveals WNT5B as an independent prognostic biomarker in LAD. Silencing WNT5B suppresses the proliferation of LAD both in vitro and in vivo by interfering G1/S cell-cycle progression and modulating amino acid metabolism, revealing its remarkable oncogenic role in LAD. Of note, we also identified miR-5587-3p as a negative upstream regulator of WNT5B in LAD, which may help develop therapies targeting LAD patients with high WNT5B expression. Taken together, our results revealed an oncogenic role of WNT5B in LAD, which could be a prognostic biomarker and promising therapeutic target for LAD patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Chen W, Sun K, Zheng R, Zeng H, Zhang S, Xia C, et al. Cancer incidence and mortality in China, 2014. Chin J cancer Res. 2018;30:1–12.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA: A Cancer J Clinicians. 2018;68:7–30.
Bergethon K, Shaw AT, Ou SH, Katayama R, Lovly CM, McDonald NT, et al. ROS1 rearrangements define a unique molecular class of lung cancers. J Clin Oncol. 2012;30:863–70.
Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–500.
Giroux-Leprieur E, Costantini A, Ding VW, He B. Hedgehog signaling in lung cancer: from oncogenesis to cancer treatment resistance. Int J Mol Sci. 2018;19:E2835.
Skronska-Wasek W, Gosens R, Konigshoff M, Baarsma HA. WNT receptor signalling in lung physiology and pathology. Pharmacol Therapeut. 2018;187:150–66.
Yuan X, Wu H, Han N, Xu H, Chu Q, Yu S, et al. Notch signaling and EMT in non-small cell lung cancer: biological significance and therapeutic application. J Hematol Oncol. 2014;7:87.
Komiya Y, Habas R. Wnt signal transduction pathways. Organogenesis. 2008;4:68–75.
Nusse R, Varmus HE. Wnt genes. Cell. 1992;69:1073–87.
Ghosh S, Roy S, Seguin C, Bryant SV, Gardiner DM. Analysis of the expression and function of Wnt-5a and Wnt-5b in developing and regenerating axolotl (Ambystoma mexicanum) limbs. Dev. Growth Differ. 2008;50:289–97.
Ozeki N, Hase N, Hiyama T, Yamaguchi H, Kawai R, Kondo A, et al. IL-1beta-induced, matrix metalloproteinase-3-regulated proliferation of embryonic stem cell-derived odontoblastic cells is mediated by the Wnt5 signaling pathway. Exp Cell Res. 2014;328:69–86.
Spanjer AI, Baarsma HA, Oostenbrink LM, Jansen SR, Kuipers CC, Lindner M, et al. TGF-beta-induced profibrotic signaling is regulated in part by the WNT receptor Frizzled-8. FASEB J. 2016;30:1823–35.
van Dijk EM, Menzen MH, Spanjer AI, Middag LD, Brandsma CA, Gosens R. Noncanonical WNT-5B signaling induces inflammatory responses in human lung fibroblasts. Am J Physiol Lung Cell Mol Physiol. 2016;310:L1166–76.
Yang L, Perez AA, Fujie S, Warden C, Li J, Wang Y, et al. Wnt modulates MCL1 to control cell survival in triple negative breast cancer. BMC Cancer. 2014;14:124.
Wang SH, Chang JS, Hsiao JR, Yen YC, Jiang SS, Liu SH, et al. Tumour cell-derived WNT5B modulates in vitro lymphangiogenesis via induction of partial endothelial-mesenchymal transition of lymphatic endothelial cells. Oncogene. 2017;36:1503–15.
Weyandt JD, Thompson CB, Giaccia AJ, Rathmell WK. Metabolic alterations in cancer and their potential as therapeutic targets. Am Soc Clin Oncol Educ book Am Soc Clin Oncol Annu Meet. 2017;37:825–32.
Cormerais Y, Giuliano S, LeFloch R, Front B, Durivault J, Tambutte E, et al. Genetic disruption of the multifunctional CD98/LAT1 complex demonstrates the key role of essential amino acid transport in the control of mTORC1 and tumor growth. Cancer Res. 2016;76:4481–92.
Cormerais Y, Massard PA, Vucetic M, Giuliano S, Tambutte E, Durivault J, et al. The glutamine transporter ASCT2 (SLC1A5) promotes tumor growth independently of the amino acid transporter LAT1 (SLC7A5). J Biol Chem. 2018;293:2877–87.
Niu X, Liu S, Jia L, Chen J. Role of MiR-3619-5p in beta-catenin-mediated non-small cell lung cancer growth and invasion. Cell Physiol Biochem: Int J Exp Cell Physiol, Biochem, Pharmacol. 2015;37:1527–36.
Xi S, Yang M, Tao Y, Xu H, Shan J, Inchauste S, et al. Cigarette smoke induces C/EBP-beta-mediated activation of miR-31 in normal human respiratory epithelia and lung cancer cells. PloS ONE. 2010;5:e13764.
Hou J, Aerts J, den Hamer B, van Ijcken W, den Bakker M, Riegman P, et al. Gene expression-based classification of non-small cell lung carcinomas and survival prediction. PloS ONE. 2010;5:e10312.
Lu TP, Tsai MH, Lee JM, Hsu CP, Chen PC, Lin CW, et al. Identification of a novel biomarker, SEMA5A, for non-small cell lung carcinoma in nonsmoking women. Cancer Epidemiol, Biomark Prev. 2010;19:2590–7.
Dato S, Hoxha E, Crocco P, Iannone F, Passarino G, Rose G. Amino acids and amino acid sensing: implication for aging and diseases. Biogerontology. 2019;20:17–31.
Krishnamurthy N, Kurzrock R. Targeting the Wnt/beta-catenin pathway in cancer: update on effectors and inhibitors. Cancer Treat Rev. 2018;62:50–60.
Nusse R. Wnt signaling in disease and in development. Cell Res. 2005;15:28–32.
Tian J, He H, Lei G. Wnt/beta-catenin pathway in bone cancers. Tumour Biol: J Int Soc Oncodev Biol Med. 2014;35:9439–45.
Behari J, Li H, Liu S, Stefanovic-Racic M, Alonso L, O’Donnell CP, et al. beta-catenin links hepatic metabolic zonation with lipid metabolism and diet-induced obesity in mice. Am J Pathol. 2014;184:3284–98.
Grant SF, Thorleifsson G, Reynisdottir I, Benediktsson R, Manolescu A, Sainz J, et al. Variant of transcription factor 7-like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nat Genet. 2006;38:320–3.
Kanazawa A, Tsukada S, Sekine A, Tsunoda T, Takahashi A, Kashiwagi A, et al. Association of the gene encoding wingless-type mammary tumor virus integration-site family member 5B (WNT5B) with type 2 diabetes. Am J Hum Genet. 2004;75:832–43.
Mani A, Radhakrishnan J, Wang H, Mani A, Mani MA, Nelson-Williams C, et al. LRP6 mutation in a family with early coronary disease and metabolic risk factors. Science. 2007;315:1278–82.
Sherwood V. WNT signaling: an emerging mediator of cancer cell metabolism? Mol Cell Biol. 2015;35:2–10.
Habas R, Dawid IB. Dishevelled and Wnt signaling: is the nucleus the final frontier? J Biol. 2005;4:2.
Wang Q, Symes AJ, Kane CA, Freeman A, Nariculam J, Munson P, et al. A novel role for Wnt/Ca2+ signaling in actin cytoskeleton remodeling and cell motility in prostate cancer. PloS ONE. 2010;5:e10456.
Dissanayake SK, Wade M, Johnson CE, O’Connell MP, Leotlela PD, French AD, et al. The Wnt5A/protein kinase C pathway mediates motility in melanoma cells via the inhibition of metastasis suppressors and initiation of an epithelial to mesenchymal transition. J Biol Chem. 2007;282:17259–71.
Rapp J, Jaromi L, Kvell K, Miskei G, Pongracz JE. WNT signaling - lung cancer is no exception. Respiratory Res. 2017;18:167.
Stewart DJ. Wnt signaling pathway in non-small cell lung cancer. J Natl Cancer Inst. 2014;106:djt356.
Watanabe K, Dai X. Winning WNT: race to Wnt signaling inhibitors. Proc Natl Acad Sci USA. 2011;108:5929–30.
Rydell-Tormanen K, Zhou XH, Hallgren O, Einarsson J, Eriksson L, Andersson-Sjoland A, et al. Aberrant nonfibrotic parenchyma in idiopathic pulmonary fibrosis is correlated with decreased beta-catenin inhibition and increased Wnt5a/b interaction. Physiological Rep. 2016;4:e12727.
Zhang Q, Fan H, Zou Q, Liu H, Wan B, Zhu S, et al. TEAD4 exerts pro-metastatic effects and is negatively regulated by miR6839-3p in lung adenocarcinoma progression. J Cell Mol Med. 2018;22:3560–71.
Zhang Q, Yuan L, Liu D, Wang J, Wang S, Zhang Q, et al. Hydrogen sulfide attenuates hypoxia-induced neurotoxicity through inhibiting microglial activation. Pharmacol Res. 2014;84:32–44.
Zhan P, Xi GM, Zhang B, Wu Y, Liu HB, Liu YF, et al. NCAPG2 promotes tumour proliferation by regulating G2/M phase and associates with poor prognosis in lung adenocarcinoma. J Cell Mol Med. 2017;21:665–76.
Zhang Q, Hu H, Liu H, Jin J, Zhu P, Wang S, et al. RNA sequencing enables systematic identification of platelet transcriptomic alterations in NSCLC patients. Biomed Pharmacother. 2018;105:204–14.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (grant numbers 81572937, 81572273, and 81772500); the16th batch “Summit of the Six Top Talents” Program of Jiangsu Province (grant number WSN-154); China Postdoctoral Science Foundation 12th batch Special fund (Postdoctoral number: 45786); China Postdoctoral Science Foundation 64th batch (Postdoctoral number: 45786); Jiangsu Provincial Postdoctoral Science Foundation (grant number 2018K049A); the Natural Science Foundation of Jiangsu province (grant numbers BK20180139 and BK20161386); Jiangsu Provincial Medical Youth Talent (grant number QNRC2016125), and the Nanjing Medical Science and Technology Development Project (No. ZKX17044), the Jiangsu Provincial Key Research and Development Program (No. BE2016721).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Zhang, Q., Fan, H., Liu, H. et al. WNT5B exerts oncogenic effects and is negatively regulated by miR-5587-3p in lung adenocarcinoma progression. Oncogene 39, 1484–1497 (2020). https://doi.org/10.1038/s41388-019-1071-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-019-1071-4
This article is cited by
-
Protein tyrosine phosphatase PTPRO represses lung adenocarcinoma progression by inducing mitochondria-dependent apoptosis and restraining tumor metastasis
Cell Death & Disease (2024)
-
WNT ligands in non-small cell lung cancer: from pathogenesis to clinical practice
Discover Oncology (2023)
-
SRPK1/2 and PP1α exert opposite functions by modulating SRSF1-guided MKNK2 alternative splicing in colon adenocarcinoma
Journal of Experimental & Clinical Cancer Research (2021)
-
Inhibition of the bromodomain and extra-terminal family of epigenetic regulators as a promising therapeutic approach for gastric cancer
Cellular Oncology (2021)