Abstract
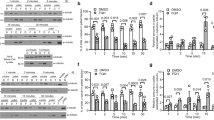
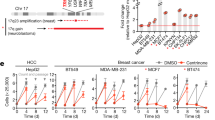
The nuclear transport receptor importin-β/karyopherin-β1 is overexpressed in cancers that display genomic instability. It is regarded as a promising cancer target and inhibitors are being developed. In addition to its role in nucleo-cytoplasmic transport, importin-β regulates mitosis, but the programmes and pathways in which it operates are defined only in part. To unravel importin-β’s mitotic functions we have developed cell lines expressing either wild-type or a mutant importin-β form in characterised residues required for nucleoporin binding. Both forms similarly disrupted spindle pole organisation, while only wild-type importin-β affected microtubule plus-end function and microtubule stability. A proteome-wide search for differential interactors identified a set of spindle regulators sensitive to mutations in the nucleoporin-binding region. Among those, HURP (hepatoma up-regulated protein) is an importin-β interactor and a microtubule-stabilising factor. We found that induction of wild type, but not mutant importin-β, under the same conditions that destabilise mitotic microtubules, delocalised HURP, indicating that the spatial distribution of HURP along the spindle requires importin-β’s nucleoporin-binding residues. Concomitantly, importin-β overexpression sensitises cells to taxanes and synergistically increases mitotic cell death. Thus, the nucleoporin-binding domain is dispensable for importin-β function in spindle pole organisation, but regulates microtubule stability, at least in part via HURP, and renders cells vulnerable to certain microtubule-targeting drugs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
van der Watt PJ, Maske CP, Hendricks DT, Parker MI, Denny L, Govender D, et al. The Karyopherin proteins, Crm1 and Karyopherinbeta1, are overexpressed in cervical cancer and are critical for cancer cell survival and proliferation. Int J Cancer. 2009;124:1829–40.
Martens de Kemp SR, Nagel R, Stigter-van Walsum M, van der Meulen IH, van BeusechemVW, Braakhuis BJ, et al. Functional genetic screens identify genes essential for tumor cell survival in head and neck and lung cancer. Clin Cancer Res. 2013;19:1994–2003.
Zhu J, Wang Y, Huang H, Yang Q, Cai J, Wang Q, et al. Upregulation of KPNβ1 in gastric cancer cell promotes tumor cell proliferation and predicts poor prognosis. Tumour Biol. 2016;37:661–72.
Yang L, Hu B, Zhang Y, Qiang S, Cai J, Huang W, et al. Suppression of the nuclear transporter- KPNβ1 expression inhibits tumor proliferation in hepatocellular carcinoma. Med Oncol. 2015;32:128.
Yang J, Guo Y, Lu C, Zhang R, Wang Y, Luo L, et al. Inhibition of Karyopherin beta 1 suppresses prostate cancer growth. Oncogene; 38:4700–14.
He S, Miao X, Wu Y, Zhu X, Miao X, Yin H, et al. Upregulation of nuclear transporter, Kpnβ1, contributes to accelerated cell proliferation- and cell adhesion-mediated drug resistance (CAM-DR) in diffuse large B-cell lymphoma. J Cancer Res Clin Oncol. 2016;142:561–72.
Yan W, Li R, He J, Du J, Hou J. importin-β1 mediates nuclear factor-κB signal transduction into the nuclei of myeloma cells and affects their proliferation and apoptosis. Cell Signal. 2015;27:851–9.
Lu T, Bao Z, Wang Y, Yang L, Lu B, Yan K, et al. Karyopherinβ1 regulates proliferation of human glioma cells via Wnt/β-catenin pathway. Biochem Biophys Res Commun. 2016;478:1189–97.
van der Watt PJ, Ngarande E, Leaner VD. Overexpression of Kpnβ1 and Kpnα2 importin proteins in cancer derives from deregulated E2F activity. PLoS ONE. 2011;6:e27723.
van der Watt PJ, Stowell CL, Leaner VD. The nuclear import receptor Kpnβ1 and its potential as an anticancer therapeutic target. Crit Rev Eukaryot Gene Expr. 2013;23:1–10.
Stelma T, Chi A, van der Watt PJ, Verrico A, Lavia P, Leaner VD. Targeting nuclear transporters in cancer: Diagnostic, prognostic and therapeutic potential. IUBMB Life. 2016;68:268–80.
Soderholm JF, Bird SL, Kalab P, Sampathkumar Y, Hasegawa K, Uehara-Bingen M, et al. Importazole, a small molecule inhibitor of the transport receptor importin-β. ACS Chem Biol. 2011;6:700–8.
Kosyna FK, Nagel M, Kluxen L, Kraushaar K, Depping R. The importin-α/β-specific inhibitor Ivermectin affects HIF-dependent hypoxia response pathways. Biol Chem. 2015;396:1357–67.
van der Watt PJ, Chi A, Stelma T, Stowell C, Strydom E, Carden S, et al. Targeting the nuclear import receptor Kpnβ1 as an anticancer therapeutic. Mol Cancer Ther. 2016;15:560–73.
Wang C, Yang SNY, Smith K, Forwood JK, Jans DA. Nuclear import inhibitor N-(4-hydroxyphenyl) retinamide targets Zika virus (ZIKV) nonstructural protein 5 to inhibit ZIKV infection. Biochem Biophys Res Commun. 2017;493:1555–59.
Ha S, Choi J, Min NY, Lee KH, Ham SW. Inhibition of importin-β1 with a 2-aminothiazole derivative resulted in G2/M cell-cycle arrest and apoptosis. Anticancer Res. 2017;37:2373–9.
Forwood JK, Lange A, Zachariae U, Marfori M, Preast C, Grubmüller H, et al. Quantitative structural analysis of importin-β flexibility: paradigm for solenoid protein structures. Structure. 2010;18:1171–83.
Christie M, Chang CW, Róna G, Smith KM, Stewart AG, Takeda AA, et al. Structural biology and regulation of protein import into the nucleus. J Mol Biol. 2016;428:2060–90.
Bayliss R, Littlewood T, Stewart M. Structural basis for the interaction between FxFG nucleoporin repeats and importin- beta in nuclear trafficking. Cell 2000;102:99–108.
Bayliss R, Littlewood T, Strawn LA, Wente SR, Stewart M. GLFG and FxFG nucleoporins bind to overlapping sites on importin-beta. J Biol Chem. 2002;277:50597–606.
Kimura M, Morinaka Y, Imai K, Kose S, Horton P, Imamoto N. Extensive cargo identification reveals distinct biological roles of the 12 importin pathways. Elife. 2017;6:e21184.
Kimura M, Okumura N, Kose S, Takao T, Imamoto N. Identification of cargo proteins specific for importin-β with importin-α applying a stable isotope labeling by amino acids in cell culture (SILAC)-based in vitro transport system. J Biol Chem. 2013;288:24540–9.
Di Francesco L, Verrico A, Asteriti IA, Rovella P, Cirigliano P, Guarguaglini G, et al. Visualization of human karyopherin beta-1/importin-beta-1 interactions with protein partners in mitotic cells by co-immunoprecipitation and proximity ligation assays. Sci Rep 2018;8:1850.
Smith AE, Slepchenko BM, Schaff JC, Loew LM, Macara IG. Systems analysis of Ran transport. Science. 2002;295:488–91.
Görlich D, Seewald MJ, Ribbeck K. Characterization of Ran-driven cargo transport and the RanGTPase system by kinetic measurements and computer simulation. EMBO J. 2003;22:1088–100.
Forbes DJ, Travesa A, Nord MS, Bernis C. Nuclear transport factors: global regulation of mitosis. Curr Opin Cell Biol. 2015;35:78–90.
Çağatay T, Chook YM. Karyopherins in cancer. Curr Opin Cell Biol. 2018;52:30–42.
Hezwani M, Fahrenkrog B. The functional versatility of the nuclear pore complex proteins. Semin Cell Dev Biol. 2017;68:2–9.
Eifler K, Vertegaal ACO. SUMOylation-mediated regulation of cell cycle progression and cancer. Trends Biochem Sci. 2015;40:779–93.
Ritterhoff T, Das H, Hofhaus G, Schröder RR, Flotho A, Melchior F. The RanBP2 /RanGAP1*SUMO1/Ubc9 SUMO E3 ligase is a disassembly machine for Crm1-dependent nuclear export complexes. Nat Commun. 2016;7:11482.
Joseph J, Liu ST, Jablonski SA, Yen TJ, Dasso M. The RanGAP1-RanBP2 complex is essential for microtubule-kinetochore interactions in vivo. Curr Biol. 2004;14:611–7.
Roscioli E, Di Francesco L, Bolognesi A, Giubettini M, Orlando S, Harel A, et al. Importin-beta negatively regulates multiple aspects of mitosis including RANGAP1 recruitment to kinetochores. J Cell Biol. 2012;196:435–50.
Gilistro E, de Turris V, Damizia M, Verrico A, Moroni S, De Santis R, et al. Importin-beta and CRM1 control a RANBP2 spatiotemporal switch essential for mitotic kinetochore function. J Cell Sci. 2017;130:256478.
Song L, Rape M. Regulated degradation of spindle assembly factors by the anaphase-promoting complex. Mol Cell. 2010;38:369–82.
Jiang H, He X, Feng D, Zhu X, Zheng Y. RanGTP aids anaphase entry through Ubr5-mediated protein turnover. J Cell Biol. 2015;211:7–18.
Tsou AP, Yang CW, Huang CY, Yu RC, Lee YC, Chang CW, et al. Identification of a novel cell cycle regulated gene, HURP, overexpressed in human hepatocellular carcinoma. Oncogene. 2003;22:298–307.
Sillje HH, Nagel S, Korner R, Nigg EA. HURP is a Ran-importin-beta-regulated protein that stabilizes kinetochore microtubules in the vicinity of chromosomes. Curr Biol. 2006;16:731–42.
Koffa MD, Casanova CM, Santarella R, Köcher T, Wilm M, Mattaj IW. HURP is part of a Ran- dependent complex involved in spindle formation. Curr Biol. 2006;16:743–54.
Ciciarello M, Mangiacasale R, Thibier C, Guarguaglini G, Marchetti E, Di Fiore B, et al. Importin-β is transported to spindle poles during mitosis and regulates Ran-dependent spindle assembly factors in mammalian cells. J Cell Sci. 2004;117:6511–22.
Wong J, Fang G. HURP controls spindle dynamics to promote proper interkinetochore tension and efficient kinetochore capture. J Cell Biol. 2006;173:879–91.
Schmitz MH, Held M, Janssens V, Hutchins JR, Hudecz O, Ivanova E, et al. Live-cell imaging RNAi screen identifies PP2A-B55alpha and importin-beta1 as key mitotic exit regulators in human cells. Nat Cell Biol. 2010;12:886–93.
Linder MI, Köhler M, Boersema P, Weberruss M, Wandke C, Marino J, et al. Mitotic disassembly of nuclear pore complexes involves CDK1- and PLK1-mediated phosphorylation of key interconnecting nucleoporins. Dev Cell. 2017;43:141–56.
Lavia P. TheGTPase RAN regulates multiple steps of the centrosome life cycle. Chromosome Res. 2016;24:53–65.
Tedeschi A, Ciciarello M, Mangiacasale R, Roscioli E, Rensen WM, Lavia P. RANBP1 localizes a subset of mitotic regulatory factors on spindle microtubules and regulates chromosome segregation in human cells. J Cell Sci. 2007;120:3748–61.
Sloss O, Topham C, Diez M, Taylor S. Mcl-1 dynamics influence mitotic slippage and death in mitosis. Oncotarget. 2016;7:5176–92.
Haschka MD, Soratroi C, Kirschnek S, Häcker G, Hilbe R, Geley S, et al. The NOXA-MCL1-BIM axis defines lifespan on extended mitotic arrest. Nat Commun. 2015;6:6891.
Lee MS, Huang YH, Huang SP, Lin RI, Wu SF, Li C. Identification of a nuclear localization signal in the polo box domain of Plk1. Biochim Biophys Acta. 2009;1793:1571–8.
Santarella RA, Koffa MD, Tittmann P, Gross H, Hoenger A. HURP wraps microtubule ends with an additional tubulin sheet that has a novel conformation of tubulin. J Mol Biol. 2007;365:1587–95.
Yu CT, Hsu JM, Lee YC, Tsou AP, Chou CK, Huang CY. Phosphorylation and stabilization of HURP by Aurora-A: implication of HURP as a transforming target of Aurora-A. Mol Cell Biol. 2005;25:5789–800.
Wu JM, Chen CT, Coumar MS, Lin WH, Chen ZJ, Hsu JT, et al. Aurora kinase inhibitors reveal mechanisms of HURP in nucleation of centrosomal and kinetochore microtubules. Proc Natl Acad Sci USA. 2013;110:E1779–87.
Wong J, Lerrigo R, Jang CY, Fang G. Aurora A regulates the activity of HURP by controlling the accessibility of its microtubule-binding domain. Mol Biol Cell. 2008;19:2083–91.
Kuo TC, Lu HP, Chao CC. The tyrosine kinase inhibitor sorafenib sensitizes hepatocellular carcinoma cells to taxol by suppressing the HURP protein. Biochem Pharmacol. 2011;82:184–94.
Rosa A, Papaioannou MD, Krzyspiak JE, Brivanlou AH. miR-373 is regulated by TGFβ signaling and promotes mesendoderm differentiation in human Embryonic Stem Cells. Dev Biol. 2014;391:81–8.
Di Cesare E, Verrico A, Miele A, Giubettini M, Rovella P, Coluccia A, et al. Mitotic cell death induction by targeting the mitotic spindle with tubulin-inhibitory indole derivative molecules. Oncotarget. 2017;8:19738 59.
Acknowledgements
This work was supported by grants from AIRC (Associazione Italiana per la Ricerca sul Cancro IG14534) and the CNR Flagship programme InterOmics (project SYNLETHER). MD was supported by a PhD fellowship from MIUR. We are grateful to our colleagues Alessandro Rosa (Department of Biology and Biotechnology, University La Sapienza, Rome) for the design and engineering of inducible expression vectors, to Enrico Cundari and Daniela Trisciuoglio (IBPM-CNR, Rome) for help with FACS experiments, to Maria Giubettini (Joint Lab CrestOptics/IIT CLNS@Sapienza, Rome) for contributing to early stages of this work, and to Veronica Morea and Andrea Ilari (IBPM-CNR and Biocrystal Facility, Rome) for insightful discussions and suggestions. We wish to dedicate this paper to our late Director and founder, Prof. Emilia Chiancone.
Author information
Authors and Affiliations
Contributions
AV, PR and MD designed and performed molecular and cell biology experiments. LDF and MES performed the proteomics studies. DSS and LLP performed bioinformatic analyses. AV, LLP, MES and PL wrote the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Verrico, A., Rovella, P., Di Francesco, L. et al. Importin-β/karyopherin-β1 modulates mitotic microtubule function and taxane sensitivity in cancer cells via its nucleoporin-binding region. Oncogene 39, 454–468 (2020). https://doi.org/10.1038/s41388-019-0989-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-019-0989-x
This article is cited by
-
Progress in the study of parvovirus entry pathway
Virology Journal (2023)
-
Inhibiting Importin 4-mediated nuclear import of CEBPD enhances chemosensitivity by repression of PRKDC-driven DNA damage repair in cervical cancer
Oncogene (2020)
-
Importin 13 promotes NSCLC progression by mediating RFPL3 nuclear translocation and hTERT expression upregulation
Cell Death & Disease (2020)